Introduction
Congenital heart disease (CHD) is the most type of
common cardiovascular disease of childhood. Left-to-right shunt CHD
results in an increase in cardiac volume load. Sustained volume
overload induces cardiac hypertrophy and ventricular remodeling,
eventually leading to decreased cardiac function, which results in
chronic heart failure (CHF); however, the pathogenesis of CHF has
not been fully elucidated. Hydrogen sulfide (H2S)
affects a wide range of physiological and pathological processes in
the cardiovascular system (1).
H2S plays an important role in the prevention of the
development and occurrence of coronary heart disease and the
protection against ischemic myocardial injury (2–4).
Exogenous H2S opens KATP channels to reduce
myocardial infarct size (5).
H2S exerts a protective effect on ischemic myocardium by
inhibiting vascular endothelial cell apoptosis and promoting the
regeneration of endothelial cells (6). In a previous study (7), we reported that increased myocardial
collagen content (particularly type I collagen) in rats with volume
overload caused CHF and treatment with sodium hydrosulfide (NaHS),
an exogenous H2S donor, resulted in a decrease of
myocardial collagen content (particularly type I collagen) in the
left-to-right shunt operation group. This suggested that
H2S plays a protective role in volume overload-induced
ventricular remodeling. However, the mechanism underlying these
changes has not been fully elucidated. Carbon monoxide (CO) is
another important endogenous signaling molecule. Mammalian tissues
continually produce CO as a result of the breakdown of heme by heme
oxygenase (HO). HO degrades the pro-oxidant heme to CO, biliverdin
and ferrous iron. HO has been reported to exist as its isoenzyme
forms, HO-1, -2 and -3. HO-3 is inactive and is not expressed in
humans. HO-1 is expressed ubiquitously at low levels and its
expression is rapidly induced by heme as well as other stresses,
including hypoxia, hyperthermia, metals, oxidized low-density
lipoprotein and inflammatory cytokines. By contrast, HO-2 is
constitutively expressed and widely distributed in the body, with
higher concentrations in the brain and testis (8). HO-1 is upregulated by a host of
oxidative stress stimuli in the cardiovascular system (9). The HO-1/CO system is beneficial in the
prevention of atherosclerotic lesion formation, protection of
ischemic myocardial injury and regulation of blood pressure
(10–15). Considering these findings, the
issues that should be addressed include whether H2S
affects the HO-1/CO system and whether the interaction between
H2S and the HO-1/CO system is involved in the regulation
of volume overload-induced heart failure. The present study was
designed in order to elucidate these issues by investigating the
expression of HO-1 in rats with left-to-right shunt and in shunted
rats treated with NaHS.
Materials and methods
Animal model of left-to-right shunt
Experiments were conducted in accordance with the
Guide to the Care and Use of Experimental Animals issued by the
Ministry of Health, the People’s Republic of China. Male
Sprague-Dawley rats were provided by the Animal Research Centre of
Peking University First Hospital. The rats were housed in plastic
cages in a room with a controlled humidity of 40%, a temperature of
22°C and a 12-h light cycle from 6:00 a.m. to 6:00 p.m. The rat
model was established by an abdominal aorta-inferior vena cava
shunt operation, as previously described by Ocampo et
al(16). Briefly, 30 male
Sprague-Dawley rats, weighing 120–140 g, were randomly divided into
four groups: the shunt group (n=8), the shunt + NaHS group (n=8),
the sham group (n=8) and the sham + NaHS group (n=6). Rats in the
shunt and shunt + NaHS groups were anesthetized with 0.25%
pentobarbital sodium (40 mg/kg, intraperitoneal injection). The
abdominal aorta and inferior vena cava were exposed and a bulldog
vascular clamp was placed across the aorta, caudal to the left
renal artery. The aorta was punctured at the union of the segment,
two-thirds caudal to the renal artery and one-third cephalic to the
aortic bifurcation, with an 18-gauge disposable needle. The needle
was slowly withdrawn and a 9-0 silk thread was used to suture the
puncture of the abdominal aortic wall. In the sham and sham + NaHS
groups, rats underwent the same experimental protocol as mentioned
above, except for the shunt procedure. Rats in the shunt + NaHS and
sham + NaHS groups were injected intraperitoneally with NaHS
(H2S donor) at 56 μmol/kg/day for 8 weeks, as
previously described (17), whereas
rats in the shunt and sham groups were injected with the same
volume of normal saline (NS).
Measurement of haemodynamic
parameters
At 8 weeks after the operation, rats in each group
were anesthetized with 25% urethane (0.5 ml/100 g, intraperitoneal
injection). After anesthesia, a cannula with a heparinized PP10 in
PP50 catheter was inserted into the left ventricle (LV) through the
right common carotid artery. The catheter was connected to a
pressure transducer. The pressure transducer was connected to a
data recording system (BL-420F, BioData Acquisition & Analysis
System; TME Technology Co., Ltd., Chengdu, China). The haemodynamic
parameters, such as left ventricular systolic pressure (LVSP), left
ventricular end-diastolic pressure (LVEDP), left ventricular peak
rate of contraction (LV+dp/dtmax) and left ventricular peak rate of
relaxation (LV-dp/dtmax), were measured as previously described
(18).
RNA extraction and cDNA synthesis
Total RNA was isolated from frozen LV tissue using
the Total RNA Extraction kit (Tiangen Biotech, Co., Ltd., Beijing
China; code no. DP419) according to the manufacturer’s protocol and
quantified by measuring the absorbance at 260 nm. The quality of
the isolated RNA was determined by measuring the 260:280 ratio.
Subsequently, first-strand cDNA was synthesized using the
First-Strand cDNA Synthesis kit (Tiangen Biotech, Co., Ltd.; code
no. KR104) according to the manufacturer’s protocol.
Relative gene expression analysis by
real-time PCR
PCR primers were designed using commercial software
(Beacon Designer; Bio-Rad Laboratories, Hercules, CA, USA) to
produce an amplicon 75–150 bp in length. Primers used in the PCR
assays are presented in Table I.
Real-time PCR was performed with the ABI Prism 7500 system (Applied
Biosystems, Carlsbad, CA, USA), using the Ultra SYBR-Green PCR kit
(Beijing CoWin Bioscience Co., Ltd., Beijing, China; code no.
CW0956). The thermal cycling conditions included an initial
denaturation step at 95°C for 10 min, followed by 40 cycles at 95°C
for 15 sec and at 60°C for 60 sec. A melting curve was determined
at the end of each cycle to confirm the specificity of the primers
and the purity of the PCR product. Results were analyzed using
Applied Biosystems 7500 Real-Time PCR System Sequence Detection
Software version 1.4 (Applied Biosystems) to obtain CT
values (threshold cycles at which a statistically significant
increase in detection of SYBR-Green emission intensity occurs).
CT values were then normalized to a
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) endogenous control
to account for variability in RNA concentrations between samples to
obtain ΔCT values. To obtain ΔΔCT values, we
subtracted the ΔCT value for the control samples from
that for the treated samples. The relative quantification value was
then calculated as 2−ΔΔCT.
 | Table IPrimers used in the study. |
Table I
Primers used in the study.
| Gene | Nucleotide
sequence |
|---|
| HO-1 | |
| Sense | 5′-AGA GTT TCC GCC
TCC AAC CA-3′ |
| Antisense | 5′-CGG GAC TGG GCT
AGT TCA GG-3′ |
| GAPDH | |
| Sense | 5′-CAA GGT CAT CCA
TGA CAA CTT TG-3′ |
| Antisense | 5′-GGG CCA TCC ACA
GTC TTC TG-3′ |
Western immunoblot analysis
LV tissue was lysed in a lysis buffer [0.2 ml 1 M
Tris-HCl (pH 8.0), 0.3 ml 5 M NaCl, 10 μl 500 mM
ethylenediaminetetraacetic acid, 0.1 ml 100 mM
phenylmethanesulfonyl fluoride and 10 μl Triton X-100, with
water added to 10 ml]. The extracts were clarified by
centrifugation at 13,400 x g for 15 min at 4°C. Protein
concentrations were determined with the BCA™ Protein Assay kit
(Thermo Fisher Scientific Inc., Waltham, MA, USA). Equal amounts of
total protein (60 μg) were resolved by sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 15% gel
and transferred onto a polyvinylidene difluoride membrane (GE
Healthcare UK Ltd., Little Chalfont, UK). The membrane was blocked
with 5% (w/v) fat-free milk in TBST [0.05% (v/v) Tween in TBST] at
room temperature for 1 h and then probed with rabbit polyclonal
antibodies (Abcam Inc., Cambridge, UK) against HO-1 at a 1:500
dilution overnight at 4°C. The membrane was then probed with
horseradish peroxidase-conjugated goat anti-rabbit IgG (1:2,500;
Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA) for 2 h at room
temperature. The reactions were developed with enhanced
chemiluminescence reagents (Beijing TransGen Biotech Co., Ltd.,
Beijing, China) and the images were obtained by exposure to X-ray
films (Kodak Life Science Imaging film, USA). The films were
digitized with Bio-Rad Gel Doc XR (Bio-Rad Laboratories) and
quantified with Quantity One software (Bio-Rad Laboratories). GAPDH
blots were used as a loading control. The bands were normalized to
GAPDH controls.
Statistical analysis
Data are expressed as the means ± standard error
(SE) and were analyzed by SPSS software, version 16.0 (SPSS Inc.,
Chicago, IL, USA). For the homogeneity of variance values, a
comparison among groups was performed with one-way analysis of
variance (ANOVA), followed by the Least Significance Difference
test. For some heterogeneity of variance values, comparison among
groups was performed with one-way ANOVA followed by Tamhane’s T2
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
LV haemodynamic parameters
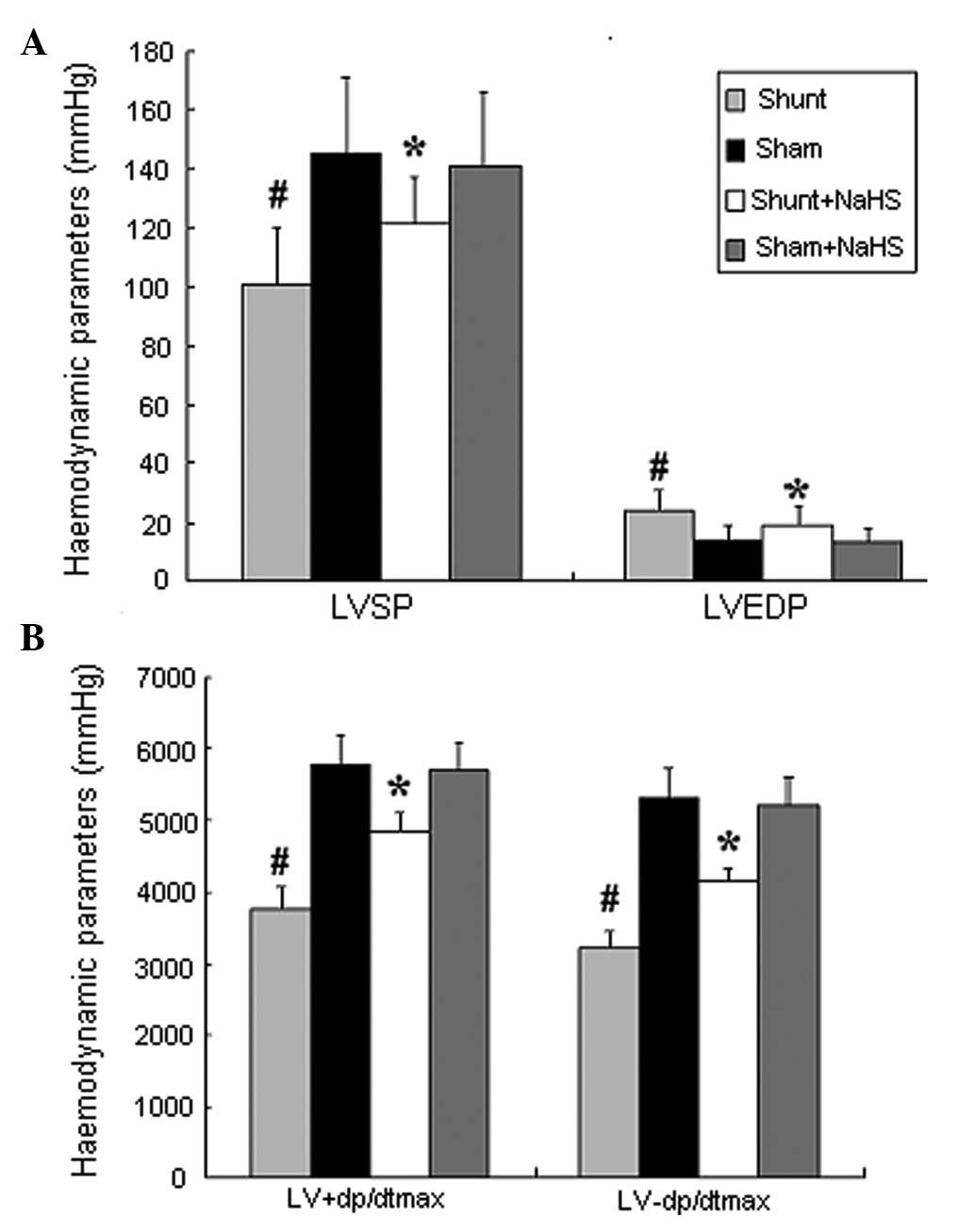
Eight weeks after the operation, the shunt group
exhibited significantly increased LVEDP (24±7 vs. 14±5 mmHg; 1
mmHg= 0.133 kPa, P<0.05) and significantly decreased LVSP and
LV±dp/dtmax (101±19 vs. 145±26 mmHg; 3768±321 vs. 5768±432
mmHg/sec; 3219±219 vs. 5312±418 mmHg/sec, all P<0.05), compared
to the sham group. In addition, the shunt + NaHS group exhibited
significantly decreased LVEDP (19±6 vs. 24±7 mmHg, P<0.05) and
significantly increased LVSP and LV±dp/dtmax (121±16 vs. 101±19
mmHg; 4865±254 vs. 3768±321 mmHg/sec; 4138±207 vs. 3219±219
mmHg/sec, all P<0.05), compared to the shunt group. There was no
significant difference between the sham and the sham + NaHS groups
(Fig. 1).
HO-1 mRNA expression in the LV
Eight weeks after the operation, HO-1 mRNA
expression tended to decrease in the shunt group compared to that
in the sham operation group (P>0.05). The shunt + NaHS group
exhibited significantly increased HO-1 mRNA expression compared to
that in the shunt group (P<0.01). There was no significant
difference in HO-1 mRNA expression between the sham and the sham +
NaHS groups (Table II).
 | Table IIHeme oxygenase-1 mRNA expression in
the left ventricle (2−ΔΔCT)a. |
Table II
Heme oxygenase-1 mRNA expression in
the left ventricle (2−ΔΔCT)a.
| Shunt | Sham | Shunt + NaHS | Sham + NaHS |
|---|
| No. | 8 | 8 | 8 | 6 |
| HO-1 | 1.86±0.29 | 2.05±0.24 | 5.86±0.61b | 2.94±0.63 |
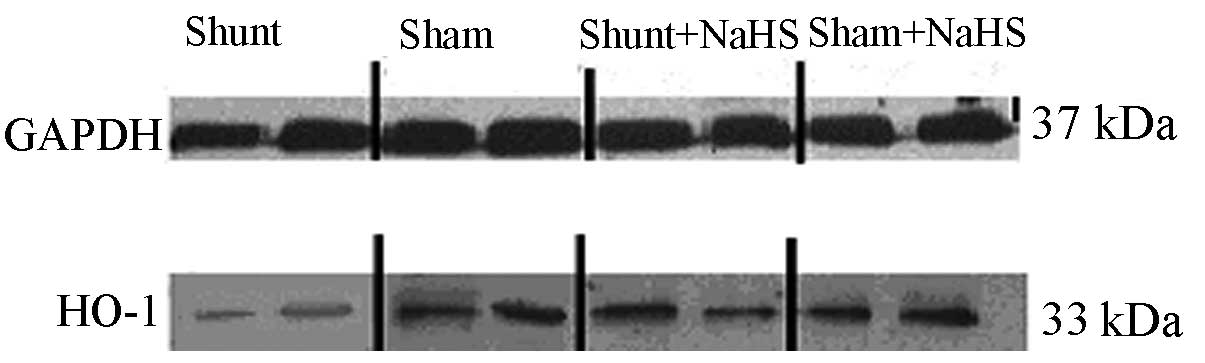
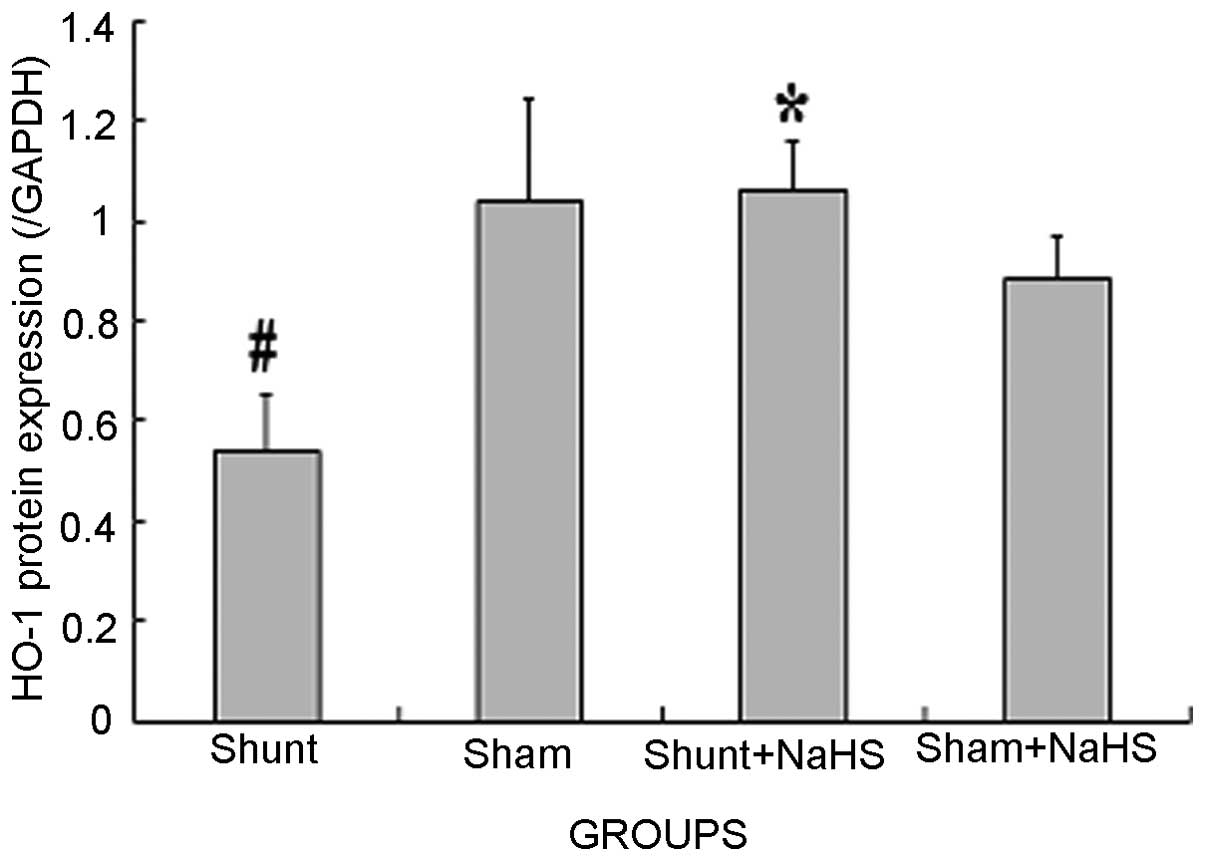
Western blot analysis results of HO-1 in
the LV
Eight weeks after the operation, HO-1 protein
expression was significantly decreased in the shunt group compared
to that in the sham operation group (0.54±0.11 vs. 1.04±0.20,
P<0.05). HO-1 protein expression was significantly increased in
the shunt + NaHS group compared to that in the shunt group
(1.06±0.10 vs. 0.54±0.11, P<0.05). There was no significant
difference in HO-1 protein expression between the sham and the sham
+ NaHS groups (Figs. 2 and 3).
Discussion
CHD is one of the most common human birth defects,
with an incidence of 6–8/1,000 live births (19). CHF is a common complication of
left-to-right shunt CHD and ventricular remodeling is an important
pathophysiological mechanism underlying volume overload-induced
CHF. With ventricular structural remodeling, cardiac function is
compromised, which results in irreversible heart failure.
Therefore, it is critical to elucidate the mechanism behind volume
overload-induced CHF and perform timely interventions.
Similar to nitric oxide (NO) and CO, which are
considered as gaseous transmitters, H2S has also been
shown to be a gaseous transmitter which plays an important role in
normal physiological processes as well as in the
process/progression of several diseases. The four most important
mammalian enzymes involved in H2S synthesis are
cystathionine-β-synthase (CBS), cystathionine-γ-lyase
(cystathionase, CSE) and cysteine aminotransferase (CAT) in
conjunction with 3-mercaptopyruvate sulfurtransferase (3-MST)
(1). H2S is synthesized
from the sulfur-containing amino acid L-cysteine by either CBS or
CSE, with pyridoxal 5′-phosphate used as a cofactor. Along with
CAT, 3-MST produces H2S using L-cysteine and
α-ketoglutarate as substrates. Both enzymes contribute to
H2S formation in the brain and vascular endothelium.
Recent experimental studies demonstrated that endogenous
H2S synthesis was lower in hearts of an arteriovenous
fistula-induced CHF model (1). In
the present study, 8 weeks after the shunt surgery, there were
significant changes in LVSP, LVEDP and LV±dp/dtmax, indicating that
H2S may improve cardiac function in volume
overload-induced CHF. Furthermore, the results indicated that
long-term treatment with NaHS may not affect the left ventricular
haemodynamic parameters in sham-operated rats, although it exerted
a significant pharmacological effect under pathological conditions
(such as CHF). This finding indicated that H2S may
protect the heart against CHF; however, its mechanism has not yet
been elucidated. Endogenous H2S is produced through the
metabolism of terminal waste of sulfur-containing amino acids in
the body. H2S may occur as gaseous H2S or
NaHS. NaHS in the body may dissociate to sodium ions
(Na+) and sulfur hydrogen ions (HS−).
HS− combines with internal hydrogen ions (H+)
to generate H2S. Therefore, H2S and NaHS are
in a type of dynamic balance. NaHS may ensure the stability of
H2S concentrations in solution and the majority of
intervention experiments on H2S used NaHS solution
(17). Therefore, in this study, we
used NaHS solution as the H2S donor.
CO is an endogenously derived gas formed from the
breakdown of heme by the HO enzyme. Although long considered an
insignificant and potentially toxic waste product of heme
catabolism, CO is now recognized as an important signaling molecule
that regulates numerous cardiovascular functions. Of note,
alterations in CO synthesis are associated with several
cardiovascular disorders, including atherosclerosis, septic shock,
hypertension, metabolic syndrome and ischemia-reperfusion injury;
restoration of physiological CO levels exerts a beneficial effect
on several of these conditions, suggesting a crucial role for CO in
the maintenance of cardiovascular homeostasis. CO causes relaxation
of numerous vascular tissues and regulates blood pressure by
activating soluble guanylate cyclase in vascular smooth muscle,
leading to the production of cyclic guanine monophosphate (20). Considerable evidence supports a
protective role for the HO-1/CO system against coronary artery
ischemia-reperfusion injury. Pharmacological induction of HO-1
expression significantly reduces infarct size and the incidence of
reperfusion arrhythmia following myocardial ischemia-reperfusion,
whereas cardiac tissue damage is exacerbated by HO inhibitors
(21–25). The induction of HO-1 may also have
therapeutic benefits during CHF. Upregulation of HO-1 expression
during heart failure serves to mitigate pathological LV remodeling
and reduce myocardial hypertrophy, oxidative stress and
inflammatory activation. HO-1 overexpression promotes
neovascularization and ameliorates apoptosis in the heart failure
model (26).
H2S and CO are involved in the regulation
of several physiological as well as pathological processes. They
have similar biological functions, as well as competitive and
antagonist actions. For example, H2S administered to
rats with experimentally induced hypoxic pulmonary hypertension
leads to an increase in plasma CO concentrations and an increase in
pulmonary artery expression of HO-1 protein and mRNA (27). In the present study, 8 weeks after
the shunt surgery, the expression of LV HO-1 mRNA and protein was
significantly increased in the shunt + NaHS group compared to those
in the shunt group. No difference in HO-1 expression was observed
between the sham group and the sham + NaHS group. These findings
demonstrated that H2S may play a protective role in
volume overload-induced heart failure by upregulating protein and
mRNA expression of HO-1.
The regulatory effect of NaHS on the HO-1/CO system
pathway has not been fully elucidated. A previous study by Oh et
al(28) demonstrated that an
H2S solution, prepared by bubbling pure H2S
gas and NaHS, dose-dependently induced HO-1 expression through the
activation of the extracellular signal-regulated kinase. However,
it has also been shown that increased expression of HO-1 is not
necessarily accompanied by increased HO activity, which should also
be measured (8). Therefore, HO-1
activity requires further investigation.
In conclusion, the present study demonstrated that
H2S upregulates mRNA and protein expression of HO-1 in
rats with volume overload-induced CHF caused by left-to-right
shunt, which may be one of the mechanisms by which H2S
attenuates volume overload-induced heart failure. However, the
specific underlying mechanisms and interaction with NO require
further investigation.
Acknowledgements
The study was supported by the
National Natural Science Foundation of China (no. 30872787),
Beijing outstanding talents training program (20081D0303200107) and
Science Foundation for High-Level Medical Talents of Beijing Health
System (2011).
References
|
1
|
Liu YH, Lu M, Hu LF, Wong PT, Webb GD and
Bian JS: Hydrogen sulfide in the mammalian cardiovascular system.
Antioxid Redox Signal. 17:141–185. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ji Y, Pang QF, Xu G, Wang L, Wang JK and
Zeng YM: Exogenous hydrogen sulfide postconditioning protects
isolated rat hearts against ischemia-reperfusion injury. Eur J
Pharmacol. 587:1–7. 2008. View Article : Google Scholar
|
|
3
|
Bliksoen M, Kaljusto ML, Vaage J and
Stenslokken KO: Effects of hydrogen sulphide on
ischaemia-reperfusion injury and ischaemic preconditioning in the
isolated, perfused rat heart. Eur J Cardiothorac Surg. 34:344–349.
2008. View Article : Google Scholar
|
|
4
|
Muellner MK, Schreier SM, Laggner H, et
al: Hydrogen sulfide destroys lipid hydroperoxides in oxidized LDL.
Biochem J. 420:277–281. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Johansen D, Ytrehus K and Baxter GF:
Exogenous hydrogen sulfide (H2S) protects against
regional myocardial ischemia-reperfusion injury - Evidence for a
role of KATP channels. Basic Res Cardiol. 101:53–60.
2006.
|
|
6
|
Zhu YZ, Wang ZJ, Ho P, et al: Hydrogen
sulfide and its possible roles in myocardial ischemia in
experimental rats. J Appl Physiol. 102:261–268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li XH, Zhang CY and Zhang T: Sodium
hydrosulfide improves cardiac functions and structures in rats with
chronic heart failure. Zhonghua Yi Xue Za Zhi. 91:3044–3049.
2011.(In Chinese).
|
|
8
|
Peterson SJ, Frishman WH and Abraham NG:
Targeting heme oxygenase: therapeutic implications for diseases of
the cardiovascular system. Cardiol Rev. 17:99–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Immenschuh S and Schroder H: Heme
oxygenase-1 and cardiovascular disease. Histol Histopathol.
21:679–685. 2006.
|
|
10
|
Johnson RA, Colombari E, Columbari DS,
Lavesa M, Talman WT and Nasjletti A: Role of endogenous carbon
monoxide in central regulation of arterial pressure. Hypertension.
30:962–967. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ndisang JF, Zhao W and Wang R: Selective
regulation of blood pressure by heme oxygenase-1 in hypertension.
Hypertension. 40:315–321. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otterbein LE, Zuckerbraun BS, Haga M, et
al: Carbon monoxide suppresses arteriosclerotic lesions associated
with chronic graft rejection and with balloon injury. Nat Med.
9:183–190. 2003. View
Article : Google Scholar
|
|
13
|
Yet SF, Layne MD, Liu X, Chen YH, Ith B,
Sibinga NE and Perrella MA: Absence of heme oxygenase-1 exacerbates
atherosclerosis lesion formation and vascular remodeling. FASEB J.
17:1759–1761. 2003.PubMed/NCBI
|
|
14
|
Fujimoto H, Ohno M, Ayabe S, et al: Carbon
monoxide protects against cardiac ischemia - reperfusion injury in
vivo via MAPK and Akt - eNOS pathways. Arterioscler Thromb Vasc
Biol. 24:1848–1853. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu X, Pachori AS, Ward CA, et al: Heme
oxygenase-1 (HO-1) inhibits postmyocardial infarct remodeling and
restores ventricular function. FASEB J. 20:207–216. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ocampo C, Ingram P, Ilbawi M, Arcilla R
and Gupta M: Revisiting the surgical creation of volume load by
aorto-caval shunt in rats. Mol Cell Biochem. 251:139–143.
2003.PubMed/NCBI
|
|
17
|
Yan H, Du J and Tang C: The possible role
of hydrogen sulfide on the pathogenesis of spontaneous hypertension
in rats. Biochem Biophys Res Commun. 313:22–27. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Wang Q, Guo W and Zhu YZ: Hydrogen
sulfide attenuates cardiac dysfunction in a rat model of heart
failure: a mechanism through cardiac mitochondrial protection.
Biosci Rep. 31:87–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sadowski SL: Congenital cardiac disease in
the newborn infant: past, present, and future. Crit Care Nurs Clin
North Am. 21:37–48. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ndisang JF, Tabien HE and Wang R: Carbon
monoxide and hypertension. J Hypertens. 22:1057–1074. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hangaishi M, Ishizaka N, Aizawa T, et al:
Induction of heme oxygenase-1 can act protectively against cardiac
ischemia/reperfusion in vivo. Biochem Biophys Res Commun.
279:582–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Masini E, Vannacci A, Marzocca C, et al:
Heme oxygenase-1 and the ischemia-reperfusion injury in the rat
heart. Exp Biol Med (Maywood). 228:546–549. 2003.PubMed/NCBI
|
|
23
|
Guo Y, Stein AB, Wu WJ, et al:
Administration of a CO-releasing molecule at the time of
reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ
Physiol. 286:H1649–H1653. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
L’Abbate A, Neglia D, Vecoli C, et al:
Beneficial effect of heme oxygenase-1 expression on myocardial
ischemia-reperfusion involves an increase in adiponectin in mildly
diabetic rats. Am J Physiol Heart Circ Physiol. 293:H3532–H3541.
2007.PubMed/NCBI
|
|
25
|
Varadi J, Lekli I, Juhasz B, et al:
Beneficial effects of carbon monoxide-releasing molecules on
post-ischemic myocardial recovery. Life Sci. 80:1619–1626. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang G, Hamid T, Keith RJ, et al:
Cardioprotective and anti-apoptotic effects of heme oxygenase-1 in
the failing heart. Circulation. 121:1912–1925. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qingyou Z, Junbao D, Weijin Z, Hui Y,
Chaoshu T and Chunyu Z: Impact of hydrogen sulfide on carbon
monoxide/heme oxygenase pathway in the pathogenesis of hypoxic
pulmonary hypertension. Biochem Biophys Res Commun. 317:30–37.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oh GS, Pae HO, Lee BS, et al: Hydrogen
sulfide inhibits nitric oxide production and nuclear factor-kappaB
via heme oxygenase-1 expression in RAW264.7 macrophages stimulated
with lipopolysaccharide. Free Radic Biol Med. 41:106–119. 2006.
View Article : Google Scholar : PubMed/NCBI
|