Introduction
Cardiovascular diseases represent >30% of all
deaths worldwide in 2008(1). Heart
failure alone, one of the consequences of ischemic heart disease,
leads to ~2% of the health expenditure in the whole western world;
therefore, the economic impact of cardiovascular diseases is huge.
Therefore, it is important to identify at-risk patients early in
order to optimize treatment and reduce costs. However, little is
known regarding the association between genetic polymorphism and
cardiovascular mortality risk.
Previous studies have shown a genetic association
between single nucleotide polymorphisms (SNPs) and cardiovascular
diseases. Special interest has been focused on the low-density
lipoprotein receptor-related protein 1 (LRP1). In particular, the
SNP rs1799986, of LRP1 is associated with increased rates of
premature cardiovascular disease in familial hypercholesterolemia
(2) and rs1466535 was identified in a
genome-wide association study as a novel polymorphism associated
with abdominal aortic aneurysm affecting the gene expression of
LRP1(3). According to Bown et
al (3) functional analyses have
demonstrated that rs1466535 might alter the sterol regulatory
element-binding protein 1 binding site and therefore influence the
activity at the locus.
Interestingly, the association between LRP1 and
platelet-derived growth factor D (PDGF-D) was elucidated by Boucher
et al (4). They demonstrated
that LRP1 forms a complex of the PDGF receptor β and that
inactivation of LRP1 causes abnormal activation of PDGF with
increased risk of atherosclerosis as a result. Therefore, as PDGF
has been shown to be associated with vascular diseases and stroke
(5), LRP1 is even more interesting to
evaluate. Therefore, the aim of this study was to investigate the
possible influence of polymorphisms in LRP1, rs1466535, on
all-cause and cardiovascular (CV) mortality in an elderly primary
health care population, and to identify possible gender differences
as the latter has not been studied before.
Materials and methods
Patient population
The study population consisted of 489 individuals
(men: 248; females: 241) with a mean age of 77.0 years (range: 18
years) living in a rural municipality in the south-east of Sweden,
who were all part of a longitudinal epidemiological study focusing
on CV risk factors (6). All the
participants in that study were invited to participate in the
present sub-study conducted from 13th January 2003 through 18th
June 2005. The blood samples were collected at the University
Hospital of Linköping (Linköping, Sweden). All those living in the
municipality within a specific age bracket were invited to
participate in the longitudinal project in order to minimize bias
in the selection process. The population that agreed to participate
donated blood samples and submitted to echocardiographic
examinations and an electrocardiogram (ECG). The New York Heart
Association functional class was evaluated by the on-site physician
based on the patient information.
All participants gave their written informed consent
and the study was conducted in accordance with the Declaration of
Helsinki principles. The study protocol was approved by the
Regional Ethical Review Board of Linköping, Sweden (Dnr 95044).
Mortality information was obtained from autopsy reports or from the
National Board of Health and Welfare in Sweden, which registers all
deaths.
Co-morbidity
In this study the following definitions have been
used; hypertension was defined as a blood pressure of >140/90
mmHg measured in the right arm with the patient in a supine
position after at least 30 min rest. Hypertension was also assumed
if the participant had previously been diagnosed with hypertension
and was receiving antihypertensive medication. Diabetes mellitus
was defined as a previous diagnosis with on-going treatment, or a
fasting blood glucose ≥7 mmol/l measured on a single occasion.
Ischemic heart disease was defined as a history of angina
pectoris/myocardial infarction or ECG-verified myocardial
infarction. Heart failure was defined as a previous diagnosis with
on-going treatment, or symptoms/signs of heart failure and
objective demonstration of reduced cardiac function in terms of
impaired cardiac function on echocardiography. CV death was defined
as death caused by fatal arrhythmias, myocardial infarction, heart
failure, or cerebrovascular insult.
Ultrasound examinations
Echocardiography examinations were performed using
an Accuson XP-128c with the patient in a left supine position.
Values for systolic function were expressed as left ventricular
ejection fraction (EF), and were split into four classes with
interclass limits of 30, 40 and 50%. Normal systolic function was
defined as EF ≥50% (7-9). Thus,
only the systolic function was evaluated.
The abdominal aorta was examined through routine
ultrasound examination, using an Accuson XP-128c ultrasound
machine.
Determination of LRP1 levels in
plasma
All blood samples (20 ml) were obtained while the
patients were at rest in a supine position and all samples were
collected in pre-chilled plastic Vacutainer tubes (Terumo EDTA
K-3); however, 2 ml was used for subsequent analysis. Plasma was
prepared by centrifugation at 3,000 x g for 10 min at 4˚C. All
samples were stored at -70˚C until used for analysis. None of the
samples were thawed more than twice.
Plasma levels of LRP1 were measured using a
commercially available ELISA kit (Cloud-Clone Corporation, Houston,
TX, USA) following the manufacturer's protocol. Plasma samples were
diluted 1:2,000.
Genotype determination
Genomic DNA was isolated from peripheral blood using
the QIAmp DNA Mini kit (Qiagen GmbH, Hilden, Germany), following
the manufacturer's protocol. Genomic determination was performed as
described in the literature (10).
Briefly, DNA (10 ng) was mixed with TaqMan genotyping master mix
coding rs1466535 in LRP1 gene (assay ID: C 7499211 10; Applied
Biosystems; Thermo Fisher Scientific, Inc.) and with TaqMan
Universal PCR Master mix II. The 7500 Fast Real-Time PCR system
(Applied Biosystems; Thermo Fisher Scientific, Inc.) was used with
initial cycle at 50˚C for 2 min, followed by one cycle at 95˚C for
10 min and 40 cycles at 95˚C for 15 sec and at 60˚C for 1 min. The
manual calling option in the allelic discrimination application ABI
PRISM 7500 SDS software, version 1.3.1 (Applied Biosystems; Thermo
Fisher Scientific, Inc.) was then used to assign the genotypes.
Statistical methods
Descriptive data are presented as percentages or
mean and standard deviation (SD). Comparative analyses were
performed using the Student's unpaired two-sided t-test, whereas
the Chi-square test was used for discrete variables. Both
univariate and multivariate Cox proportional hazard regression
analyses were used to analyze and illustrate the risk of mortality
during the follow-up period, where both all-cause mortality and CV
mortality were analyzed. Kaplan-Meier graphs were used to
illustrate CV mortality as a function of follow-up time. Censored
patients were those who were still alive at the end of the study
period or who had died of causes other than CV disease. Completed
patients comprised those who had died due to CV disease. In the
multivariate multivariable regression model, adjustments were made
for the following co-variates: Age, hypertension, ischemic heart
disease, diabetes, atrial fibrillation, Hb <120 g/l, EF <40%,
ACE-inhibitors/Angiotensin receptor blockers, beta-blockers,
diuretics, s-cholesterol, s-triglycerides, s-low density
lipoporotein (LDL) and s-high density lipoporoteins (HDL).
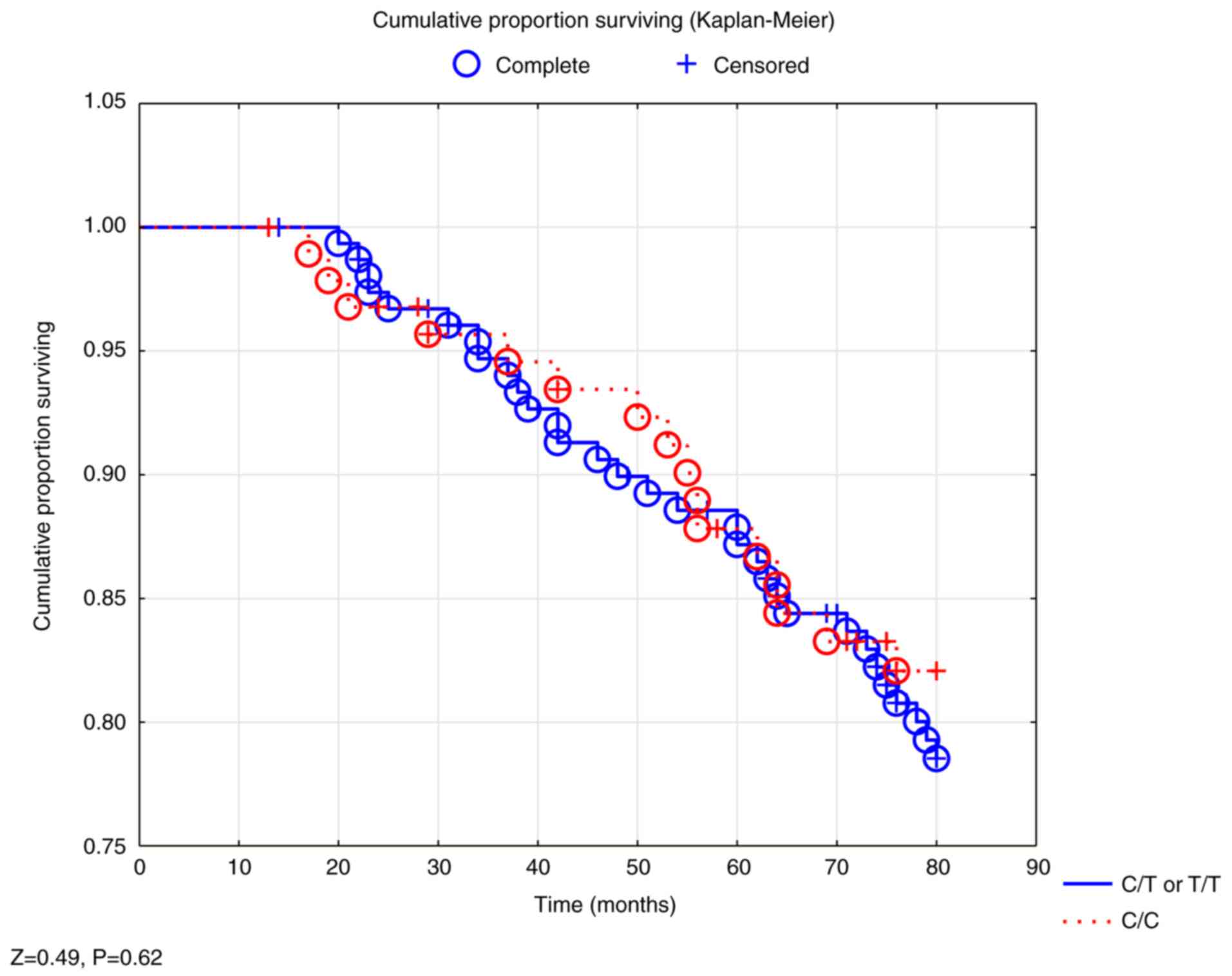
In the Cox regressions, the C/T and T/T genotypes
were amalgamated because almost identical mortality figures were
found during the follow-up with the two groups, and because the
group size of the T/T group was small.
P<0.05 was considered to indicate a statistically
significant difference. All data were analyzed using standard
software packages (Statistica v. 13.1; Statsoft, Inc., Tulsa, OK,
USA).
Results
The basic characteristics of the study population
divided into gender and genotypes of LPR1 are presented in Table I.
 | Table IBasal characteristics of the study
population divided into the three polymorphisms of LRP1. |
Table I
Basal characteristics of the study
population divided into the three polymorphisms of LRP1.
| | LRP1 | |
|---|
| Variable | TT | CT | CC | P-value |
|---|
| n | 65 | 222 | 202 | |
|
Males/females,
n | 25/40 | 107/115 | 105/97 | |
|
Age, mean
(SD) | 77.4 (4.0) | 77.3 (3.6) | 77.7 (3.9) | |
| History | | | | |
|
Diabetes, n
(%) | 13 (20.0) | 56 (25.2) | 47 (23.3) | 0.66 |
|
Hypertension,
n (%) | 49 (75.4) | 171 (77.0) | 163 (80.7) | 0.99 |
|
IHD, n
(%) | 12 (18.5) | 52 (23.4) | 51 (25.2) | 0.53 |
|
NYHA I, n
(%) | 33 (50.8) | 112 (50.5) | 95 (47.0) | 0.75 |
|
NYHA II, n
(%) | 17 (26.2) | 67 (30.2) | 72 (35.6) | 0.27 |
|
NYHA III, n
(%) | 13 (20.0) | 41 (18.5) | 35 (17.3) | 0.88 |
|
NYHA IV, n
(%) | 2 (3.1) | 1 (0.5) | 0 | |
|
Unclassified,
n | 0 | 1 (0.5) | 0 | |
| Medication | | | | |
|
Beta
blockers, n (%) | 24(37) | 70(32) | 93(46) | 0.08 |
|
ACEI/AII, n
(%) | 12(19) | 57(26) | 65(32) | 0.07 |
|
Diuretics, n
(%) | 21(32) | 81(36) | 73(36) | 0.81 |
| Examinations | | | | |
|
BP, systolic
mm Hg, mean (SD) | 151(20) | 148(24) | 150(19) | 0.62 |
|
BP,
diastolic mmHg, mean (SD) | 75(10) | 75(12) | 75(10) | 0.97 |
|
IMT,
thickness, mm (SD) | 0.61 (0.22) | 0.54 (0.16) | 0.59 (0.18) | 0.02 |
|
EF <40%,
n (%) | 5(8) | 14(6) | 20(10) | 0.31 |
Almost equal numbers of males and females were
included (237 vs. 252). In the total study population, 383/489
(78.3%) of the participants had hypertension, 116/489 (23.7%) had
diabetes, 115/489 (23.5%) had ischemic heart disease and 134/489
(27.4%) were receiving treatment with ACE-inhibitors or angiotensin
receptor blockers. Of the population, 187/489 (38.2%) were
receiving treatment with beta-blockers and 175/489 (35.8%) were
receiving treatment with diuretics.
The abdominal aorta of in the study population was
examined and a systolic dimension between 29.3 to 9.7 mm was found.
Based on these measurements there were no signs of abdominal aortic
aneurysms in the individuals examined.
When analyzing the two genders, it was found that
significantly more females with the C/T genotype were receiving
treatment with diuretics compared with the corresponding genotype
among males (47.2% vs. 26.3%; P=0.001), which is in concurrence
with other gender-divided reports (6). In the population with the C/C genotype,
a higher systolic blood pressure was recorded among the females
compared with the males with the same genotype. Also, it could be
demonstrated that there was a larger proportion of participants
with an impaired heart function out of the male population with the
genotypes C/T and C/C, compared with the females with the
corresponding genotypes.
Plasma levels of LRP1
In a small subsection of the study population
(n=221) the level of LRP1 was evaluated using an ELISA. When
evaluating the three genotypes, it could also be seen that the C/C
genotype had a reduced level of LRP1 in comparison with the T/T
genotype [mean 20,994 (SD 7131) pg/ml vs. 23,409 (SD 5651) pg/ml;
P=0.043) and the C/T genotype with a level mean 21534 (SD 7,969)
pg/ml]. No other differences in levels of LRP1 between clinical or
gender groups could be found.
Mortality and genotypes
The median follow-up time of the population was 80
months (6.7 years) and during that time 116 (23.7%) all-cause and
75 (15.3%) CV deaths were registered. Analyzing the three genotypes
and the total number of mortalities, significant differences could
be detected in the distribution of both all-cause and CV mortality,
with a higher mortality in the male population with the C/C
genotype (Table II).
 | Table IICardiovascular and all-cause
mortality in the study population distributed in the three
genotypes of LRP1, and gender, during a follow-up period of 80
months. |
Table II
Cardiovascular and all-cause
mortality in the study population distributed in the three
genotypes of LRP1, and gender, during a follow-up period of 80
months.
| | CV mortality | All-cause
mortality |
|---|
| LRP1 | Females | Males | P-value | Females | Males | P-value |
|---|
| C/C, n (%) | 14/97 (14.4) | 32/105 (30.5) |
Χ2=7.38 | 22/97 (22.7) | 47/105 (44.8) |
Χ2=10.9 |
| | | | P=0.007 | | | P=0.0009 |
| C/T, n (%) | 20/115 (17.4) | 21/107 (19.6) | P=0.67 | 35/115 (30.4) | 37/107 (34.6) | 0.51 |
| T/T, n (%) | 6/40 (15.0) | 7/25 (28.0) | 0.20 | 9/40 (23.0) | 11/25 (44.0) | 0.07 |
In a survival analysis, no significant difference in
survival could be demonstrated between the three genotypes in the
total study population, nor in the male population (Fig. 1).
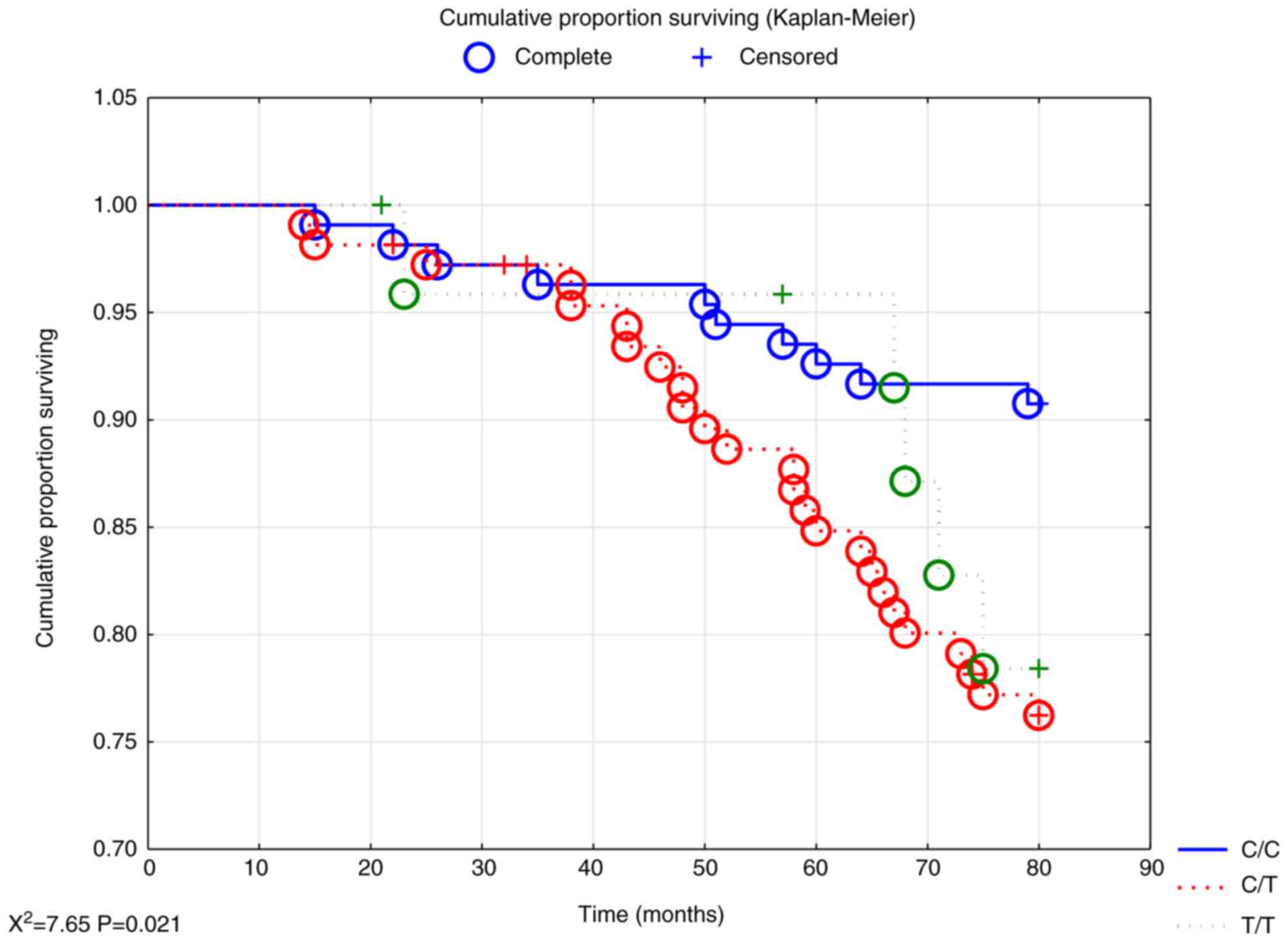
However, evaluating the female population revealed
significant differences in survival between the three genotypes,
where increased all-cause mortality could be seen in the T/T and
C/T genotypes (P<0.05; Fig.
2).
Analyzing mortality risk during the 6.7 years of
follow-up in a multivariate model demonstrated a significantly
increased risk both of all-cause mortality (HR 1.68; 95% CI
1.12-2.52; P=0.01) and CV mortality (HR 2.07; 95% CI 1.23-3.49;
P=0.006) of the total study population for those with the T/T or
C/T genotype (Table III).
 | Table IIIMultivariate Cox proportional hazard
regression illustrating risk of cardiovascular and all-cause
mortality in the total population and with a follow-up period of 80
months. |
Table III
Multivariate Cox proportional hazard
regression illustrating risk of cardiovascular and all-cause
mortality in the total population and with a follow-up period of 80
months.
| | CV-mortality | All cause
mortality |
|---|
| Variables | Hazard ratio | 95% confidence
interval | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age | 1.07 | 1.00-1.15 | 0.04 | 1.06 | 1.01-1.12 | 0.02 |
| Hypertension | 1.34 | 0.75-2.40 | 0.32 | 1.61 | 0.99-2.61 | 0.06 |
| IHD | 1.21 | 0.70-2.11 | 0.50 | 1.34 | 0.85-2.09 | 0.21 |
| Atrial
fibrillation | 2.39 | 1.29-4.46 | 0.006 | 2.27 | 1.37-3.76 | 0.001 |
| Diabetes | 1.64 | 0.97-2.77 | 0.07 | 1.51 | 0.98-2.32 | 0.06 |
| Hb <120 g/l | 1.69 | 0.90-3.17 | 0.10 | 1.26 | 0.71-2.22 | 0.43 |
| EF <40% | 1.72 | 0.82-3.57 | 0.15 | 1.81 | 1.02-3.23 | 0.04 |
| ACEI/ARB | 0.79 | 0.46-1.38 | 0.41 | 0.75 | 0.48-1.18 | 0.21 |
| Beta blockers | 1.27 | 0.76-2.10 | 0.36 | 1.09 | 0.72-1.65 | 0.67 |
| Diuretics | 1.00 | 0.60-1.69 | 0.99 | 1.09 | 0.72-1.65 | 0.69 |
| s-Chol | 0.59 | 0.02-22.4 | 0.78 | 0.83 | 0.19-3.54 | 0.80 |
| s-Triglyc | 0.99 | 0.18-5.36 | 0.99 | 0.86 | 0.40-1.82 | 0.69 |
| s-LDL | 1.73 | 0.04-66.7 | 0.77 | 1.11 | 0.26-4.74 | 0.89 |
| s-HDL | 1.49 | 0.04-54.4 | 0.83 | 1.08 | 0.23-5.04 | 0.92 |
| LRP1 C/T or
T/T | 2.07 | 1.23-3.49 | 0.006 | 1.68 | 1.12-2.52 | 0.01 |
Evaluating the female population with the same
multivariate model showed increased risk for all-cause mortality
(HR 2.75; 95% CI 1.28-5.90; P=0.01) and for CV mortality (HR 5.61;
95% CI 1.98-15.85; P=0.001) for those with the T/T or C/T
genotypes, as compared to those with the C/C genotype (Table IV).
 | Table IVMultivariate Cox proportional hazard
regression illustrating risk of cardiovascular and all-cause
mortality in the female population and with a follow-up period of
80 months. |
Table IV
Multivariate Cox proportional hazard
regression illustrating risk of cardiovascular and all-cause
mortality in the female population and with a follow-up period of
80 months.
| | CV-mortality | All cause
mortality |
|---|
| Variables | Hazard ratio | 95% confidence
interval | P-value | Hazard ratio | 95% confidence
interval | P-value |
|---|
| Age | 0.98 | 0.88-1.10 | 0.77 | 1.04 | 0.95-1.15 | 0.38 |
| Hypertension | 1.18 | 0.43-3.23 | 0.74 | 1.29 | 0.56-2.98 | 0.55 |
| IHD | 1.69 | 0.60-4.77 | 0.32 | 1.90 | 0.86-4.19 | 0.11 |
| Atrial
fibrillation | 3.38 | 0.95-12.00 | 0.06 | 2.41 | 0.89-6.53 | 0.08 |
| Diabetes | 3.23 | 1.26-8.30 | 0.01 | 1.99 | 0.95-4.14 | 0.07 |
| Hb <120 g/l | 4.12 | 1.62-10.51 | 0.03 | 2.57 | 1.11-5.94 | 0.03 |
| EF<40% | 2.14 | 0.41.11.08 | 0.36 | 0.90 | 0.19-4.35 | 0.90 |
| ACEI/ARB | 0.60 | 0.22-1.65 | 0.32 | 1.08 | 0.50-2.32 | 0.85 |
| Beta blockers | 1.40 | 0.56-3.48 | 0.47 | 1.09 | 0.53-2.24 | 0.81 |
| Diuretics | 2.04 | 0.81-5.15 | 0.13 | 1.85 | 0.86-3.98 | 0.11 |
| s-Chol | 1.08 | 0.19-6.15 | 0.93 | 0.89 | 0.20-4.01 | 0.88 |
| s-Triglyc | 1.17 | 0.37-3.76 | 0.79 | 0.98 | 0.36-2.68 | 0.96 |
| s-LDL | 0.91 | 0.16-5.07 | 0.91 | 0.93 | 0.24-4.74 | 0.93 |
| s-HDL | 2.82 | 0.35-22.91 | 0.33 | 1.56 | 0.26-9.48 | 0.63 |
| LRP1 C/T or
T/T | 5.61 | 1.98-15.85 | 0.001 | 2.75 | 1.28-5.90 | 0.01 |
No corresponding increased risk could be seen in the
male population (for all-cause mortality HR 1.19; 95% CI 0.72-1.97;
P=0.49; for CV mortality HR 1.39; 95% CI 0.74-2.60; P=0.31).
Discussion
In this study three genotypes of LRP1 were
evaluated; C/C, C/T and T/T, and different risks of both all-cause,
and CV mortality were demonstrated in different genotypes of the
female population, but not in males. It should be noted that the
population evaluated in this study was an elderly community-living
group and there, a representative one even though the sample size
was not large. The results are interesting as they show
gender-specific differences both regarding all-cause and to a
greater extent CV mortality. The main project has been presented in
several previous publications (11-22).
That genetic variance could result in effects on
CV-associated mortality is well-known from the literature (23-25).
However, to the best of our knowledge, this is the
first time that differences in gender-specific risk in mortality
that are associated with a genetic variance have been
demonstrated.
In recent years, special interest has been focused
on LRP1 and its possible association with CV diseases. Gonias
(26) demonstrated in an excellent
review the important relationship between LRP1 and smooth muscle
cell properties. In a report from Italy, Galora et al
(27) demonstrated an interesting
association between the T allele of rs1466535 and abdominal aortic
aneurysms, but not with the other alleles investigated. The present
study was able to confirm that the T allele also had an association
with mortality, but only in one of the two genders. Ye et al
(28) in a study of abdominal
aneurysms, demonstrated significant sex differences, where a faster
expansion of the aneurysms could be seen in the female group and
therefore the present study intended to evaluate any sex
differences in CV mortality in the different genotypes of
rs1466535.
As Giusti et al (29) reported, the present study also found
that the majority of participants had the C/C genotype and only a
small number had the T/T genotype. After combining the T/T and the
C/T genotypes, Giusti was able to report a greater proportion of
patients suffering from carotid stenosis in this group compared
with the C/C genotype. The same amalgamating process of the T/T and
C/T genotypes in the evaluations was performed in the present
study.
In the literature there are reports of a study with
a larger number of participants compared to the present study, such
as the huge genome-wide association study for coronary artery
disease reported by Webb et al (30), which included more than 120,000
participants. However, the important difference compared with the
present study is that mortality data for all participants is
included; no one was lost during the follow-up period of 6.7 years.
CV mortality is regarded as the definite endpoint of the CV risks
that the participants were exposed to, rather than getting
diagnosed with coronary artery disease, as was done in the
genome-wide association study mentioned above, which is why the
small study sample reported in the present study is notable.
In the ELISA evaluation of the levels of LRP1, a
significant difference was demonstrated in the levels between the
T/T and the C/C genotypes, a fact that has not been reported before
to the best of our knowledge. In the genome-wide study by Bown
et al they reported a tendency toward an increased level of
the C/C genotype of LRP1 in comparison with the T/T genotype
(3). However, Bown et al
reported on a population with abdominal aortic aneurysms, something
that the population of the present study did not have and thus it
is not surprising that the opposite results were reported regarding
the level of the C/C genotype of LRP1.
The female population of individuals with the T/T or
C/T genotypes of LRP1 demonstrated a 2.75-fold increased risk of
all-cause mortality and a 5.61-fold increased risk of CV mortality
when applying a multivariate model adjusting for certain well-known
variables influencing the CV mortality risk. However, it should be
pointed out that the confidence interval is wide in the evaluation,
so it is important to interpret the point estimate of the HR with
caution. The wide confidence interval is probably the result of the
limited size of the different genotype groups analyzed. The
important message is that an increased mortality risk exists in the
C/T and T/T genotypes in comparison with the C/C genotype. In the
male population no such prognostic differences between the
different genotypes of LRP1 could be found. This could imply that
irrespective of the LRP1 genotype, there are stronger factors that
determine the mortality risk in the male group, for example
mortality based on ischemic heart disease, a condition with a
substantial preponderance in the male population.
In the literature there are a multitude of reports
indicating that there are gender differences regarding CV mortality
in general and a higher mortality is generally reported in the male
population of corresponding age, compared to the female population
(31,32). The major reason for this is the higher
incidence of ischemic heart disease among males (33). It is possible that the SNP may in some
(secondary) way be influenced by variation in genes situated on the
sex chromosomes, or by hormonal differences between men and women,
and this should be a focus of further study.
The presented gender differences in some of the
genotypes of LRP1 are of importance in clinical routine since
individuals with increased risk for CV mortality can be identified.
Even if it is not possible to modify the genetic constitution, it
is possible to provide more intense prevention procedures and an
individualized follow-up program for patients, which also may
reduce CV mortality and health care costs.
It should also be noted that the genetic analyses
exhibited in the present study were conducted at a cost that was
below the cost for analyses of the golden-standard biomarker used
to identify patients with heart failure, the N-terminal fragment of
the natriuretic peptide proBNP. Thus, it was concluded that the
presented genetic analyses are also cost-effective in the
clinic.
It could be argued that it would only be relevant to
evaluate the at-risk genotypes in an at-risk population. However,
this assumption is only correct in a population with known or
assumed increased risk and this is usually not the case in the
clinical routine. Therefore, elderly persons from a
community-living population with mixed CV risks were included to
better reflect the situation that clinicians are faced with every
day. Despite this fact, it was possible to obtain significant and
interesting data indicating that certain individuals are exposed to
an increased risk that can be identified through genotype
evaluation.
Therefore, out of the female group, those with the
C/T or T/T genotypes of the LPR1 gene, with a more than 5-fold
increased risk of CV mortality, constituted a high-risk population
and thus it was important to identify them, even though the
obtained point estimate should be interpreted with caution due to
the limited sample size.
However, from a clinical and economical point of
view it is also interesting to identify those with a low risk of
mortality. As the present study demonstrated above, the females
with the C/C genotype of LRP1 only exhibited a 9.3% all-cause and a
4.6% CV mortality during almost seven years of follow-up in spite
of being an elderly patient group. From this it could be concluded
that identifying both those at high as well as those at low risk of
death is important from a clinical point of view and the use of
genetic information could further aid in this identification
process.
The presented research is a community-based study
and thus includes most patients with discrete or no CV symptoms.
Therefore, those with CV disease are in a minority and thus the
size of the CV group is small, which results in wide CIs in risk
evaluations, thus making their interpretation uncertain.
Also, the presented study population is an elderly
one and therefore it is not possible to extrapolate the obtained
results into other age groups without uncertainty.
Finally, as the subgroups of those with certain
genotypes are small, the results of some of the evaluations should
be interpreted with caution and the results should be regarded as
hypothesis-generating. However, the results of the present study
are in line with and strengthen previous findings and a hypothesis
that the investigated genes and SNPs could play an important role
as markers to identify individuals at risk.
In conclusion, information regarding the LRP1 gene
was applied to an elderly primary health care population that was
followed for almost seven years and where all mortality was
registered. Specific gender differences were noted, and in the
female group those with the LRP1 T/T or C/T genotype had a
5.61-fold increased risk for CV mortality in the multivariate
analysis.
In the male group however, no such gender difference
could be found.
Applying a combined analysis using, for the females,
those with LRP1 C/T or T/T could substantially increase the
possibility of identifying individuals with high CV risk and
thereby help to optimize CV prevention and treatment.
Acknowledgements
The authors would like to thank research nurse Mrs.
Anette Gylling for all her valuable help in preparation of the
blood samples.
Funding
The present study was supported by grants from the
County Council of Östergötland, University of Linköping, Linköping,
Sweden and the Swedish Heart and Lung Foundation.
Availability of data and materials
Under Swedish Law, the datasets generated/analyzed
are not publicly available but are available from the corresponding
author upon reasonable request and with permission of the
University of Linköping.
Authors' contributions
Conceived and designed the experiments: UA and DW;
performed the experiments: UA and DW; analyzed the data: UA and DW;
contributed reagents/material/analysis tools: DW; wrote the
manuscript: UA and DW.
Ethics approval and consent to
participate
All participants gave their written informed consent
and the study was conducted in accordance with the Declaration of
Helsinki principles. The study protocol was approved by the
Regional Ethical Review Board of Linköping, Sweden (Dnr 95044).
Patient consent for publication
All participants gave their written informed
consent.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alwan A: Global report on noncommunicable
diseases 2010. Italy, World Health Organization. 2011.
|
|
2
|
Aledo R, Alonso R, Mata P, Llorente-Cortés
V, Padró T and Badimon L: LRP1 gene polymorphisms are associated
with premature risk of cardiovascular disease in patients with
familial hypercholesterolemia. Rev Esp Cardiol (Engl Ed).
65:807–812. 2012.(In English, Spanish). PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bown MJ, Jones GT, Harrison SC, Wright BJ,
Bumpstead S, Baas AF, Gretarsdottir S, Badger SA, Bradley DT,
Burnand K, et al: Abdominal aortic aneurysm is associated with a
variant in low-density lipoprotein receptor-related protein 1. Am J
Hum Genet. 89:619–627. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boucher P, Gotthardt M, Li WP, Anderson RG
and Herz J: LRP: Role in vascular wall integrity and protection
from atherosclerosis. Science. 300:329–332. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Folestad E, Kunath A and Wågsäter D:
PDGF-C and PDGF-D signaling in vascular diseases and animal models.
Mol Aspects Med. 62:1–11. 2018.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alehagen U, Ericsson A and Dahlström U:
Are there any significant differences between females and males in
the management of heart failure? Gender aspects of an elderly
population with symptoms associated with heart failure. J Card
Fail. 15:501–507. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Pombo JF, Troy BL and Russell RO Jr: Left
ventricular volumes and ejection fraction by echocardiography.
Circulation. 43:480–490. 1971. View Article : Google Scholar
|
|
8
|
Wahr DW, Wang YS and Schiller NB: Left
ventricular volumes determined by two-dimensional echocardiography
in a normal adult population. J Am Coll Cardiol. 1:863–868.
1983.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gibbons RJ, Lee KL, Cobb F and Jones RH:
Ejection fraction response to exercise in patients with chest pain
and normal coronary arteriograms. Circulation. 64:952–957.
1981.PubMed/NCBI
|
|
10
|
Shamoun L, Skarstedt M, Andersson RE,
Wågsäter D and Dimberg J: Association study on IL-4, IL-4Rα and
IL-13 genetic polymorphisms in Swedish patients with colorectal
cancer. Clin Chim Acta. 487:101–106. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Ljungberg L, Alehagen U, Länne T, Björck
H, De Basso R, Dahlström U and Persson K: The association between
circulating angiotensin-converting enzyme and cardiovascular risk
in the elderly: A cross-sectional study. J Renin Angiotensin
Aldosterone Syst. 12:281–289. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Björck HM, Länne T, Alehagen U, Persson K,
Rundkvist L, Hamsten A, Dahlström U and Eriksson P: Association of
genetic variation on chromosome 9p21.3 and arterial stiffness. J
Intern Med. 265:373–381. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ljungberg LU, De Basso R, Alehagen U,
Björck HM, Persson K, Dahlström U and Länne T: Impaired abdominal
aortic wall integrity in elderly men carrying the
angiotensin-converting enzyme D allele. Eur J Vasc Endovasc Surg.
42:309–319. 2011.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Johansson P, Alehagen U, Ulander M,
Svanborg E, Dahlström U and Broström A: Sleep disordered breathing
in community dwelling elderly: Associations with cardiovascular
disease, impaired systolic function, and mortality after a six-year
follow-up. Sleep Med. 12:748–753. 2011.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chisalita SI, Dahlström U, Arnqvist HJ and
Alehagen U: Increased IGF1 levels in relation to heart failure and
cardiovascular mortality in an elderly population: Impact of ACE
inhibitors. Eur J Endocrinol. 165:891–898. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Johansson P, Alehagen U, Svanborg E,
Dahlström U and Broström A: Clinical characteristics and mortality
risk in relation to obstructive and central sleep apnoea in
community-dwelling elderly individuals: A 7-year follow-up. Age
Ageing. 41:468–474. 2012.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ljungberg LU, Alehagen U, De Basso R,
Persson K, Dahlström U and Länne T: Circulating
angiotensin-converting enzyme is associated with left ventricular
dysfunction, but not with central aortic hemodynamics. Int J
Cardiol. 166:540–541. 2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Johansson P, Riegel B, Svensson E,
Broström A, Alehagen U, Dahlström U and Jaarsma T: The contribution
of heart failure to sleep disturbances and depressive symptoms in
older adults. J Geriatr Psychiatry Neurol. 25:179–187.
2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Johansson P, Riegel B, Svensson E,
Broström A, Alehagen U, Dahlström U and Jaarsma T: Sickness
behavior in community-dwelling elderly: Associations with impaired
cardiac function and inflammation. Biol Res Nurs. 16:105–113.
2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Johansson P, Svensson E, Alehagen U,
Jaarsma T and Broström A: The contribution of hypoxia to the
association between sleep apnoea, insomnia, and cardiovascular
mortality in community-dwelling elderly with and without
cardiovascular disease. Eur J Cardiovasc Nurs. 14:222–231.
2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chisalita SI, Dahlström U, Arnqvist HJ and
Alehagen U: Proinsulin and IGFBP-1 predicts mortality in an elderly
population. Int J Cardiol. 174:260–267. 2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Alehagen U, Vorkapic E, Ljungberg L, Länne
T and Wågsäter D: Gender difference in adiponectin associated with
cardiovascular mortality. BMC Med Genet. 16:37:2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Eppinga RN, Hagemeijer Y, Burgess S, Hinds
DA, Stefansson K, Gudbjartsson DF, van Veldhuisen DJ, Munroe PB,
Verweij N and van der Harst P: Identification of genomic loci
associated with resting heart rate and shared genetic predictors
with all-cause mortality. Nat Genet. 48:1557–1563. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
24
|
Ansani L, Marchesini J, Pestelli G, Luisi
GA, Scillitani G, Longo G, Milani D, Serino ML, Tisato V and
Gemmati D: F13A1 gene variant (V34L) and residual circulating
FXIIIA levels predict Short- and Long-term mortality in acute
myocardial infarction after coronary angioplasty. Int J Mol Sci.
19pii. (E2766)2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Heijmans BT, Westendorp RG, Knook DL,
Kluft C and Slagboom PE: Angiotensin I-converting enzyme and
plasminogen activator inhibitor-1 gene variants: Risk of mortality
and fatal cardiovascular disease in an elderly population-based
cohort. J Am Coll Cardiol. 34:1176–1183. 1999.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Gonias SL: Mechanisms by which LRP1
(Low-Density Lipoprotein Receptor-Related Protein-1) maintains
arterial integrity. Arterioscler Thromb Vasc Biol. 38:2548–2549.
2018.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Galora S, Saracini C, Pratesi G, Sticchi
E, Pulli R, Pratesi C, Abbate R and Giusti B: Association of
rs1466535 LRP1 but not rs3019885 SLC30A8 and rs6674171 TDRD10 gene
polymorphisms with abdominal aortic aneurysm in Italian patients. J
Vasc Surg. 61:787–792. 2015.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ye Z, Austin E, Schaid DJ, Bailey KR,
Pellikka PA and Kullo IJ: A DAB2IP genotype: Sex interaction is
associated with abdominal aortic aneurysm expansion. J Investig
Med. 65:1077–1082. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Giusti B, Galora S, Saracini C, Pratesi G,
Gensini GF, Pulli R, Pratesi C and Abbate R: Role of rs1466535 low
density lipoprotein receptor-related protein 1 (LRP1) gene
polymorphism in carotid artery disease. Atherosclerosis.
237:135–137. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Webb TR, Erdmann J, Stirrups KE, Stitziel
NO, Masca NG, Jansen H, Kanoni S, Nelson CP, Ferrario PG, König IR,
et al: Systematic evaluation of pleiotropy identifies 6 further
loci associated with coronary artery disease. J Am Coll Cardiol.
69:823–836. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Kappert K, Böhm M, Schmieder R, Schumacher
H, Teo K, Yusuf S, Sleight P and Unger T: ONTARGET/TRANSCEND
Investigators. Impact of sex on cardiovascular outcome in patients
at high cardiovascular risk: Analysis of the Telmisartan randomized
assessment study in ACE-intolerant subjects with cardiovascular
disease (TRANSCEND) and the ongoing telmisartan alone and in
combination with ramipril global end point trial (ONTARGET).
Circulation. 126:934–941. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Regitz-Zagrosek V, Oertelt-Prigione S,
Seeland U and Hetzer R: Sex and gender differences in myocardial
hypertrophy and heart failure. Circ J. 74:1265–1273.
2010.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Dudas K, Björck L, Jernberg T, Lappas G,
Wallentin L and Rosengren A: Differences between acute myocardial
infarction and unstable angina: A longitudinal cohort study
reporting findings from the Register of Information and Knowledge
about Swedish Heart Intensive Care Admissions (RIKS-HIA). BMJ Open.
3pii. (e002155)2013.PubMed/NCBI View Article : Google Scholar
|