1. Introduction
Morbidity and mortality rates associated with
diabetes mellitus (DM) are high and result from
hyperglycemia-related complications that can develop in various
organ systems. The mechanisms involved in the development of
complications are versatile and varied, with each complication
being organ-specific (1). Diabetic
neuropathy (DNP) is one of the most prevalent complications of DM,
which affects >50% of individuals with long-standing DM. It is a
microvascular complication characterized by pain and high morbidity
rates, mostly caused by damage to the somatosensory nervous system.
The longevity of diabetes and hemoglobin A1c levels are two key
predictors of DNP. The latter is often linked to poor glycemic
control, genetic predisposition, environmental variables, metabolic
factors and cardiovascular risk factors (2).
Even though there are several factors at play in the
development of DNP and the precise pathogenic process remains
unknown, numerous theories can be described. Hyperglycemia and a
complex metabolic imbalance, primarily oxidative stress (OS), are
current hypotheses (3). Since
neurons obtain glucose via facilitated concentration-dependent
transport, they are probably more vulnerable to glucose flow, which
leads to elevated levels of OS. The polyol pathway, diacylglycerol
(DAG)/protein kinase C (PKC), hexosamine biosynthetic pathway
(HBP), advanced glycation end products (AGEs)/inflammation and
nitric oxide all play critical roles in DNP. Evidence suggests that
OS plays a role in each of the aforementioned pathways (4-6).
Despite concerted efforts to detect and prevent the
progression of DNP, there are presently only a limited number of
alleviative medications available; the remainder essentially
provide symptomatic improvement. In the meantime, the present
objective of DNP treatment is to improve quality of life and
functionality of patients, while reducing pain. However, beyond
glycemic control, several antidiabetic medications can alleviate
DNP and prevent its progression (7-9).
The aim of the present review was to synthesize currently available
knowledge on OS-related mechanisms in DNP, while critically
appraising the evidence on the antioxidant potential of various
antidiabetic drug classes. By integrating mechanistic insights with
clinical outcomes, the present review aimed to bridge the gap
between basic the pathophysiological understanding and therapeutic
applications for this condition. Unlike prior reviews, the present
review discusses antidiabetic agents, such as metformin,
pioglitazone, dapagliflozin and others, with emphasis on OS
modulation and mechanistic pathways linked to diabetic neuropathy
and integrates clinical prescribing recommendations, providing a
practical translation of mechanistic insights into the management
of diabetes.
2. Clinical spectrum of DNP
The dorsal root ganglia neurons are subjected to
systemic metabolic and hypoxic stresses, rendering them very
sensitive to damage. The structure of the sensory system outside
the blood-brain barrier may also explain its extraordinary
vulnerability. DNP presents as a diffuse neuropathy (distal
symmetrical polyneuropathy or autonomic neuropathy), as well as
mononeuropathy or radiculopathy/polyradiculopathy. Distal symmetric
polyneuropathy (DSPN), the most common form of DNP, is mostly
accompanied by pain and distal sensory loss. However, approximately
half of the patients do not experience any symptoms (10). The symptoms are usually manifested
as a perception of tingling, numbness, sharpness, or burning that
begins in the feet and extends proximally. The clinical feature of
DSPN is that symptoms manifest in the lower extremities at rest and
worsen at night. Paresthesia, hyperesthesia and dysesthesia may
also occur. The pain becomes less severe over time with DSPN;
however, there is still a loss of sensory function, and motor
dysfunction may appear (11).
Another form of DNP is autonomic neuropathy, which
is presented as autonomic dysfunction and is manifested in patients
with long-term DM, affecting both the parasympathetic and
sympathetic nervous systems. Thus, it can affect a number of
systems, including the cardiovascular system. Complications of
cardiac autonomic neuropathy include resting tachycardia, exercise
intolerance, orthostatic hypotension associated with dizziness,
silent myocardial ischemia and an increased risk of sudden death
syndrome. Other systems that are also affected include the
gastrointestinal (bloating, diarrhea, or constipation),
genitourinary (infections, bladder dysfunction, or sexual
dysfunction) and metabolic (sweating abnormalities, and trouble
identifying low blood sugar) systems (12). Mononeuropathy, a less prevalent
form of DNP, manifests as a painful sensation and a lack of
strength in the muscles along with motor dysfunction in a
particular nerve. Mononeuropathy involves the malfunctioning of
isolated cranial or peripheral nerves and may be non-compressive or
arise at entrapment sites such as as the carpal tunnel. Other
examples of peripheral mononeuropathies include ulnar neuropathy at
the elbow, peroneal neuropathy at the fibular head, radial
neuropathy causing wrist drop, and femoral neuropathy with
quadriceps weakness (13).
3. Oxidative stress in diabetes
OS describes a condition when there is an imbalance
between the generation of oxidants inside the body and the
endogenous antioxidant system. Free radicals or other oxidants
mediate OS. Free radicals include reactive oxygen species (ROS),
which are the most critical, and reactive nitrogen species (RNS).
Previous population studies on DM and its chronic complications
have provided evidence of an association between DM and OS
(14-16).
ROS are naturally occurring oxygen-containing free
radicals that result from oxygen metabolism as a byproduct. These
include hydrogen peroxide, superoxide anion radicals, hypochlorite,
oxygen singlet and hydroxyl radicals. They arise inside organelles,
such as the mitochondria, endoplasmic reticulum and peroxisomes.
Mitochondrial OS impairs insulin signaling, leading to insulin
resistance, and contributes to pancreatic β-cell dysfunction and
death (15). When the protein
folding capacity of the endoplasmic reticulum is overwhelmed, it
triggers an unfolded protein response that, if prolonged, can lead
to inflammation and the apoptosis of insulin-producing cells and
insulin resistance. The dysfunction of peroxisomes worsens the
metabolic imbalances observed in diabetic patients and interferes
with insulin secretion. However, RNS comprise nitric oxide, the
nitroxyl ion, peroxynitrite anion, nitrosyl-containing compounds,
and organic hydroperoxide (17).
To blunt or scavenge the excessive generation of
ROS, and consequently OS, cells have a variety of defensive
mechanisms. Antioxidant enzymes, such as glutathione peroxidase,
catalase, and superoxide dismutase (SOD) are among these (18). Glutathione peroxidase eliminates
hydrogen peroxide and lipid peroxides via detoxification, whereas
SOD scavenges superoxide radicals by promoting their conversion
into hydrogen peroxide. Catalase catalyzes the decomposition of
hydrogen peroxide into oxygen and water. Hyperglycemia can suppress
the endogenous antioxidant defense system, which may alter the
activity of antioxidant enzymes. For instance, SOD is known to be
inactivated by increased hydrogen peroxide concentration, although
its activity may also be reduced by glycosylation of the enzyme
and/or loss of copper, an essential cofactor. OS in diabetic
patients may arise due to either an increase in the generation of
free radicals or a decline in the protective mechanisms of
antioxidants (19). Another
mechanism for scavenging the excessive generation of ROS is the
non-enzymatic antioxidants. These include metal-binding proteins
such as ferritin, which sequester pro-oxidant metals; glutathione,
a primary cellular reductant that neutralizes free radicals; uric
acid, a potent scavenger of hydroxyl radicals; melatonin, a potent
antioxidant and free radical scavenger that easily crosses cell
membranes; bilirubin, which provides lipophilic antioxidant
protection; and polyamines, which stabilize cellular structures and
directly scavenge ROS (20).
4. Mechanisms of oxidant generation in
DNP
Evidence is presented to support the hypothesis that
both chronic and acute high blood sugar levels lead to OS in the
peripheral nervous system, which may contribute to the onset of
DNP. Various damaging molecular mechanisms may clarify the adverse
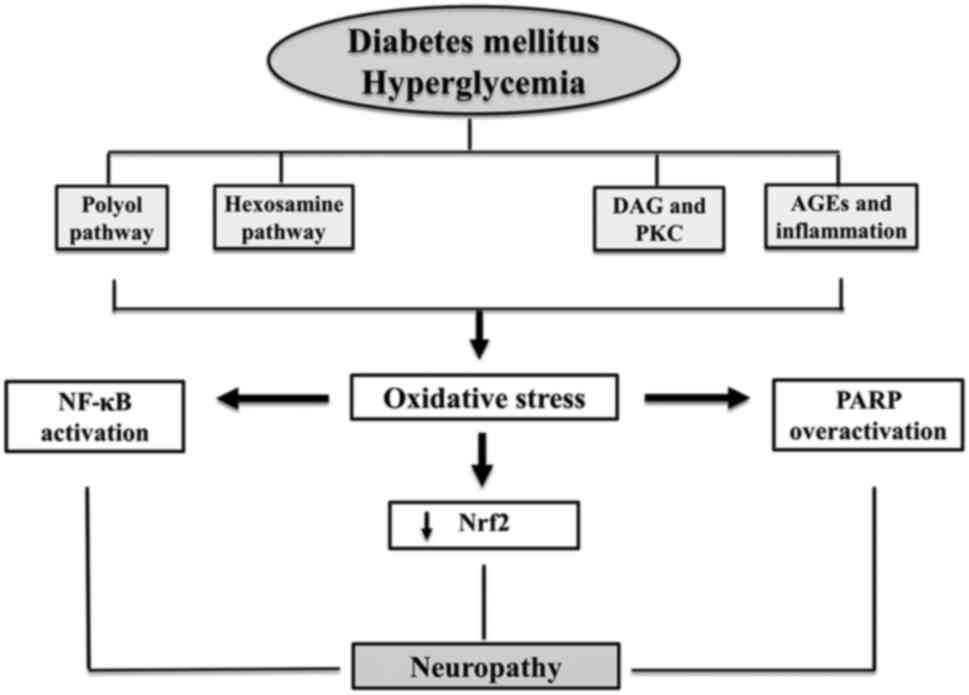
consequences of reactive oxidants in DNP generated by hyperglycemia
(Fig. 1). These mechanisms include
the polyol pathway and HBP, which have consistently been recognized
in patients with DNP. The AGE and PKC pathways exert direct or
indirect effects on proteins, lipids, and DNAs via glucose. All
these are associated with DNP through the excessive production of
ROS, a distinguishing characteristic found in all cell types
affected by hyperglycemia (21).
OS, when combined with hyperglycemia, triggers the
induction of poly(ADP-ribose) polymerase (PARP), which then breaks
down nicotinamide adenine dinucleotide (NAD+) into
nicotinamide and ADP-ribose fragments. This process proceeds via
the interaction with nuclear proteins, leading to alterations in
gene expression and transcription, depletion of NAD+,
and the redirection of glycolytic products towards other
disease-causing mechanisms, such as PKC and AGEs (21,22).
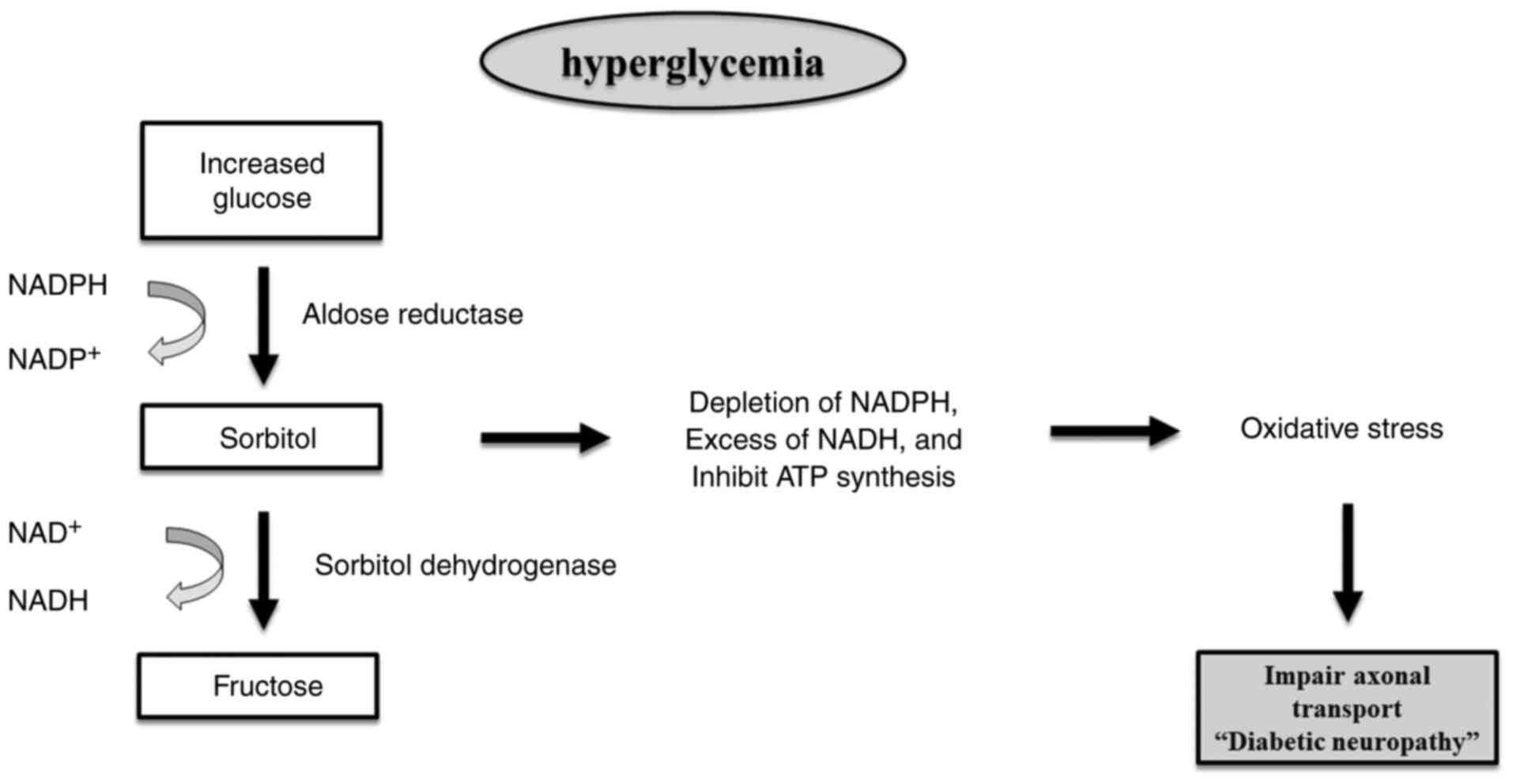
Activated polyol pathway
Under conditions of hyperglycemia, excess glucose
saturates glycolysis in nerve cells, diverting it into the polyol
pathway (Fig. 2). This pathway,
involving aldose reductase and sorbitol dehydrogenase, converts
glucose to sorbitol and then fructose, consuming nicotinamide
adenine dinucleotide phosphate (NADPH) and generating NADH. This
process markedly contributes to ROS production, driving OS
(23).
The polyol pathway results in a decline in
intracellular NADPH levels and an accumulation of NADH. The greater
production of NADH serves as a substrate for NADH oxidase, leading
to the production of ROS. Intracellular hyperosmolarity occurs due
to an elevated polyol flow, which leads to the buildup of
impermeable sorbitol and the efflux of other osmolytes.
Consequently, the inhibition of adenosine triphosphate (ATP)
production occurs (24). The
conversion of glucose to sorbitol by the action of aldose reductase
leads to the utilization of NADPH. Since NADPH is necessary for the
reformation of reduced glutathione, this process directly adds to
OS generation. Furthermore, the conversion of sorbitol into
fructose contributes to glycation, leading to reduced NADPH
availability and elevated AGEs, all of which contribute to a
significant disruption in redox balance (25).
As regards DNP, the peripheral nerves of diabetic
patients have been shown to exhibit an accumulation of sorbitol and
fructose. Additionally, the shunting of glycolytic intermediates to
the polyol pathway increases glycation and the generation of DAG in
the dorsal root ganglia. These processes minimize the activity of
Na+/K+-ATPase, suppressed axonal transport
and the structural deterioration of nerves, ultimately manifesting
as an abnormal action potential. Therefore, the suppression of the
polyol pathway remains a focal point for therapeutic development in
the control of diabetic neuropathy (26,27).
HBP
Diabetic complications, including DNP, may be caused
by the shunting of excess glucose in nerve cells into another
pathway rather than glycolysis, including the HBP, in addition to
the polyol pathway (28). The most
commonly proposed mechanism by which HBP contributes to DNP is the
effect of intracellular uridine-5-diphosphat-N-acetylglucosamine
(UDP-GlcNAc) on the modification of proteins (Fig. 3). Under healthy conditions, the HBP
represents a minor pathway of the glycolytic system, with
glutamine-fructose-6-phosphate aminotransferase (GFAT), the
rate-limiting enzyme, converting only 2 to 5% of
fructose-6-phosphate to glucosamine-6-phosphate (29).
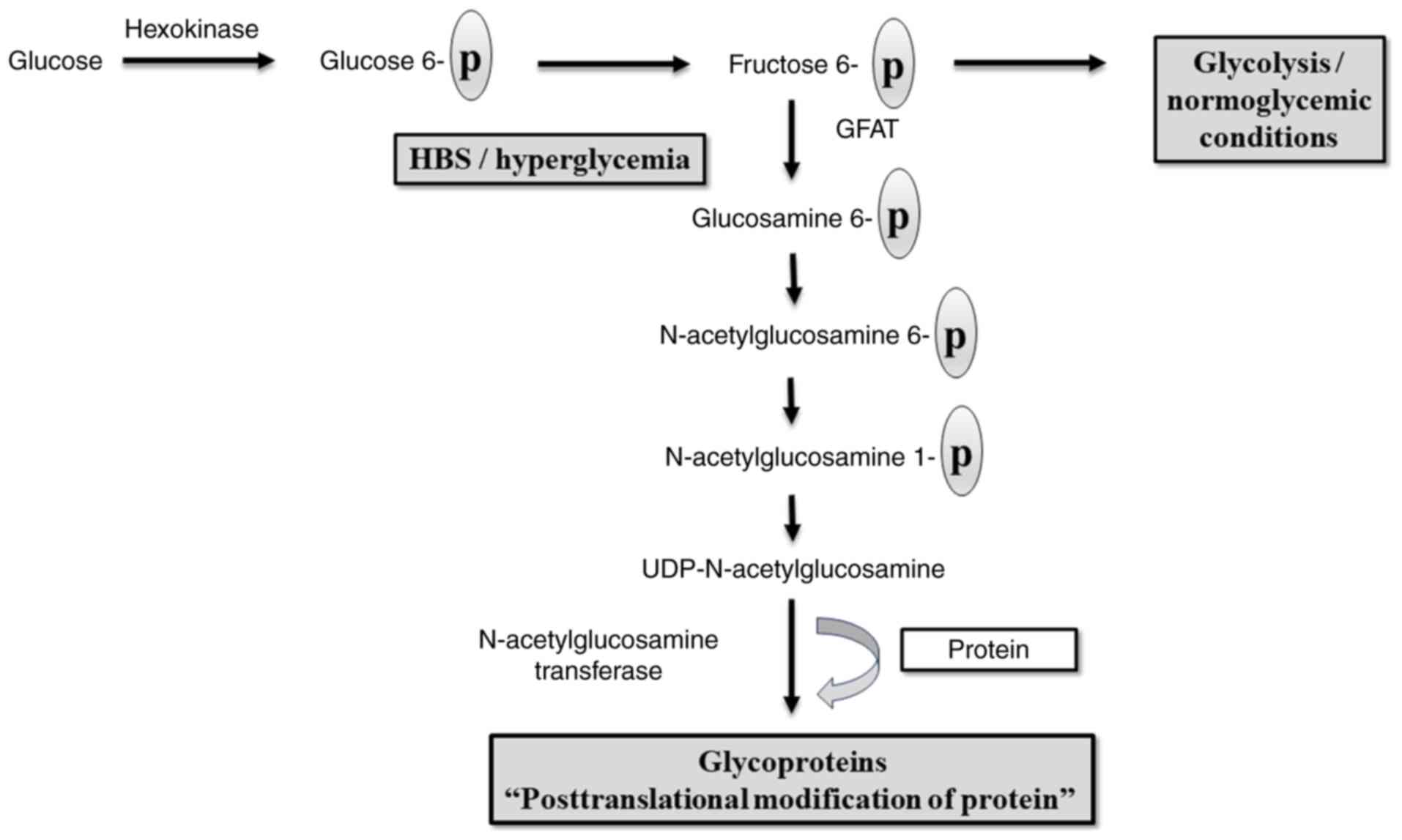 | Figure 3Hexosamine biosynthetic pathway:
Under normal conditions, glucose undergoes a phosphorylation
process catalyzed by hexokinase, and phosphorylated glucose is
formed which is then converted to fructose that undergoes
glycolysis. In hyperglycemia, shifting from glycolysis into a
hexosamine biosynthetic pathway occurs resulting in the formation
of UDP-N-acetylglucosamine, the rate-limiting step is catalyzed by
GFAT. UDP-N-acetylglucosamine interferes with the action of
N-acetylglucosamine transferase, an enzyme that interferes with the
action of a number of proteins, including insulin receptor
substrate and glucose transporter protein (102,103). GFAT,
glutamine-fructose-6-phosphate aminotransferase; HBS, hexosamine
biosynthetic pathway; UDP, uridine diphosphate. |
However, under conditions of hyperglycemia, the
increased generation of ROS inside the mitochondria hinders the
action of the glycolytic enzyme glyceraldehyde-3-phosphate
dehydrogenase, which inhibits fructose-6-phosphate from flowing
through glycolysis (30).
Subsequently, UDP-GlcNAc is established from
glucosamine-6-phosphate, acetyl-CoA, and uridine-5-triphosphate.
UDP-GlcNAc regulates the activity of O-linked
N-acetylglucosamine transferase, which is present in both
the nucleus and cytosol. The latter is an enzyme that transfers
N-acetylglucosamine (GlcNAc) to certain serine and threonine
residues on proteins, allowing for a reversible posttranslational
modification. Notable proteins that have undergone O-GlcNAcylation,
including glucose transporter 4 and insulin receptor substrates 1
and 2, result in insulin resistance (31,32).
Concerning DNP, there was a noticeable increase in
GFAT activity and UDP-GlcNAc levels in the muscle of ob/ob mice. By
contrast, mice with continuous caloric restriction exhibit a
decrease in the UDP-GlcNAc concentration, which is accompanied by
an improvement in insulin sensitivity in their muscles (33). Since peripheral nerves are highly
metabolically active and insulin-dependent, this disruption
directly links insulin resistance to neuronal injury, leading to
axon degeneration, demyelination, and ultimately, DNP. However, the
particular peripheral nerve proteins that can be altered by the
activated HBP in response to DM have yet to be identified.
Therefore, further investigations are required to fully elucidate
the interplay between HBP and DNP (21).
AGEs and inflammation
Hyperglycemia increases the formation of AGEs by
non-enzymatic reactions between reducing sugars and proteins,
nucleic acids, or lipids. These groups tend to impair the
biological activity of proteins, affecting cellular function. AGEs
promote modification through glycation of the extracellular matrix
protein laminin, which causes impaired regenerative activity in
DNP. In addition, AGEs induce segmental demyelination; as a result,
the nerves become susceptible to phagocytosis by macrophages.
Axonal atrophy, degeneration and impaired axonal transport are
consequences of AGE-modified major axonal cytoskeletal proteins
such as tubulin, neurofilamen and actin (34). In the peripheral nerves of
diabetics, the AGE receptor [receptor of advanced glycation end
products (RAGE)] has been shown to colocalize with AGEs. It appears
that AGEs and their interactions with RAGE cause OS in DNP
(35). Consequently, this leads to
an increase in nuclear factor κB (NF-κB) and numerous
pro-inflammatory genes regulated by NF-κB. Furthermore, the blood
of individuals with DM contains inflammatory mediators, such as
C-reactive protein and TNF-α (5,36).
The combined effects of AGE-induced biochemical damage include
reduced neurotrophic support, nerve blood flow impairment, neuronal
integrity disruption and compromised repair mechanisms (37).
DAG and PKC pathway
Chronically increased levels of DAG occur in
hyperglycemia as a result of an increased glycolytic intermediary,
dihydroxyacetone phosphate. This intermediate is converted into
glycerol-3-phosphate, which then enhances the production of DAG by
de novo synthesis. The PKC family consists of 11 isoforms,
of which nine are activated by DAG. The activation of the DAG-PKC
pathway increases ROS production via NADPH oxidase, reinforcing OS,
while simultaneously disrupting mitochondrial electron transport,
uncoupling endothelial nitric oxide synthetase and influencing
transcription factors, such as NF-κB, which promotes
pro-inflammatory signaling (38).
Animal studies support the role of PKC in DNP, as
inhibition using LY333531 has been shown to improve sciatic nerve
blood flow, conduction and hyperalgesia (39). PKC activation contributes to
neuropathy via two mechanisms: Reduced activity limits blood flow
and alters conduction, while excessive activity disrupts neuronal
function by affecting neurochemical signaling. Several PKC
inhibitors, similar to aldose reductase inhibitors, also
demonstrate antioxidant properties antioxidant (40).
PARP overactivation
PARP is a nuclear enzyme that facilitates the
attachment of ADP-ribose units to DNA, histones and other DNA
repair enzymes. This process has an impact on cellular functions
(41). Recent evidence indicates
that the overactivation of PARP and the occurrence of OS are two
interconnected pathways. PARP activity is minimal under normal
physiological circumstances. Nevertheless, in the presence of OS,
DNA single-strand breaks become abundant and result in excessive
activation of PARP. As regards DNP, research has demonstrated that
the overactivation of PARP may contribute to the development of
DNP, whereas preventing its function may impede the progression of
this condition (42). PARP is
found in both endothelial cells and Schwann cells inside the
peripheral nerve. The activation of PARP is evident in DM and plays
a role in diabetic endothelial dysfunction, which is a key
contributor to DNP. Activation of PARP induces marked metabolic
alterations and influences the expression of genes. Furthermore,
PARP is necessary for the translocation of apoptosis-provoking
factors from the mitochondria to the nucleus. This process plays a
crucial role in PARP-mediated programmed cell death, which has
recently been linked to the development of DNP (43).
Nuclear factor erythroid 2-related
factor 2 (Nrf2) in DNP
Nrf2 is a major leucine zipper protein that mainly
acts as a defense mechanism against cellular OS. It functions as a
transcription factor to regulate the development of cytoprotective
enzymes. Typically, Nrf2 is not active within cells; however, it
becomes activated when there is stress or an increase in the
generation of free radicals (44).
Upon activation, Nrf2 translocates to the cell nucleus and
selectively interacts with the DNA at the antioxidant response
element, decreasing free radicals and OS. Similarly, in the
presence of DM, elevated blood sugar levels lead to the activation
of several neuroinflammatory pathways and the generation of free
radicals. During the first phases of hyperglycemia, Nrf2 signaling
plays a crucial role in controlling the activation of several
cytoprotective genes. However, in cases of prolonged hyperglycemia,
the levels of Nrf2 decline, leading to the development of DNP via
linked neuroinflammatory pathways (3).
5. Impact of antidiabetics on DNP and
oxidative stress
Generally, antidiabetics cannot interfere directly
with the polyol pathway or HBP, which are the main pathways of the
generation of free radicals in DNP (45). Nevertheless, antidiabetic
medications can decrease the production of AGEs and disrupt the
activation of the PKC pathway. In addition, antidiabetics tend to
suppress the inflammatory condition that often induces OS (46-48).
Some antidiabetics exert antioxidant effects either by improving
the endogenous antioxidant enzymes, such as SOD and catalase, or by
reducing the production of ROS. The antioxidant effects of
antidiabetics are accompanied by a reduction in the occurrence of
diabetic complications, including DNP. Thus, in the case of DNP or
high-risk conditions, some antidiabetics may be recommended over
others (Table I) (49).
 | Table IEffects of antidiabetics on diabetic
neuropathy along with antioxidant effects. |
Table I
Effects of antidiabetics on diabetic
neuropathy along with antioxidant effects.
| Drug group | Antioxidant
effect | Effect on diabetic
neuropathy | (Refs.) |
|---|
| Metformin | Present | Inconclusive
effects: Several studies have proven positive benefits; on the
other hand, it may be an iatrogenic cause of DNP exacerbation | (55-58,60) |
| Sulfonylurea | Present | Beneficial effects
(gliclazide) | (66) |
|
Thiazolidinediones | Present | Beneficial
effects | (7,71) |
| SGLT-2
inhibitors | Present | Beneficial
effects | (75) |
| DDP-IV
inhibitors | Present | Beneficial
effects | (91-93) |
| GLP-1 receptor
agonists | Present | Inconclusive
effects: Some studies have proven positive effects, while others
have failed to demonstrate improvements in the clinical
presentations of DNP | (80,81,83-85) |
| Alpha-glucosidase
inhibitors | No effect | No effects | (65) |
| Meglitinides | No effect | No effects | (65) |
Metformin
Metformin, a biguanide derivative, is primarily used
for controlling type 2 DM. Adenosine monophosphate-activated
protein kinase (AMPK) is a key prospective target of metformin
since it serves as a cellular energy sensor that becomes activated
in response to metabolic stress, leading to improved glucose uptake
(50). Metformin can significantly
reduce the OS associated with diabetic patients. Previous studies
have demonstrated that metformin has the ability to inhibit
mitochondrial complex I (NADH:ubiquinone oxidoreductase), which
contributes to the antioxidant effect of metformin (51-53).
The cellular production of ROS may be greatly
influenced by mitochondrial complex I. There is much documentation
indicating that a blockage of this complex results in a decrease in
the generation of reactive species. This is caused by a reduction
in the transportation of electrons from NADH plus hydrogen. In
addition, metformin has been demonstrated to scavenge oxygenated
free radicals produced in vitro directly and to inhibit the
opening of the mitochondrial permeability transition pore in both
intact and permeabilized human epithelial carcinoma cells (KB
cells), as well as in permeabilized human microvascular endothelial
cells (HMEC-1 cells) (51,54).
The use of metformin for the treatment of the
manifestations of DNP in both animals and humans has yielded
inconclusive results to date. Several studies have proven the
positive benefits of metformin on DNP (55-58).
Metformin has been shown to reduce the accumulation of AGEs in the
sciatic nerves of rats with streptozotocin (STZ)-induced diabetes
by activating AMPK, leading to improved nerve conduction velocity,
and the attenuation of heat and mechanical hyperalgesia (59). It has also been shown to decrease
serum malondialdehyde (MDA) levels and enhance SOD activity,
highlighting its role in counteracting diabetes-induced OS
(59).
Conversely, other studies suggest that metformin may
worsen neuropathic outcomes, with reports of it functioning as an
iatrogenic factor contributing to more severe neuropathy in type 2
DM (60). This negative effect has
been partly attributed to the association of long-term metformin
use with vitamin B12 deficiency, a known risk factor for DNP. These
contrasting findings underscore the complexity of the effects of
metformin on DNP, indicating a dual role where protective
mechanisms against OS may be counterbalanced by adverse effects
under certain conditions (61).
Sulfonylureas
Sulfonylureas promote insulin secretion by their
interaction with the ATP-sensitive potassium channel located on the
β-cell of the pancreas. Sulfonylureas decrease the level of AGEs
indirectly by controlling blood glucose levels (62). The administration of glimepiride
has been shown to cause a decrease in the levels of peroxides and
MDA, and an increase in the activity of SOD and glutathione
peroxidase in rats following the administration of STZ; this
suggests that glimepiride may effectively inhibit the development
of OS in DM (63). Gliclazide also
exerts a prominent antioxidant effect mainly due to the free
radical scavenging effect. The main mechanism for this effect is
not yet clearly understood; however, the characteristic of an
azabicyclo-octyl ring grafted on a hydrazide group, a structure
unique to gliclazide, may provide the compound with free radical
scavenging properties (64).
Glibenclamide, glipizide, tolazamide and other sulfonylureas do not
exert antioxidant effects. Similar to sulfonylureas, meglitinides
such as repaglinide and nateglinide form a bond with the KATP
channel on the pancreatic beta cells, but at a different binding
location. However, it has not been shown that these medications
have any antioxidant properties (65).
In the aspect of DNP control, gliclazide is a novel
sulfonylurea that inhibits the development of DNP. Regardless of
blood glucose levels in mice with STZ-induced diabetes, gliclazide
considerably reduces peripheral nerve morphological alterations and
improves the slowing of motor nerve conduction velocity. The
morphological alterations observed in diabetic rats compared to
non-diabetic rats include an increase in nerve fiber density and a
reduction in fascicular area, axon/myelin ratio and nerve fiber
area (66). Animal research has
demonstrated that potassium-ATP channel blockage by sulfonylurea
may enhance glutamate-induced superoxide generation and
neurotoxicity by selectively intensifying mitochondrial inhibitors;
however, no such data have been reported for humans, at least to
the best of our knowledge (67).
Thiazolidinediones (TZDs)
TZDs exert their insulin-sensitizing effects through
the activation of the peroxisome proliferator-activated receptor γ
(PPAR-γ) nuclear receptor, hence reducing insulin resistance. TZDs
possess exert antioxidant effects. The potential mechanism may be
attributed to the triggering of the transcription of several genes,
including NADPH, SOD and catalase, via the activation of PPAR-γ
receptors, resulting in the improvement of mitochondrial health
(68). Some TZDs, such as
troglitazone, provide direct antioxidant effects via a side chain
that resembles α-tocopherol, in addition to their ability to
indirectly upregulate antioxidant genes. Due to their ability to
decrease NO production via the trans-repression of inducible nitric
oxide synthetase, all TZDs exert intracellular antioxidant effects.
Pioglitazone is beneficial in reducing OS via correction of the PKC
pathway and pro-inflammatory process (69).
It has been shown that the administration of
pioglitazone improves biochemical markers via reduced MDA levels
and improved GSH and SOD activities (70). Pioglitazone has also been shown to
be more effective than metformin in reducing OS, as seen by a
decrease in MDA levels. However, only metformin exerts an
antioxidant effect through an increase in SOD levels. The distinct
mechanisms of action of the two medications on OS support the
concurrent prescription of both treatments to enhance the result in
ameliorating insulin resistance and diabetes complications
(49). TZDs are associated with
increased total serum soluble RAGE levels that may lead to a
decrease in the harmful effects of AGEs. Furthermore, TZDs reduce
the tissue expression of RAGE, resulting in decreased
proinflammatory effects of AGEs (62).
Several studies have been conducted to demonstrate
the efficacy of TZD in slowing or preventing the progression of
DNP. In STZ-treated rats, troglitazone protected against nerve
conduction velocity slowing and maintained normal myelinated fiber
architecture and number (71).
Pioglitazone has the triple advantage of lowering central
sensitization, hyperglycemia and hyperalgesia; hence, TZDs are a
desirable pharmacotherapy for those with neuropathic pain related
to type 2 DM (7). Furthermore,
pioglitazone has neuroprotective properties by enhancing nerve
conduction velocity and diminishing macrophage infiltration in the
sciatic nerve (72). Rosiglitazone
reduces the OS in the sciatic nerve, thus reducing the progression
of DNP (73).
Sodium-glucose co-transporter (SGLT-2)
inhibitors
SGLT-2 inhibitors block SGLT2, which are responsible
for glucose reabsorption in renal proximal convoluted tubules,
leading to glycosuria and a reduction in blood glucose level.
Recently, SGLT-2 inhibitors have been identified as potent
antioxidant agents that can prevent oxidative damage to tissues by
lowering glucose levels, generating fewer free radicals, or by
potentiating the antioxidant system (Table II). The observed therapeutic
advantages of SGLT-2 inhibitors in diabetic complications may be
attributed to the reduction of OS (74).
 | Table IIMechanisms of SGLT-2 inhibitors in
reducing oxidative stress (74). |
Table II
Mechanisms of SGLT-2 inhibitors in
reducing oxidative stress (74).
| Effect | Mechanisms |
|---|
| Reduction of
free-radical generation | Decreasing the
level of prooxidant enzymes, such as |
| | Nox and eNOS |
| | Reduction of levels
of AGEs |
| | Lowering the level
of proinflammatory cytokines |
| | Improvement of
insulin sensitivity |
| | Normalization of
hemodynamic condition |
| | Enhancement of
mitochondrial function |
| Antioxidant
effect | Directly via
increasing the endogenous antioxidant system and scavenging the
free radicals or indirectly by inducing normoglycemia. |
A previous meta-analysis of 89 articles in the field
of DNP demonstrated that SGLT-2 inhibitors have the potential to
preserve the nerves by significantly enhancing the speed at which
sensory and motor nerves conduct signals (75). This improvement in nerve function
leads to improved clinical manifestations for patients with DNP,
with a reduction in the activity of the sympathetic nervous system
(75). A follow-up study
demonstrated that the use of SGLT-2 inhibitors for >3 years led
to significant improvements in certain measures of neuropathy
(76).
Combining dapagliflozin and mecobalamin may
considerably reduce clinical symptoms in patients with DNP. This
combination may lower blood glucose, control MDA, SOD and
cyclooxygenase 2 levels, and prevent nerve cell damage.
Additionally, it promotes sensory and motor nerve transmission. The
approach of using dapagliflozin and mecobalamin is safe and
warrants clinical promotion (77).
Glucagon-like peptide 1 (GLP-1)
agonists
GLP-1 receptor agonists, as a class of antidiabetic
medications, have been observed to enhance the secretion of insulin
in response to glucose stimulation, inhibit the release of
glucagon, and delay the process of stomach emptying. GLP-1 can
reverse the oxidative action, as the administration of GLP-1 or its
receptor agonist has been found to have a beneficial effect on OS
markers, such as SOD, glutathione peroxidase, glutathione amount,
glutathione reductase, catalase, lipid peroxidation and
non-enzymatic glycosylated proteins, stimulated by different stress
factors (78). The mechanism by
which GLP-1 reduces OS in DM involves the activation of cyclic
adenosine monophosphate (cAMP), PI3K and PKC pathways via
receptors, as well as the activation of Nrf-2, increasing
antioxidant capacity. These findings indicate that activating Nrf2
by GLP-1 and its subsequent antioxidative effects may have
potential benefits in preventing and treating DM, as well as
reducing the likelihood of complications. Also, GLP-1 receptor
agonists were suggested to suppress OS generation induced by
AGEs-RAGE and reduce tissue expression of RAGE via activation of
cAMP pathways (79).
However, the effect of GLP-1s receptor agonists in
DNP remain uncertain. Some clinical studies support the role of
GLP-1 receptor agonists in improving DNP through a number of
mechanisms including the antioxidant effect, the anti-inflammatory
signaling through microglia/astrocyte modulation, improvement in
peripheral nerve blood flow and endothelial function, and metabolic
improvement (80,81). The antioxidant effect is one of the
most effective mechanisms. GLP-1 receptor agonists activate SOD;
however, they do not cause alterations in the distribution pattern
of neuronal markers. Exenatide protects cells against apoptosis
caused by OS and promotes neurite. These findings suggest that
GLP-1 receptor agonists function as neuroprotective agents,
considering their direct effects on neurons (82). Another study documented that the
use of liraglutide alleviated DNP through antioxidant and
anti-inflammatory effects, as well as via the remodeling of the
extracellular matrix (81).
On the other hand, other research has demonstrated
that GLP-1 receptor agonists have no significant effect in
improving DNP (8). Despite the
anti-inflammatory and antioxidant effects of liraglutide, no
clinical improvement in autonomic neuropathy or polyneuropathy has
been observed (83). In addition,
exenatide has failed to provide a significant effect on DNP
(84,85).
Dipeptidyl peptidase IV (DDP-IV)
inhibitors
DPP-IV inhibitors prevent the breakdown of
endogenous GLP-1, consequently enhancing the incretin action.
DPP-IV, a cell surface enzyme found in endothelial cells and some
lymphocytes, is responsible for the degradation of various
peptides. DPP-IV inhibitors have been observed to stimulate insulin
secretion without causing hypoglycemia or weight gain (86). Clinical studies have demonstrated
that DPP-IV inhibitors exert antioxidant effects by reducing ROS
generation and promoting the activity of antioxidant enzymes,
including increased nitric oxide, SOD, catalase and reduced
glutathione (87,88).
Furthermore, it has been discovered that the
production of ROS caused by the AGE-RAGE interaction leads to the
release of DPP-4. DPP-4 inhibitors prevent this release (89). Additionally, another study
demonstrated that the use of vildagliptin, a DPP-4 inhibitor, was
associated with decreased AGEs to a certain extent (90).
As regards DNP, sitagliptin plays protective roles
on neurons via activating GLP-1 receptor, resulting in an
anti-apoptotic effect, improving microtubule stabilization and axon
regeneration, ameliorating mitochondrial dysfunction, and promoting
locomotor functional recovery. The mechanism of action of
sitagliptin in DNP is related to AMPK/peroxisome
proliferator-activated receptor coactivator 1α (PGC-1α) signaling
pathway. The activation of the AMPK/PGC-1α signaling pathway
results in the development of neurites and the regeneration of
axons (9). It is well-established
that when teneligliptin is orally administered, it produces
analgesic properties in humans specifically against thermal pain.
It has been demonstrated that teneligliptin exerts mild
antinociceptive effects in response to acute pain; however, it
exerts, significant analgesic effects against DNP. Furthermore,
teneligliptin can improve the synthesis of glutathione antioxidants
inside the cellular environment (91,92).
Vildagliptin improves glucose intolerance and increases serum
insulin and GLP-1 levels, accompanied by the amelioration of
delayed nerve conduction velocity and neuronal atrophy (93).
Alpha-glucosidase inhibitors
Alpha-glucosidase inhibitors attenuate the process
of starch digestion in the gastrointestinal tract, resulting in a
slow release of glucose into the circulation. The currently used
alpha-glucosidase inhibitors consist of acarbose and miglitol. Thus
far, there have been no documented antioxidant properties observed
for these medications (65).
However, a previous study demonstrated that miglitol improved the
activity of catalase, SOD, glutathione peroxidase, glutathione
reductase and thioredoxin reductase (94).
6. Clinical significance, prescribing
recommendations and future directions
Clinical significance
Glycemic control is the primary goal of DNP
treatment. The pathogenesis of the complication is regarded as the
primary focus of interest while developing pharmaceutical DNP
targets. Although there is no curative treatment for DNP, several
medications can alleviate the symptoms. When managing diabetic
neuropathy, it is important to select antidiabetics with
antioxidant properties that attenuate the progression of the
disease, in addition to controlling the blood sugar levels
(75-77,95).
Prescribing recommendations
The use of metformin should be accompanied by
routine vitamin B12 monitoring and supplementation to maximize its
benefit in DNP, while minimizing deficiency-related risks. The use
of pioglitazone may be considered in patients with neuropathic pain
due to its neuroprotective potential, although caution is advised
due to its side-effect profile. Combination therapy with metformin,
pioglitazone and vitamin B12 supplementation may provide the most
prominent protective effect against DNP (7,49,72,96).
Concerning sulfonylureas, gliclazide is a novel sulfonylurea that
inhibits the development of DNP (65). However, the use of sulfonylureas or
insulin in combination with metformin and pioglitazone may worsen
DNP (75,76,97).
SGLT-2 inhibitors, particularly dapagliflozin, may be incorporated
into diabetes therapy where neuropathy is a concern, with potential
added benefit when combined with cobalamin (77).
The effect of GLP-1 receptor agonists in DNP is
uncertain, and the use of such medications in DNP requires further
investigations (8,80,81,83-85).
Sitagliptin, as a DPP-IV inhibitor, on the other hand, plays a
protective role in neurons by improving the actions of GLP-1. The
administration of sitagliptin in conjunction with metformin leads
to enhanced grip strength and increased pain sensitivity, while
also demonstrating neuroprotective effects (95).
Future directions
Future studies for the management of DNP are
required to focus on identifying medications that can directly
interfere with the main pathways of ROS generation, including the
polyol pathway and HBP. Furthermore, additional studies may be
required to provide sufficient insight into the role of
antidiabetic combinations in preventing DNP, and to determine which
combinations are the most effective.
7. Conclusion
Hyperglycemia and a complex metabolic imbalance,
primarily OS, are current hypotheses for the progression of DNP. A
number of antidiabetics exert antioxidant effects that can reduce
OS in patients with DM. The antioxidant effects of antidiabetics
are accompanied by a reduction in the occurrence of diabetic
complications, including DNP. Some antidiabetics may be beneficial
in preventing the progression of DNP, while others have no effect
or could trigger or worsen the existing DNP. Thus, in the case of
DNP or high-risk conditions, some antidiabetics may be recommended
over others.
Acknowledgements
The authors would like to extend their deepest
appreciation to the University of Mosul and the College of Pharmacy
(University of Mosul), Mosul, Iraq, for their critical advice, and
invaluable academic guidance.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
KAH and MKZ were involved in the writing, reviewing
and editing of the manuscript, as well as in the writing and
preparation of the original draft of the manuscript, and the
conceptualization of the study. FAA and MNA supervised the study,
and were also involved in project administration, in the literature
search and in the conceptualization of the study. All authors have
read and approved the final manuscript. Data authentication is not
applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ahmed GM, Abed MN and Alassaf FA: Impact
of calcium channel blockers and angiotensin receptor blockers on
hematological parameters in type 2 diabetic patients. Naunyn
Schmiedebergs Arch Pharmacol. 397:1817–1828. 2024.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Liu X, Xu Y, An M and Zeng Q: The risk
factors for diabetic peripheral neuropathy: A meta-analysis. PLoS
One. 14(e0212574)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
González P, Lozano P, Ros G and Solano F:
Hyperglycemia and oxidative stress: An integral, updated and
critical overview of their metabolic interconnections. Int J Mol
Sci. 24(9352)2023.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Al-dabbagh BM, Abed MN, Mahmood NM,
Alassaf FA, Jasim MH, Alfahad MA and Thanoon IAJ:
Anti-inflammatory, antioxidant and hepatoprotective potential of
milk thistle in albino rats. Lat Am J Pharm. 41:1832–1841.
2022.
|
|
5
|
Hadid KA, Alassaf FA and Abed MN:
Mechanisms and linkage of insulin signaling, resistance, and
inflammation. Iraqi J Pharm. 21:1–8. 2024.
|
|
6
|
Lin Q, Li K, Chen Y, Xie J, Wu C, Cui C
and Deng B: Oxidative stress in diabetic peripheral neuropathy:
Pathway and mechanism-based treatment. Mol Neurobiol. 60:4574–4594.
2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Griggs RB, Donahue RR, Adkins BG, Anderson
KL, Thibault O and Taylor BK: Pioglitazone inhibits the development
of hyperalgesia and sensitization of spinal nociresponsive neurons
in type 2 diabetes. J Pain. 17:359–373. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
García-Casares N, González-González G, de
la Cruz-Cosme C, Garzón-Maldonado FJ, de Rojas-Leal C, Ariza MJ,
Narváez M, Barbancho MÁ, García-Arnés JA and Tinahones FJ: Effects
of GLP-1 receptor agonists on neurological complications of
diabetes. Rev Endocr Metab Disord. 24:655–672. 2023.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Han W, Li Y, Cheng J, Zhang J, Chen D,
Fang M, Xiang G, Wu Y, Zhang H, Xu K, et al: Sitagliptin improves
functional recovery via GLP-1R-induced anti-apoptosis and
facilitation of axonal regeneration after spinal cord injury. J
Cell Mol Med. 24:8687–8702. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Maeda-Gutiérrez V, Galván-Tejada CE, Cruz
M, Valladares-Salgado A, Galván-Tejada JI, Gamboa-Rosales H,
García-Hernández A, Luna-García H, Gonzalez-Curiel I and
Martínez-Acuña M: Distal symmetric polyneuropathy identification in
type 2 diabetes subjects: A random forest approach. Healthcare
(Basel). 9(138)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Oh J: Clinical spectrum and diagnosis of
diabetic neuropathies. Korean J Intern Med. 35:1059–1069.
2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Sharma JK, Rohatgi A and Sharma D:
Diabetic autonomic neuropathy: A clinical update. J R Coll
Physicians Edinb. 50:269–273. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bell DSH: Diabetic mononeuropathies and
diabetic amyotrophy. Diabetes Ther. 13:1715–1722. 2022.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ahmed GM, Abed MN and Alassaf FA: The
diabetic-anemia nexus: Implications for clinical practice. Mil Med
Sci Lett. 92:1–11. 2023.
|
|
15
|
Bhatti JS, Sehrawat A, Mishra J, Sidhu IS,
Navik U, Khullar N, Kumar S, Bhatti GK and Reddy PH: Oxidative
stress in the pathophysiology of type 2 diabetes and related
complications: Current therapeutics strategies and future
perspectives. Free Radic Biol Med. 184:114–134. 2022.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mosquera-Sulbarán JA and Hernández-Fonseca
JP: Advanced glycation end products in diabetes. In: Patel VB,
Preedy VR (eds) Biomarkers in Diabetes. Biomarkers in Disease:
Methods, Discoveries and Applications. Springer, Cham, pp171-194,
2023.
|
|
17
|
Abed MN, Alassaf FA, Jasim MHM, Alfahad M
and Qazzaz ME: Comparison of antioxidant effects of the proton
pump-inhibiting drugs omeprazole, esomeprazole, lansoprazole,
pantoprazole, and rabeprazole. Pharmacology. 105:645–651.
2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Alfahad M, Qazzaz ME, Abed MN, Alassaf FA
and Jasim MHM: Comparison of anti-oxidant activity of different
brands of esomeprazole available in Iraqi pharmacies. Syst Rev
Pharm. 11:330–33. 2020.
|
|
19
|
Dilworth L, Stennett D, Facey A, Omoruyi
F, Mohansingh S and Omoruyi FO: Diabetes and the associated
complications: The role of antioxidants in diabetes therapy and
care. Biomed Pharmacother. 181(117641)2024.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Ganjifrockwala FA, Joseph JT and George G:
Decreased total antioxidant levels and increased oxidative stress
in South African type 2 diabetes mellitus patients. J Endocrinol
Metab Diabetes South Africa. 22:21–25. 2017.
|
|
21
|
Pang L, Lian X, Liu H, Zhang Y, Li Q, Cai
Y, Ma H and Yu X: Understanding diabetic neuropathy: Focus on
oxidative stress. Oxid Med Cell Longev.
2020(9524635)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Zampieri M, Bacalini MG, Barchetta I,
Scalea S, Cimini FA, Bertoccini L, Tagliatesta S, De Matteis G,
Zardo G, Cavallo MG and Reale A: Increased PARylation impacts the
DNA methylation process in type 2 diabetes mellitus. Clin
Epigenetics. 13(114)2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chung SSM, Ho ECM, Lam KSL and Chung SK:
Contribution of polyol pathway to diabetes-induced oxidative
stress. J Am Soc Nephrol. 14 (8 Suppl 3):S233–S236. 2003.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Lv Y, Yao X, Li X, Ouyang Y, Fan C and
Qian Y: Cell metabolism pathways involved in the pathophysiological
changes of diabetic peripheral neuropathy. Neural Regen Res.
19:598–605. 2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gugliucci A: Formation of
fructose-mediated advanced glycation end products and their roles
in metabolic and inflammatory diseases. Adv Nutr. 8:54–62.
2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Rendell MS: The time to develop treatments
for diabetic neuropathy. Expert Opin Investig Drugs. 30:119–130.
2021.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Niimi N, Yako H, Takaku S, Chung SK and
Sango K: Aldose reductase and the polyol pathway in schwann cells:
Old and new problems. Int J Mol Sci. 22(1031)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Brownlee M: Biochemistry and molecular
cell biology of diabetic complications. Nature. 414:813–820.
2001.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Nagel AK and Ball LE: Intracellular
protein O-GlcNAc modification integrates nutrient status with
transcriptional and metabolic regulation. Adv Cancer Res.
126:137–166. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Paneque A, Fortus H, Zheng J, Werlen G and
Jacinto E: The hexosamine biosynthesis pathway: Regulation and
function. Genes (Basel). 14(933)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Nelson ZM, Leonard GD and Fehl C: Tools
for investigating O-GlcNAc in signaling and other fundamental
biological pathways. J Biol Chem. 300(105615)2024.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Alasaf FA, Jasim MHM, Alfahad M, Qazzaz
ME, Abed MN and Thanoo IAJ: Effects of bee propolis on FBG, HbA1c,
and insulin resistance in healthy volunteers. Turkish J Pharm Sci.
18:405–409. 2021.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Buse MG, Robinson KA, Gettys TW, McMahon
EG and Gulve EA: Increased activity of the hexosamine synthesis
pathway in muscles of insulin-resistant ob/ob mice. Am J Physiol.
272:E1080–E1088. 1997.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Papachristou S, Pafili K and Papanas N:
Skin AGEs and diabetic neuropathy. BMC Endocr Disord.
21(28)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Sugimoto K, Yasujima M and Yagihashi S:
Role of advanced glycation end products in diabetic neuropathy.
Curr Pharm Des. 14:953–961. 2008.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Jasim MHM, Alfahad M, Al-Dabbagh BM,
Alassaf FA, Abed MN and Mustafa YF: Synthesis, characterization,
ADME study and in-vitro anti-inflammatory activity of aspirin amino
acid conjugates. Pharm Chem J. 57:243–249. 2023.
|
|
37
|
Kim J and Lee J: Role of obesity-induced
inflammation in the development of insulin resistance and type 2
diabetes: History of the research and remaining questions. Ann
Pediatr Endocrinol Metab. 26:1–13. 2021.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Kolczynska K, Loza-Valdes A, Hawro I and
Sumara G: Diacylglycerol-evoked activation of PKC and PKD isoforms
in regulation of glucose and lipid metabolism: A review. Lipids
Health Dis. 19(113)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Kim H, Sasaki T, Maeda K, Koya D,
Kashiwagi A and Yasuda H: Protein kinase Cbeta selective inhibitor
LY333531 attenuates diabetic hyperalgesia through ameliorating cGMP
level of dorsal root ganglion neurons. Diabetes. 52:2102–2109.
2003.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oyenihi AB, Ayeleso AO, Mukwevho E and
Masola B: Antioxidant strategies in the management of diabetic
neuropathy. Biomed Res Int. 2015(515042)2015.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Arruri VK, Gundu C, Khan I, Khatri DK and
Singh SB: PARP overactivation in neurological disorders. Mol Biol
Rep. 48:2833–2841. 2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Brady PN, Goel A and Johnson MA:
Poly(ADP-ribose) polymerases in host-pathogen interactions,
inflammation, and immunity. Microbiol Mol Biol Rev. 83:e00038–18.
2018.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Yuan P, Song F, Zhu P, Fan K, Liao Q,
Huang L and Liu Z: Poly (ADP-ribose) polymerase 1-mediated
defective mitophagy contributes to painful diabetic neuropathy in
the db/db model. J Neurochem. 162:276–289. 2022.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Kumar A and Mittal R: Nrf2: A potential
therapeutic target for diabetic neuropathy. Inflammopharmacology.
25:393–402. 2017.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Gawli K and Bojja KS: Molecules and
targets of antidiabetic interest. Phytomedicine Plus.
4(100506)2024.
|
|
46
|
Alnaser RI, Alassaf FA and Abed MN:
Adulteration of hypoglycemic products: The silent threat. Rom J Med
Pract. 18:157–160. 2023.
|
|
47
|
Abdollahi E, Keyhanfar F, Delbandi AA,
Falak R, Hajimiresmaiel SJ and Shafiei M: Dapagliflozin exerts
anti-inflammatory effects via inhibition of LPS-induced TLR-4
overexpression and NF-κB activation in human endothelial cells and
differentiated macrophages. Eur J Pharmacol.
918(174715)2022.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Mehdi SF, Pusapati S, Anwar MS, Lohana D,
Kumar P, Nandula SA, Nawaz FK, Tracey K, Yang H, LeRoith D, et al:
Glucagon-like peptide-1: A multi-faceted anti-inflammatory agent.
Front Immunol. 14(1148209)2023.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Singh RK, Gupta B, Tripathi K and Singh
SK: Anti oxidant potential of metformin and pioglitazone in type 2
diabetes mellitus: Beyond their anti glycemic effect. Diabetes
Metab Syndr. 10:102–104. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Rena G, Hardie DG and Pearson ER: The
mechanisms of action of metformin. Diabetologia. 60:1577–1585.
2017.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Fontaine E: Metformin-induced
mitochondrial complex I Inhibition: Facts, uncertainties, and
consequences. Front Endocrinol (Lausanne). 9(753)2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Martín-Rodríguez S, de Pablos-Velasco P
and Calbet JAL: Mitochondrial complex I inhibition by metformin:
Drug-exercise interactions. Trends Endocrinol Metab. 31:269–271.
2020.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Cameron AR, Logie L, Patel K, Erhardt S,
Bacon S, Middleton P, Harthill J, Forteath C, Coats JT, Kerr C, et
al: Metformin selectively targets redox control of complex I energy
transduction. Redox Biol. 14:187–197. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Carvalho C, Correia S, Santos MS, Seiça R,
Oliveira CR and Moreira PI: Metformin promotes isolated rat liver
mitochondria impairment. Mol Cell Biochem. 308:75–83.
2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Wei J, Wei Y, Huang M, Wang P and Jia S:
Is metformin a possible treatment for diabetic neuropathy? J
Diabetes. 14:658–669. 2022.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Lós DB, Oliveira WH, Duarte-Silva E,
Sougey WWD, Freitas EDSR, de Oliveira AGV, Braga CF, França MER,
Araújo SMDR, Rodrigues GB, et al: Preventive role of metformin on
peripheral neuropathy induced by diabetes. Int Immunopharmacol.
74(105672)2019.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Alcántara Montero A, Goicoechea García C,
Pacheco de Vasconcelos SR and Alvarado PMH: Potential benefits of
metformin in the treatment of chronic pain. Neurol Perspect.
2:107–109. 2022.
|
|
58
|
Kim SH, Park TS and Jin HY: Metformin
preserves peripheral nerve damage with comparable effects to alpha
lipoic acid in streptozotocin/high-fat diet induced diabetic rats.
Diabetes Metab J. 44:842–853. 2020.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Kakhki FSH, Asghari A, Bardaghi Z,
Anaeigoudari A, Beheshti F, Salmani H and Hosseini M: The
antidiabetic drug metformin attenuated depressive and anxiety-like
behaviors and oxidative stress in the brain in a rodent model of
inflammation induced by lipopolysaccharide in male rats. Endocr
Metab Immune Disord Drug Targets. 24:1525–1537. 2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Yang R, Yu H, Wu J, Chen H, Wang M, Wang
S, Qin X, Wu T, Wu Y and Hu Y: Metformin treatment and risk of
diabetic peripheral neuropathy in patients with type 2 diabetes
mellitus in Beijing, China. Front Endocrinol (Lausanne).
14(1082720)2023.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Alvarez M, Sierra OR, Saavedra G and
Moreno S: Vitamin B12 deficiency and diabetic neuropathy in
patients taking metformin: A cross-sectional study. Endocr Connect.
8:1324–1329. 2019.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Ragazzi E, Burlina S, Cosma C, Chilelli
NC, Lapolla A and Sartore G: Anti-diabetic combination therapy with
pioglitazone or glimepiride added to metformin on the AGE-RAGE
axis: A randomized prospective study. Front Endocrinol (Lausanne).
14(1163554)2023.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Krauss H, Koźlik J, Grzymisławski M,
Sosnowski P, Mikrut K, Piatek J and Paluszak J: The influence of
glimepiride on the oxidative state of rats with
streptozotocin-induced hyperglycemia. Med Sci Monit. 9:BR389–BR393.
2003.PubMed/NCBI
|
|
64
|
O'Brien RC, Luo M, Balazs N and Mercuri J:
In vitro and in vivo antioxidant properties of gliclazide. J
Diabetes Complications. 14:201–206. 2000.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Choi SW and Ho CK: Antioxidant properties
of drugs used in type 2 diabetes management: Could they contribute
to, confound or conceal effects of antioxidant therapy? Redox Rep.
23:1–24. 2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Qiang X, Satoh J, Sagara M, Fukuzawa M,
Masuda T, Miyaguchi S, Takahashi K and Toyota T: Gliclazide
inhibits diabetic neuropathy irrespective of blood glucose levels
in streptozotocin-induced diabetic rats. Metabolism. 47:977–981.
1998.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Kou J, Klorig DC and Bloomquist JR:
Potentiating effect of the ATP-sensitive potassium channel blocker
glibenclamide on complex I inhibitor neurotoxicity in vitro and in
vivo. Neurotoxicology. 27:826–834. 2006.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Hwang J, Kleinhenz DJ, Rupnow HL, Campbell
AG, Thulé PM, Sutliff RL and Hart CM: The PPARgamma ligand,
rosiglitazone, reduces vascular oxidative stress and NADPH oxidase
expression in diabetic mice. Vascul Pharmacol. 46:456–462.
2007.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Alhowail A, Alsikhan R, Alsaud M,
Aldubayan M and Rabbani SI: Protective effects of pioglitazone on
cognitive impairment and the underlying mechanisms: A review of
literature. Drug Des Devel Ther. 16:2919–2931. 2022.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Al-Muzafar HM, Alshehri FS and Amin KA:
The role of pioglitazone in antioxidant, anti-inflammatory, and
insulin sensitivity in a high fat-carbohydrate diet-induced rat
model of insulin resistance. Braz J Med Biol Res.
54(e10782)2021.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Qiang X, Satoh J, Sagara M, Fukuzawa M,
Masuda T, Sakata Y, Muto G, Muto Y, Takahashi K and Toyota T:
Inhibitory effect of troglitazone on diabetic neuropathy in
streptozotocin-induced diabetic rats. Diabetologia. 41:1321–1326.
1998.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Yamagishi S, Ogasawara S, Mizukami H,
Yajima N, Wada R, Sugawara A and Yagihashi S: Correction of protein
kinase C activity and macrophage migration in peripheral nerve by
pioglitazone, proliferator activated-gamma-ligand, in
insulin-deficient diabetic rats. J Neurochem. 104:491–499.
2008.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Wiggin TD, Kretzler M, Pennathur S,
Sullivan KA, Brosius FC and Feldman EL: Rosiglitazone treatment
reduces diabetic neuropathy in streptozotocin-treated DBA/2J mice.
Endocrinology. 149:4928–4937. 2008.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Nabrdalik-Leśniak D, Nabrdalik K,
Sedlaczek K, Główczyński P, Kwiendacz H, Sawczyn T, Hajzler W,
Drożdż K, Hendel M, Irlik K, et al: Influence of SGLT2 inhibitor
treatment on urine antioxidant status in type 2 diabetic patients:
A pilot study. Oxid Med Cell Longev. 2021(5593589)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Kandeel M: The outcomes of sodium-glucose
co-transporter 2 inhibitors (SGLT2I) on diabetes-associated
neuropathy: A systematic review and meta-analysis. Front Pharmacol.
13(926717)2022.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Ishibashi F, Kosaka A and Tavakoli M:
Sodium glucose cotransporter-2 inhibitor protects against diabetic
neuropathy and nephropathy in modestly controlled type 2 diabetes:
Follow-up study. Front Endocrinol (Lausanne).
13(864332)2022.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Wang C, Pan H, Wang W and Xu A: Effect of
dapagliflozin combined with mecobalamin on blood glucose
concentration and serum MDA, SOD, and COX-2 in patients with type 2
diabetes mellitus complicated with peripheral neuropathy. Acta
medica Mediterr. 35:2211–2215. 2019.
|
|
78
|
Bray JJH, Foster-Davies H, Salem A, Hoole
AL, Obaid DR, Halcox JPJ and Stephens JW: Glucagon-like peptide-1
receptor agonists improve biomarkers of inflammation and oxidative
stress: A systematic review and meta-analysis of randomised
controlled trials. Diabetes, Obes Metab. 23:1806–1822.
2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Oh Y and Jun HS: Effects of glucagon-like
peptide-1 on oxidative stress and Nrf2 signaling. Int J Mol Sci.
19(26)2017.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Ma J, Shi M, Zhang X, Liu X, Chen J, Zhang
R, Wang X and Zhang H: GLP-1R agonists ameliorate peripheral nerve
dysfunction and inflammation via p38 MAPK/NF-κB signaling pathways
in streptozotocin-induced diabetic rats. Int J Mol Med.
41:2977–2985. 2018.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Moustafa PE, Abdelkader NF, El Awdan SA,
El-Shabrawy OA and Zaki HF: Liraglutide ameliorated peripheral
neuropathy in diabetic rats: Involvement of oxidative stress,
inflammation and extracellular matrix remodeling. J Neurochem.
146:173–185. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Mohiuddin MS, Himeno T, Inoue R,
Miura-Yura E, Yamada Y, Nakai-Shimoda H, Asano S, Kato M, Motegi M,
Kondo M, et al: Glucagon-like peptide-1 receptor agonist protects
dorsal root ganglion neurons against oxidative insult. J Diabetes
Res. 2019(9426014)2019.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Brock C, Hansen CS, Karmisholt J, Møller
HJ, Juhl A, Farmer AD, Drewes AM, Riahi S, Lervang HH, Jakobsen PE
and Brock B: Liraglutide treatment reduced interleukin-6 in adults
with type 1 diabetes but did not improve established autonomic or
polyneuropathy. Br J Clin Pharmacol. 85:2512–2523. 2019.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Jaiswal M, Martin CL, Brown MB, Callaghan
B, Albers JW, Feldman EL and Pop-Busui R: Effects of exenatide on
measures of diabetic neuropathy in subjects with type 2 diabetes:
Results from an 18-month proof-of-concept open-label randomized
study. J Diabetes Complications. 29:1287–1294. 2015.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Ponirakis G, Abdul-Ghani MA, Jayyousi A,
Almuhannadi H, Petropoulos IN, Khan A, Gad H, Migahid O, Megahed A,
DeFronzo R, et al: Effect of treatment with exenatide and
pioglitazone or basal-bolus insulin on diabetic neuropathy: A
substudy of the Qatar study. BMJ Open Diabetes Res Care.
8(e001420)2020.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Saini K, Sharma S and Khan Y: DPP-4
inhibitors for treating T2DM-hype or hope? An analysis based on the
current literature. Front Mol Biosci. 10(1130625)2023.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Bolevich S, Milosavljevic I, Draginic N,
Andjic M, Jeremic N, Bolevich S, Litvitskiy PF and Jakovljevic V:
The effect of the chronic administration of dpp4-inhibitors on
systemic oxidative stress in rats with diabetes type 2. Serbian J
Exp Clin Res. 20:199–206. 2019.
|
|
88
|
Choi SW and Ho CK-C: Antioxidant
properties of drugs used in type 2 diabetes management. In:
Diabetes. 2nd Edition. Academic Press, pp139-148, 2020.
|
|
89
|
Nakashima S, Matsui T, Takeuchi M and
Yamagishi SI: Linagliptin blocks renal damage in type 1 diabetic
rats by suppressing advanced glycation end products-receptor axis.
Horm Metab Res. 46:717–721. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Zhang L, Li P, Tang Z, Dou Q and Feng B:
Effects of GLP-1 receptor analogue liraglutide and DPP-4 inhibitor
vildagliptin on the bone metabolism in ApoE-/- mice. Ann
Transl Med. 7(369)2019.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Kuthati Y, Rao VN, Busa P and Wong CS:
Teneligliptin exerts antinociceptive effects in rat model of
partial sciatic nerve transection induced neuropathic pain.
Antioxidants (Basel). 10(1438)2021.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Busa P, Kuthati Y, Huang N and Wong CS:
New advances on pathophysiology of diabetes neuropathy and pain
management: Potential role of melatonin and DPP-4 inhibitors. Front
Pharmacol. 13(864088)2022.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Tsuboi K, Mizukami H, Inaba W, Baba M and
Yagihashi S: The dipeptidyl peptidase IV inhibitor vildagliptin
suppresses development of neuropathy in diabetic rodents: Effects
on peripheral sensory nerve function, structure and molecular
changes. J Neurochem. 136:859–870. 2016.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Shrivastava A, Chaturvedi U, Singh SV,
Saxena JK and Bhatia G: Lipid lowering and antioxidant effect of
miglitol in triton treated hyperlipidemic and high fat diet induced
obese rats. Lipids. 48:597–607. 2013.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Sharma AK, Sharma A, Kumari R, Kishore K,
Sharma D, Srinivasan BP, Sharma A, Singh SK, Gaur S, Jatav VS, et
al: Sitagliptin, sitagliptin and metformin, or sitagliptin and
amitriptyline attenuate streptozotocin-nicotinamide induced
diabetic neuropathy in rats. J Biomed Res. 26:200–210.
2012.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Pecikoza U, Tomić M, Nastić K, Micov A and
Stepanović-Petrović R: Synergism between metformin and
analgesics/vitamin B12 in a model of painful diabetic
neuropathy. Biomed Pharmacother. 153(113441)2022.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Pop-Busui R, Lu J, Lopes N and Jones TLZ:
BARI 2D Investigators. Prevalence of diabetic peripheral neuropathy
and relation to glycemic control therapies at baseline in the BARI
2D cohort. J Peripher Nerv Syst. 14:1–13. 2009.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Singh R, Farooq SA, Mannan A, Singh TG,
Najda A, Grażyna Z, Albadrani GM, Sayed AA and Abdel-Daim MM:
Animal models of diabetic microvascular complications: Relevance to
clinical features. Biomed Pharmacother. 145(112305)2022.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Ahmad AA, Barani SSI and Sultan SJ: Role
of the IL-10 gene and its genetic variations in the development of
diabetes mellitus in women. World Acad Sci J. 7:1–7. 2025.
|
|
100
|
Singh M, Kapoor A and Bhatnagar A:
Physiological and pathological roles of aldose reductase.
Metabolites. 11(655)2021.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Mohammad RA, Saeed MK and Ali WK:
Evaluation of sorbitol dehydrogenase and some biochemical
parameters in patients with hepatitis. Kirkuk Univ J Sci Stud.
14:21–32. 2019.
|
|
102
|
Chiaradonna F, Ricciardiello F and
Palorini R: The nutrient-sensing hexosamine biosynthetic pathway as
the hub of cancer metabolic rewiring. Cells. 7(53)2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Mustafa YF: Harmful free radicals in
aging: A narrative review of their detrimental effects on health.
Indian J Clin Biochem. 39:154–167. 2024.PubMed/NCBI View Article : Google Scholar
|