Contents
Introduction
Material selection and production of
nano-microcapsules
Factors affecting the targeted delivery efficiency
of nano-microcapsules
Research on overcoming the hindrances of
nanoparticle delivery
Research progress on the delivery efficiency of
drug-loaded nano-microcapsules
Possible mechanisms for the promotion of
nano-microcapsule delivery
Research progress on nano-microcapsules delivery
system mediated by UTMD
Conclusion
Introduction
An optimal drug delivery method is required to
ensure safety and high efficiency of delivery. The nanoparticle has
recently become one of the most popular and promising non-viral
vectors (1) and has several
advantages compared to viral vectors, such as lack of
pathogenicity, lack of immunogenicity, biodegradability, wide range
of host cells or tissues and diversification of loadings. The
diameter of nano particles is 1/60–1/60,000 that of a cell.
Therefore, drug-loaded nano-microcapsules are able to pass through
several insurmountable obstacles and ingress the interior of cells
and tissues for targeted therapy (1,2).
However, drug delivery efficiency does not appear to reach
satisfactory therapeutic levels, particularly under specific
physiological or pathological conditions.
The oscillation and destruction of microbubbles, as
well as microstreaming and radiation forces generated by
ultra-sound-targeted microbubble destruction (UTMD) may result in
the rupture of stalwart barriers, such as the blood-brain barrier,
dense connective tissue and the cell membrane structure, allowing
more nano-microcapsules into cells and tissues. Recent studies
demonstrated that UTMD has considerably improved the efficiency of
the nano-microcapsules drug delivery system (3–7).
Material selection and production of
nano-microcapsules
Material selection
The materials used for manufacturing
nano-microcapsules are classified as non-biodegradable and
biodegradable. Non-biodegradable materials are able to protect DNA
and RNA from digestion by enzymes, however, they may result in
severe cytotoxicity and tissue necrosis (8–10).
Biodegradable materials are highly biocompatible and are able to be
decomposed by hydrolytic enzymes in the body and absorbed,
ultimately metabolize to carbon dioxide and water through the
tricarboxylic acid cycle and are excreted by the lungs, kidneys and
skin. Therefore, biodegradable materials are considered the optimal
choice and are widely used (11).
Poly(lactic-co-glycolic acid) (PLGA) copolymer (12,13),
one of the most commonly used biodegradable polyester materials,
may be used in all types of drug-loaded nano-microcapsules
embedding proteins (3), amino acids
(3), genes (3), vaccines (9), antigens and growth factors (4). PLGA has been approved by the FDA for
human medical use, and is non-toxic and harmless (10,14,15).
Its crystallinity, solubility and water absorption capacity are
regulated by modifying the proportion of polylactic and
polyglycolic acid to control the rate of degradation, in order to
meet the needs of the release of different embedded drugs (16–19).
Production of nano-microcapsules
Nanoparticle-producing technologies are currently
classified into three categories, the mechanical pulverization,
physical dispersion and chemical synthesis methods. Different types
of nanoparticles are manufactured by different techniques and
processes. The mechanical smashing method is a technique during
which the mass is broken into nanoparticles by a high-speed rotary
mill, jet mill, ultrasound, ball mill or colloid mill. The solvent
evaporation and emulsification/solvent diffusion methods (physical
methods) are suitable for producing nanosuspensions. The chemical
synthesis method uses the hydrophobic segments of polymers to
synthesize surface-active block copolymers. Several studies on
nanoparticles successfully loading DNA (7), siRNA (7), anticancer drugs such as cisplatin
(20) and mitoxantrone (21), and antiparasitic drugs such as
pentamidine (22) and albendazole
(22) were recently published. The
encapsulation efficiency of drugs is affected by factors such as
material and emulsifier concentration and intensity of the
ultrasonic irradiation and the release rate is regulated by the
proportion of various components of the nanomaterial and the pH
(20,23).
Factors affecting the targeted delivery
efficiency of nano-microcapsules
Size of the nano-microcapsule
Nano-microcapsules may be used for the treatment of
a variety of diseases, particularly tumors. Different sizes of
nanoparticles are selective for different tumor tissues. In
general, nano-microcapsules ∼150–300 nm readily accumulate in the
liver and spleen and nano-microcapsules ∼30–150 nm are prone to
accumulate in the bone marrow, heart and kidneys. Particularly
small nano-microcapsules, with a diameter of ∼20–30 nm are usually
cleared by the kidneys prior to ingressing the target tissues
(24).
Electric charges borne on the surface of
nano-microcapsules
The negative electric charges on the surface of
nano-micro-capsules limit their combination with certain gene drugs
as well as with several target tissues and cells, particularly
tumor cells (25,23).
Monitoring of the immune system
Nano-microcapsules that enter the human body may be
cleared away as foreign bodies by the mononuclear phagocytes of the
reticuloendothelial system in the liver and spleen (24).
High expression of specific antigens or
receptors on the surface of tumor cells
High expression of specific antigens or receptors on
the surface of tumor cells or tumor vascular endothelial cells is
moderately or not expressed on the suface of normal cells or normal
vascular endothelial cells (26,27).
Research on overcoming the hindrances of
nanoparticle delivery
Prolonging the circulation time of
nano-microcapsules
Modifying PLGA with monomethyl ether polyethylene
glycol (mPEG) may shield some of the surface charges of the complex
and evade clearance by the body’s immune system, consequently
prolonging the nano-microcapsules residence time in the systemic
circulation (28,29).
Increasing the rate of gene drug
encapsulation by increasing the amount of surface positive charges
to promote delivery efficiency
The positive charges on the surface of PLGA are
distinctly increased following its combination with poly-L-lysine
(PLL), which is able to generate electrostatic interactions with
the negative charges carried by DNA/siRNA to improve the loading
effect (27).
Active targeted delivery by targeting
molecules modifying nano-microcapsules
Recently, several investigators reported that
drug-loaded nano-microcapsules modified with specific target
antibodies may actively recognize target tissues or target cells,
increasing the efficiency of drug delivery (26,27,30).
The integrin αvβ3 is a receptor that is highly expressed on the
surface of a variety of tumor cells or malignant tumor vascular
endothelial cells and not expressed or detected in normal tissue
cells or mature vascular endothelial cells. mPEG-PLGA-PLL polymers
modified with ligand analogs that contain the Arg-Gly-AsP sequence
combine with αvβ3 as antagonists to modify targeted delivery
(29). Yoo et al (31) successfully constructed PEG-PLGA
polymers modified with folic acid that encapsulated adriacin. Human
oral squamous cell carcinoma cells exhibited increased uptake of
nano-microcapsules modified with folic acid compared to unmodified
ones in an in vitro study (32).
Research progress on the delivery efficiency
of drug-loaded nano-microcapsules
Nano-microcapsule-targeted delivery technology has
achieved some success; however, the gene transfection efficiency
and drug delivery efficiency remain low and do not satisfy the
treatment demands.
A previous study conducted by de la Fuente et
al (33) reported that plasmid
DNA was delivered to the cornea and conjunctiva cells by a new type
of nanocarrier synthesized by the bioadhesive polysaccharides
hyaluronic acid and calcium silicate, the transfection efficiency
of which was 15%. Chen et al (20) suggested that the targeted therapy
effect of nano-microcapsules containing mitoxantrone was slightly
superior to intravenous chemotherapy in mouse breast cancer only to
a certain extent. A previous study demonstrated that drug-loaded
nano-microcapsules were extremely difficult to pass through the
vitreous cavity, a grid-like barrier consisting of collagen fibers
bridged by proteoglycans (34).
However, the treatment of retinal diseases requires drugs to cross
this barrier, which remains an intractable problem. Similarly, in
pancreatic cancer (referred to as ‘the king of cancer’), which
exhibits a special pathological anatomy structure, drug-loaded
nano-microcapsules faced significant resistance. A previous study
(31) demonstrated that the
peripancreatic tissue of normal pancreas as well as the leaf gap
tissues that act as ingress and egress pathways to the blood,
nerves and lymphatics of the normal pancreas are loose connective
tissues. Furthermore, little leaf gap tissues of normal pancreas
are connected to the retroperitoneal and peripancreatic loose
connective tissues. By contrast, the tissues surrounding pancreatic
cancer are dense connective tissues and the little leaf gaps of
pancreatic cancer tissues are immersed in a substantial amount of
fibrous tissue and lymphocytes. Therefore, it is difficult for
nano-microcapsules to ingress pancreatic cancer tissues and
identifying a way to promote nano-microcapsule delivery efficiency
is of utmost importance. UTMD was recently verified to be a helpful
tool to enhance nano-microcapsule delivery, for which possible
mechanisms have been described.
Possible mechanisms of UTMD for the
promotion of nano-microcapsule delivery
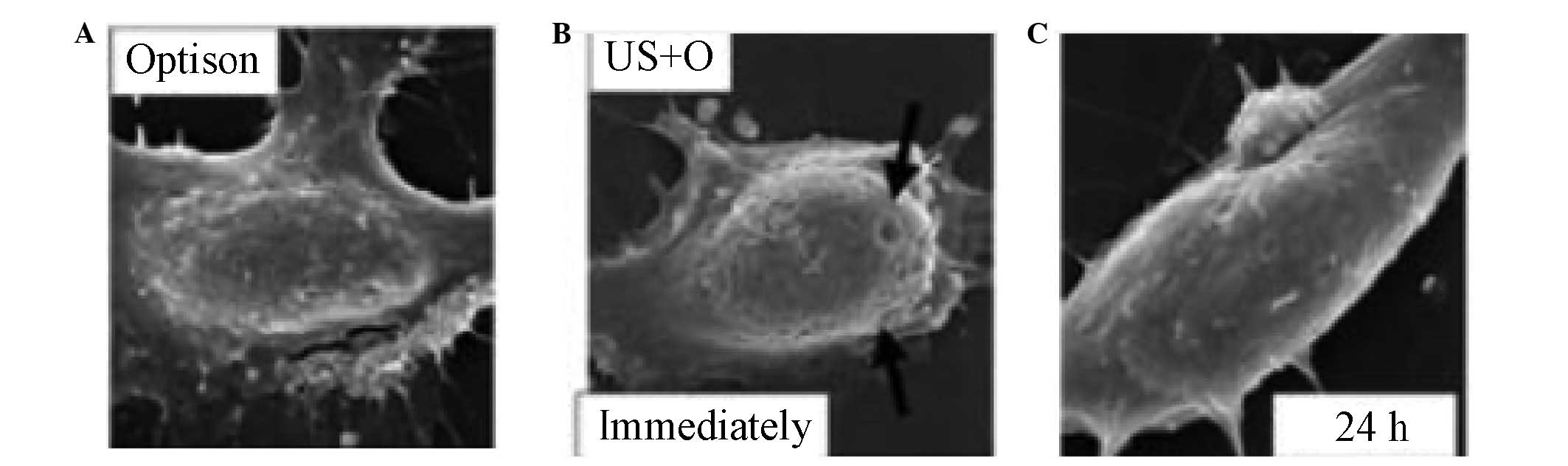
First, UTMD leads to the formation of transient
openings on the surfaces of cell membranes through which
nano-microcapsules are able to enter cells and deliver drugs and
genes (35–38) (Fig.
1). Second, the greatly increased oxyradical generation in
cells under the effect of ultrasound (US) improves the permeability
of cell membranes and promotes cellular uptake of
nano-microcapsules (39). Third, US
may increase endocytosis and activate cell membrane transport, thus
enhancing the uptake of nano-microcapsules (40). US enables the local temperature of
cell membrane to rise, which alters the liquidity of the membrane
phospholipid bilayer and maximizes the cell membrane
permeability.
Although the mechanism underlying its action has not
been fully elucidated, UTMD has played a significant role in
mediating drug and/or gene delivery to several targets, such as
eyes, tumors, skeletal muscle, heart and bone marrow stem cells
(3,4,34,40,41–46).
Research progress on nano-microcapsules
delivery system mediated by UTMD
Eye
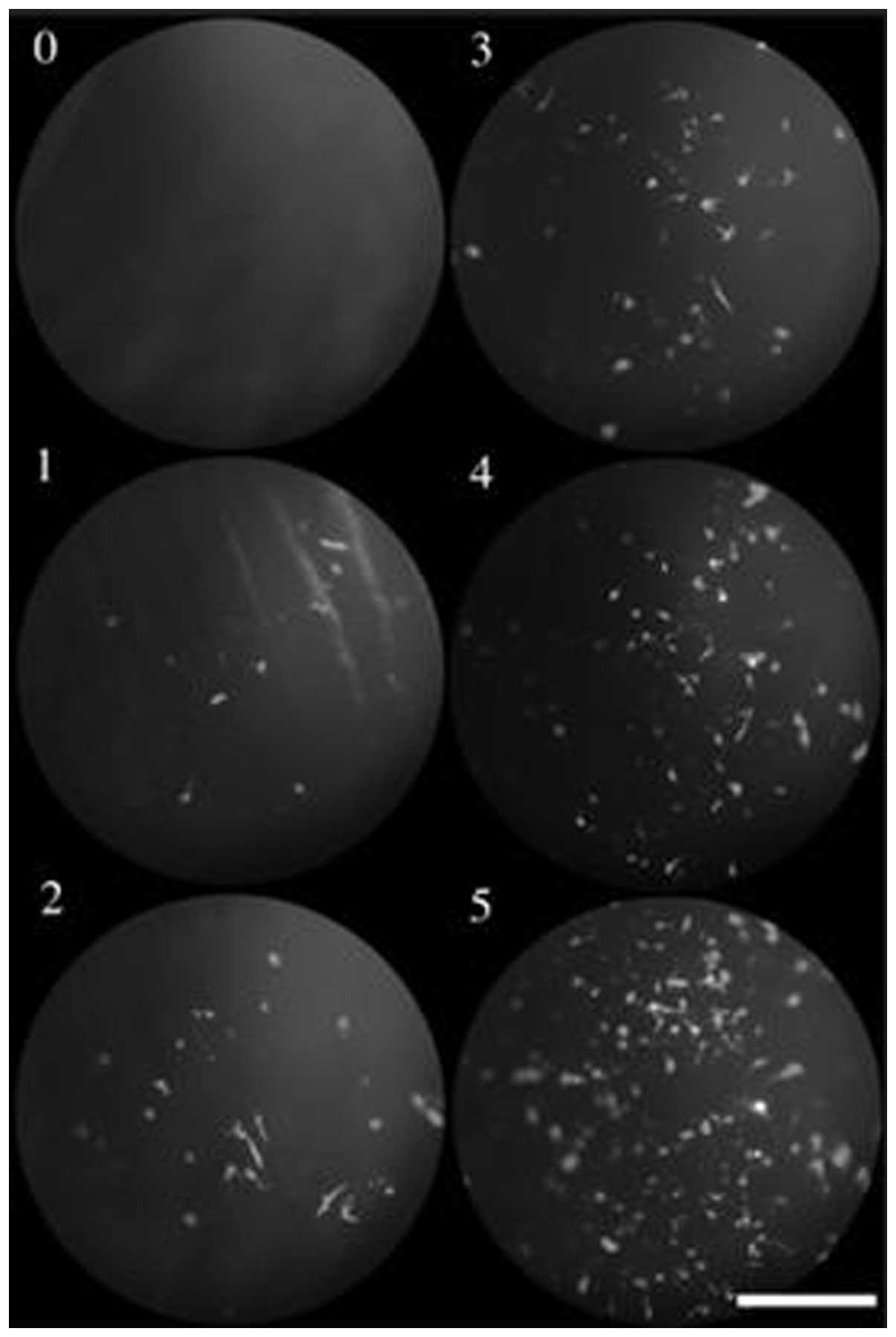
Sonoda et al (41) demonstrated that under the
combination of US and Optison albumin-coated microbubbles, the
green fluorescent protein (GFP) gene transfer to in vivo and
in vitro rabbit corneal cells was greatly increased without
apparent tissue damage, whereas US alone exerted a minimal
enhancing effect on gene transfer (Fig.
2). Wu et al (42) also
reported that using US in conjunction with commercially available
SonoVue microbubbles safely enhanced GFP plasmid transfer to the
mouse cornea in vivo. Another example of a successful gene
transfer to the ocular surface mediated by UTMD was a study
conducted by de la Fuente et al (33). By using a novel hyaluronic
acid-Chitosan nanoparticle mediated by UTMD successful transfection
of plasmid DNA in retinal pigment epithelium (RPE) cells in
vitro and in vivo was achieved. Du et al
(43) reported that UTMD is able to
safely and effectively enhance siRNA-loaded nano-microcapsule
delivery to RPE cells. Moreover, the most notable benefit of
UTMD-mediated Cy3-siRNA loaded by nano-microcapsules was using the
least amount of nano-microcapsules while maintaining a higher rate
of uptake, which was achieved in rats in vivo and in
vitro.
Tumor
Chumakova et al (44) reported that DNA-loaded
nano-microcapsules produced from PLGA and PEI triggered by UTMD
were successfully delivered to tumor cells in vivo. In
addition, the gene transfection rate with UTMD was at least 8 times
higher compared to that without UTMD. Hosseinkhani et al
(45) demonstrated that cationic
Dextran modified by PEG and US may target transfer plasmid DNA to
fibrosarcoma cells efficiently. Rapoport et al (46) succeeded in synthesizing
doxorubicin-containing polymer microcapsules and nano-microbubbles
filled with gas, which were used for the treatment of mice bearing
xenograft breast tumors, triggered by US. Doxorubicin was released
from the polymer micro-capsules to infiltrate target tumor
interstitial tissues, leading to significant tumor shrinkage. Hauff
et al (47) demonstrated
that plasmid pU t651-MB packaged in inflatable nanoparticles
combined with UTMD was effective in treating hepatocellular
carcinoma in rats and gene expression in liver cancer cells was
significantly increased. In the same manner, plasmid p16 as an
anti-oncogene may effectively inhibit the growth of human
pancreatic cancer cells. Yang et al (3) reported that gene-loaded Chitosan
alginate particles combined with US significantly promoted the
transfection efficacy of plasmid GFP in HeLa and 293T cells.
Heart and muscle
Bekeredjian et al (48) reported that luciferase reporter gene
was target delivered to rat heart cells mediated by UTMD. After
measuring the activity of luciferase and mRNA at different time
points within 4 weeks, the investigators observed that the heart
gene transfection efficiency mediated by UTMD was higher than that
mediated by virus. Moreover, the transfection rate peaked after the
first 4 days. Chappell et al (4) suggested that nano-microcapsules
containing fibroblast growth factor 2 were largely deposited on the
muscle tissue of rats mediated by UTMD.
Conclusion
Nano-microcapsule drug-loaded systems triggered by
UTMD prolong the circulation time of the drug in the body and
improve the drug concentration in target tissues, thus enhancing
their efficacy. In addition, they reduce the frequency of drug
administration. Therefore, they are regarded as fairly promising,
particularly in cases with intractable malignant neoplasms. A
previous study demonstrated that tumor cells were visualized
through magnetic resonance concurrently with nano-microcapsule
targeted therapy (49). Ke et
al (50) of the Third People’s
Hospital affiliated with Peking University and Harbin Industry
University, have synthesized a type of novel drug-loaded gold
nano-microcapsule which may be useful for diagnosis and treatment.
The gold nano-microcapsule combines the function of ultrasound
contrast imaging with the function of photothermal therapy
triggered by UTMD. Tumor position and size during the course of
treatment is visualized and evaluated by enhanced ultrasound
imaging of the polymer microcapsules. In addition, gold shells
irradiated by laser generate high temperatures and destroy tumor
tissues. However, the size of the gold nano-microcapsule is so
minute that lesions may be visuaized only by using a great number
of nanoparticles.
In conclusion, nano-microcapsules drug-loaded
systems triggered by UTMD may play a critical role in therapy as
well as imaging, which is a subject requiring further
investigation.
Acknowledgements
The study was supported by grants from
the National Natural Science Foundation of China (nos. 81171352 and
81271596).
References
|
1
|
Yang K, Feng L, Shi X and Liu Z:
Nano-graphene in biomedicine: theranostic applications. Chem Soc
Rev. 10:530–547. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eldar-Boock A, Miller K, Sanchis J, et al:
Integrin-assisted drug delivery of nano-scaled polymer therapeutics
bearing paclitaxel. Biomaterials. 32:3862–3874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang SJ, Chang SM, Tsai KC, et al: Effect
of chitosan-alginate nanoparticles and ultrasound on the efficiency
of gene transfection of human cancer cells. J Gene Med. 12:168–179.
2010.PubMed/NCBI
|
|
4
|
Chappell JC, Song J, Burke CW, et al:
Targeted delivery of nanoparticles bearing fibroblast growth
factor-2 by ultrasonic microbubble destruction for therapeutic
arteriogenesis. Small. 4:1769–1777. 2008. View Article : Google Scholar
|
|
5
|
Park HJ, Lee J, Kim MJ, et al: Sonic
hedgehog intradermal gene therapy using a biodegradable
poly(β-amino esters) nanoparticle to enhance wound healing.
Biomaterials. 33:9148–9156. 2012.PubMed/NCBI
|
|
6
|
Kummitha CM, Malamas AS and Lu ZR: Albumin
pre-coating enhances intracellular siRNA delivery of
multifunctional amphiphile/siRNA nanoparticles. Int J Nanomedicine.
7:5205–5214. 2012.PubMed/NCBI
|
|
7
|
Fields RJ, Cheng CJ, Quijano E, et al:
Surface modified poly(β-amino ester)-containing nanoparticles for
plasmid DNA delivery. J Control Release. 164:41–48. 2012.
|
|
8
|
Kumari A, Yadav SK and Yadav SC:
Biodegradable polymeric nanoparticles based drug delivery systems.
Colloids Surf B Biointerfaces. 75:1–18. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kang BC, Kang KS and Lee YS:
Biocompatibility and long-term toxicity of lnnoPol implant a
biodegradable polymer scaffold. Exp Anim. 54:37–52. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Itaka K, Ishii T, Hasegawa Y and Kataoka
K: Biodegradable polyamino acid-based polycations as safe and
effective gene carrier minimizing cumulative toxicity.
Biomaterials. 31:3707–3714. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jere D, Jiang HL, Arote R, et al:
Degradable polyethylenimines as DNA and small interfering RNA
carriers. Expert Opin Drug Deliv. 6:827–834. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nounou MI, Emmanouil K, Chung S, et al:
Novel reducible linear L-lysine-modified copolymers as efficient
nonviral vectors. J Control Release. 143:326–334. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Chan HF and Leong KW: Advanced
materials and processing for drug delivery: The past and the
future. Adv Drug Deliv Rev. 65:104–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Duan Y, Xu J, Lin Y, et al: A preliminary
study on MeO-PEG- PLGA-PEG-OMe nanoparticles as intravenous
carriers. J Biomed Mater Res A. 87:515–523. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brazeau GA, Sciame M, al-Suwayeh SA and
Fattal E: Evaluation of PLGA microsphere size effect on myotoxicity
using the isolated rodent skeletal muscle model. Pharm Dev Technol.
1:279–283. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Falamarzian A and Lavasanifar A:
Optimization of the hydrophobic domain in poly(ethylene
oxide)-poly(varepsiloncaprolactone) based nano-carriers for the
solubilization and delivery of Amphotericin B. Colloids Surf B
Biointerfaces. 81:313–320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gabler F, Frauenschuh S and Ringe J:
Emulsion-based synthesis of PLGA-microspheres for the in vitro
expansion of porcine chondrocytes. Biomol Eng. 24:515–520. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Falamarzian A and Lavasanifar A: Chemical
modification of hydrophobic block in poly(ethylene oxide)
poly(caprolactone) based nanocarriers: effect on the solubilization
and hemolytic activity of amphotericin B. Macromol Biosci.
10:648–656. 2010. View Article : Google Scholar
|
|
19
|
Fan L, Li F, Zhang H, Wang Y, Cheng C, Li
X, Gu CH, Yang Q, Wu H and Zhang S: Co-delivery of PDTC and
doxorubicin by multifunctional micellar nanoparticles to achieve
active targeted drug delivery and overcome multidrug resistance.
Biomaterials. 31:5634–5642. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chen M, Cooper HM, Zhou JZ, et al:
Reduction in the size of layered double hydroxide nanoparticles
enhances the efficiency of siRNA delivery. J Colloid Interface Sci.
390:275–281. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Du J, Shi QS, Sun Y, Liu PF, Zhu MJ, Du LF
and Duan YR: Enhanced delivery of monomethoxypoly(ethylene glycol)-
poly(lactic-co-glycolic acid)-poly l-lysine nanoparticles loading
platelet-derived growth factor BB small interfering RNA by
ultrasound and/or microbubbles to rat retinal pigment epithelium
cells. J Gene Med. 13:312–323. 2011. View
Article : Google Scholar
|
|
22
|
Duncan R: Polymer conjugates as anticancer
nanomedicines. Nat Rev Cancer. 6:688–701. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee SH and Shin H: Matrices and scaffolds
for delivery of bioactive molecules in bone and cartilage tissue
engineering. Adv Drug Deliv Rev. 59:339–359. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Moghimi SM: Mechanisms of splenic
clearance of blood cells and particles: towards development of new
splenotropic agents. Adv Drug Deliv Rev. 17:103–115. 1995.
View Article : Google Scholar
|
|
25
|
Nafee N, Taetz S, Schneider M, et al:
Chitosan-coated PLGA nanoparticles for DNA/RNA delivery: effect of
the formulation parameters on complexation and transfection of
antisense oligonucleotides. Nanomedicine. 3:173–183. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Loomis K, Smith B, Feng Y, Garg H,
Yavlovich A, Campbell-Massa R, Dimitroy DS, Blumenthal R, Xiao X
and Puri A: Specific targeting to B cells by lipid-based
nanoparticles conjugated with a novel CD22-ScFv. Exp Mol Pathol.
88:238–249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Wu JJ and Huang L: Nanoparticles
targeted with NGR motif deliver c-myc siRNA and doxorubicin for
anticancer therapy. Mol Ther. 18:828–834. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Patil Y and Panyam J: Polymeric
nanoparticles for siRNA delivery and gene silencing. Int J Pharm.
367:195–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tahara K, Yamamoto H and Hirashima N:
Chitosan-modified poly(D,L-lactide-co-glycolide) nanospheres for
improving siRNA delivery and gene-silencing effects. Eur J Pharm
Biopharm. 74:421–426. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gunaseelan S, Gunaseelan K, Deshmukh M, et
al: Surface modifications of nanocarriers for effective
intracellular delivery of anti-HIV drugs. Adv Drug Deliv Rev.
62:518–531. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yoo HS and Park TG: Folate receptor
targeted biodegradable polymeric doxorubicin micelles. J Control
Release. 96:273–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yoo KS and Park TG: Folate receptor
targeted biodegradable polymeric doxorubicin micelles. J Controlled
Release. 96:273–283. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de la Fuente M, Seijo B and Alonso MJ:
Novel hyaluronic acid-chitosan nanoparticles for ocular gene
therapy. Invest Ophthalmol Vis Sci. 49:2016–2024. 2008.PubMed/NCBI
|
|
34
|
Bishop P: The biochemical structure of
mammalian vitreous. Eye (Lond). 10:664–670. 1996. View Article : Google Scholar
|
|
35
|
Prentice P, Cushieri A, Dholakia K,
Prausnitz M and Campbell P: Membrane disruption by optically
controlled microbubble cavitation. Nat Phys. 1:107–110. 2005.
View Article : Google Scholar
|
|
36
|
Tachlibana K, Uchida T, Ogawa K, et al:
Induction of cell- membrane porosity by ultrasound. Lancet.
353:14091999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
van Wamel A, Kooiman K, Harteveld M, et
al: Vibrating micro-bubbles poking individual cells: drug transfer
into cells via sonoporation. J Control Release. 112:149–155.
2006.PubMed/NCBI
|
|
38
|
Schlicher RK, Radhakrishna H, Tolentino
TP, et al: Mechanism of intracellular delivery by acoustic
cavitation. Ultrasound Med Biol. 32:915–924. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Juffermans LJ, Dijkmans PA, Musters RJ, et
al: Transient permeabilization of cell membranes by
ultrasound-exposed microbubbles is related to formation of hydrogen
peroxide. Am J Physiol Heart Circ Physiol. 291:H1595–H1601. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Miller DL and Gies RA: The interaction of
ultrasonic heating and cavitation in vascular bioeffects on mouse
intestine. Ultrasound Med Biol. 24:123–128. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Sonoda S, Tachibana K and Uchino E: Gene
transfer to corneal epithelium and keratocytes mediated by
ultrasound with micro-bubbles. Invest Ophthalmol Vis Sci.
47:558–564. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wu Y, Du LF, Chen YD, et al: SonoVue and
ultrasound-mediated pEGFP-N1 transfection to mouse cornea in an in
vivo study. Chin J Ultrasonogr. 17:350–353. 2008.
|
|
43
|
Du J, Sun Y, Shi QS, Liu PF, Zhu MJ, Wang
CH, Du LF and Duan YR: Biodegradable nanoparticles of mPEG-PLGA-PLL
triblock copolymers as novel non-viral vectors for improving siRNA
delivery and gene silencing. Int J Mol Sci. 13:516–533. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chumakova OV, Liopo AV, Andreev VG, et al:
Composition of PLGA and PEI/DNA nanoparticles improves
ultrasound-mediated gene delivery in solid tumors in vivo. Cancer
Lett. 261:215–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hosseinkhani H and Tabata Y: Ultrasound
enhances in vivo tumor expression of plasmid DNA by PEG-introduced
cationized dextran. J Control Release. 108:540–556. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Rapoport N, Gao Z and Kennedy A:
Multifunctional nanoparticles for combining ultrasonic tumor
imaging and targeted chemotherapy. J Natl Cancer Inst.
99:1095–1106. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Hauff P, Seemann S, Reszka R, et al:
Evaluation of gas-filled microparticles and sonoporation as gene
delivery system: feasibility study in rodent tumor models.
Radiology. 236:572–578. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Bekeredjian R, Chen S, Frenkel PA, et al:
Ultrasound-targeted microbubble destruction can repeatedly direct
highly specific plasmid expression to the heart. Circulation.
108:1022–1026. 2003. View Article : Google Scholar
|
|
49
|
Torchilin VP: Passive and active drug
targeting: drug delivery to tumors as an example. Handb Exp
Pharmacol. 197:3–53. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Ke H, Wang J, Dai Z, et al:
Gold-nanoshelled microcapsules: a theranostic agent for ultrasound
contrast imaging and photo-thermal therapy. Angew Chem Int Ed Engl.
50:3017–3021. 2011. View Article : Google Scholar : PubMed/NCBI
|