1. Introduction
Recurrent aphthous stomatitis (RAS), also known as
canker sores, is the most common disease of the oral mucosa
(1). This review presents key aspects
of RAS, integrating clinical, histological and molecular concepts
that are important for every medical professional that encounters
this disease to understand.
The clinical picture of RAS is characterized by
recurrent episodes of solitary or multiple painful ulcerations
(2) without an association with
systemic diseases (3). The latter is
relevant to ensure that RAS is not confused with aphthous
ulcerations.
2. Differential diagnosis and
epidemiology
Aphthous ulcerations (or RAS-like ulcerations) have
an underlying systemic cause; therefore, they should be considered
as a distinct medical condition (3).
The differential diagnoses should be established with
autoinflammatory syndromes, including periodic fever with adenitis,
pharyngitis and aphthae (PFAPA) syndrome, Behçet's syndrome and
Crohn's disease; and immunodeficiency states, including nutritional
defects (such as celiac disease and other gastrointestinal
disorders), immune defects (such as human immunodeficiency virus
infection/acquired immune deficiency syndrome) and neutrophil
defects (such as cyclic neutropenia) (4). The term RAS should be used for
ulceration present in the absence of systemic disease.
The prevalence of RAS varies between 0.9 and 78% in
different groups examined. In the US, for the period of 1988-1994
the prevalence was 0.89% in adults (5) and 1.64% in children (6). In Iran (2005), Jordan (2008), India
(2010-2012) and China (2013-2017) reported prevalence was 25.2%
(7), 70% (8), 21.7% (9)
and 27.17% (10), respectively. Its
onset appears to peak between 10 and 19 years of age (11) and its frequency decreases with
advancing age (12).
3. Pathogenesis
The etiology and pathogenesis of RAS remain unclear.
Multiple factors are associated with the establishment of this
disease, including a positive family history, food
hypersensitivity, smoking cessation, psychological stress and
immune disturbance (11,13). However, for this evidence, there is
often an absence of statistical risk analysis. Immune dysregulation
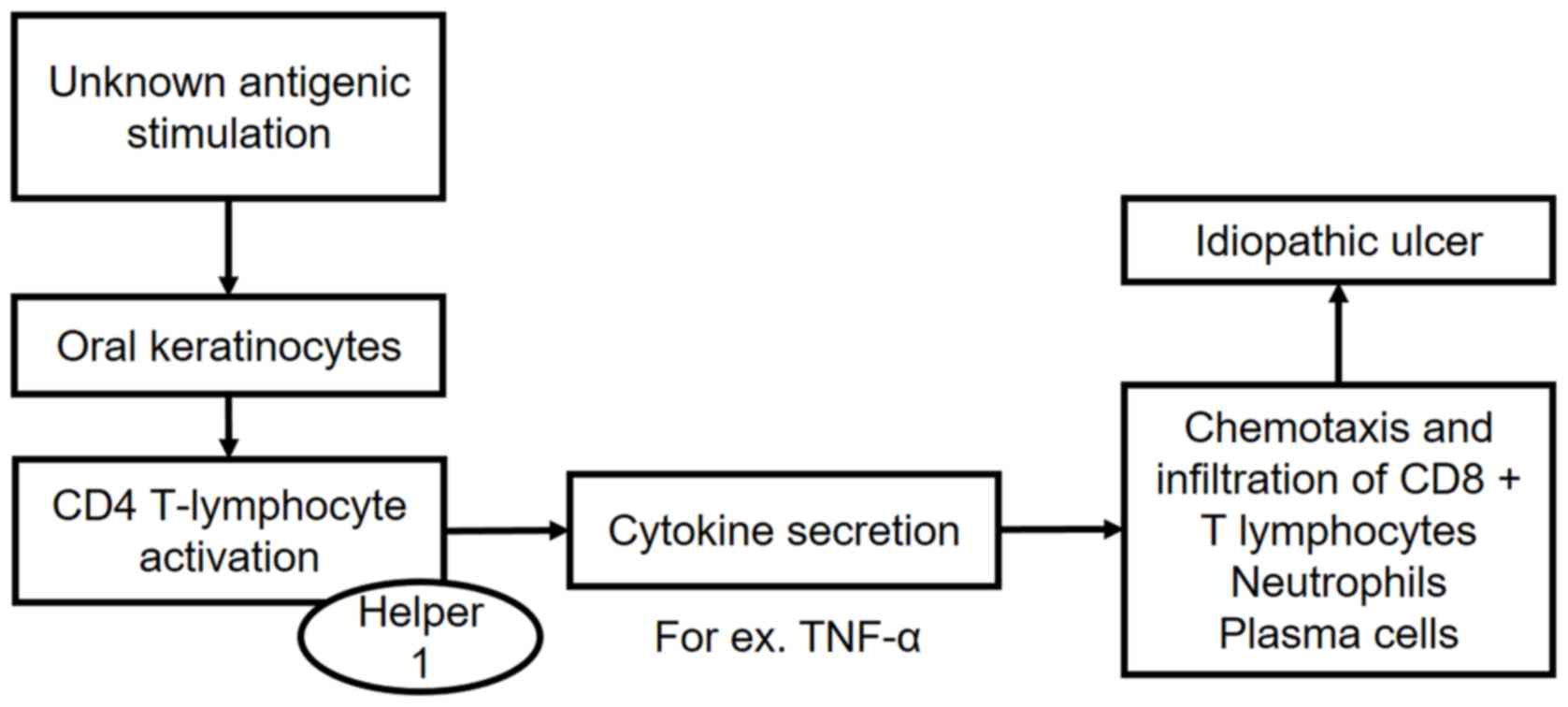
linked to several triggers may facilitate the development of RAS.
The roles of the immune system and inflammatory processes have been
confirmed in recent large-scale bioinformatics analyses (14,15). It is
known that a Th1-type hyperimmune response favors the appearance of
inflammatory reactions that precede ulcerations (Fig. 1) (16,17). In
addition, genetic risk factors can determine individual
susceptibility to RAS; in particular, several DNA polymorphisms of
the NOD-like receptor 3(18),
toll-like receptor 4(19),
interleukin (IL)-6(20), E-selectin
(21), IL-1β and TNF-α genes
(22). However, despite the large
number of factors examined, the underlying cause triggering the
episodes of ulcers remains to be elucidated. Therefore, clinically,
the emergence of new lesions cannot be avoided at present.
4. Clinical characteristics
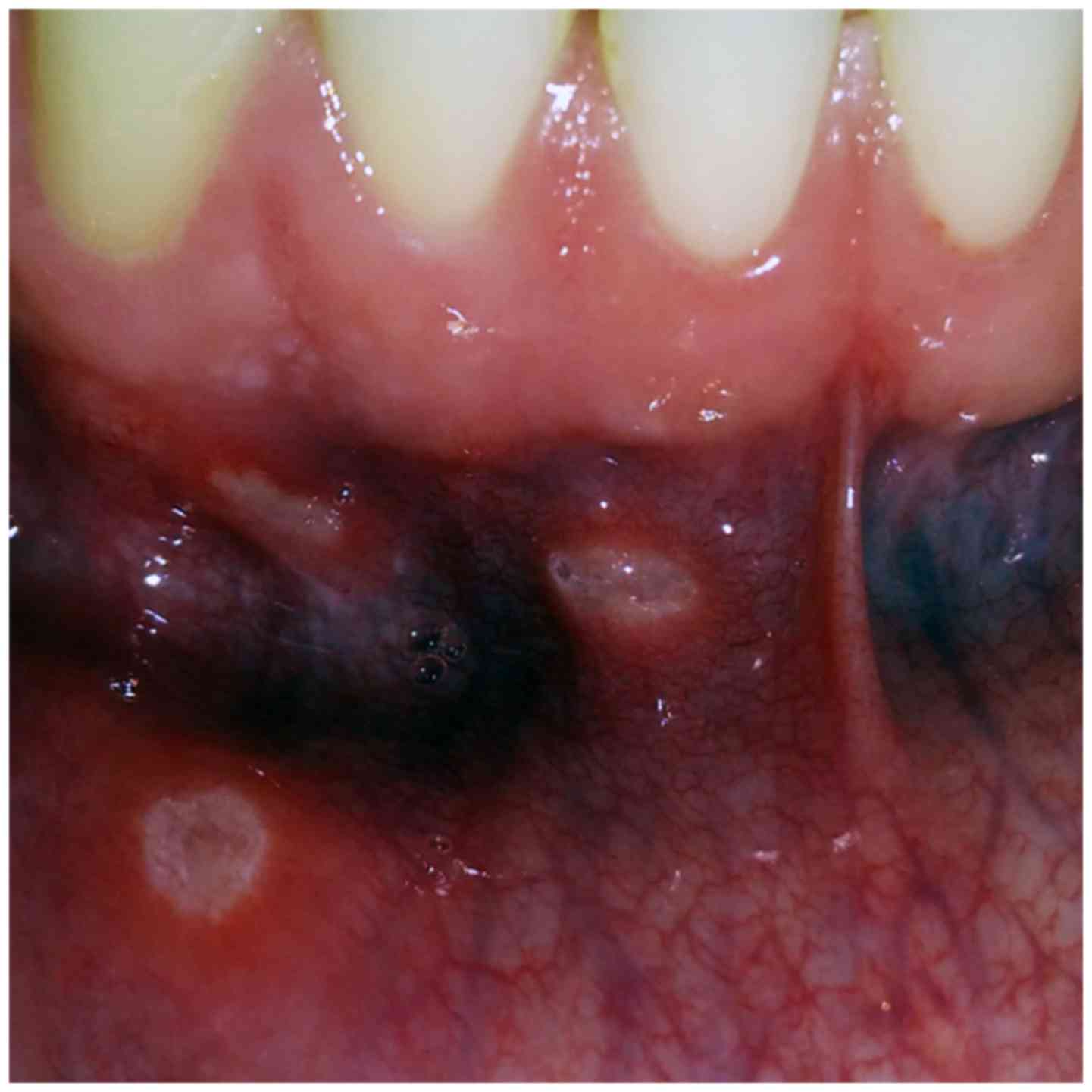
RAS is known to be particularly painful (15). These idiopathic ulcerations are oval
lesions of different sizes with clean edges surrounded by an
erythematous halo. At the center of the ulceration, the necrotic
fundus is covered with a yellow-white fibrinous exudate (23). The ulcers typically present in the
non-masticatory mucosa of the cheeks, lips, ventral and lateral
surfaces of the tongue, non-attached gingiva, and occasionally, the
soft palate (24). RAS lesions are
self-limiting (simple aphthosis), resolving within 1-2 weeks in the
majority of patients (25). In those
affected by the disease, the ulcers can compromise important daily
functions, including nutrition, speech and oral hygiene (26), and affect quality of life (27). This is important, considering that the
lesions can last >2 weeks, with recurrent episodes in a period
of 1-4 months (11). RAS occurs in
three morphological presentations: Minor-type (Mikulicz ulcers,
2-10 mm in diameter), which is the most common (Fig. 2); major aphthous, also termed Sutton
ulcers or periadenitis necrotic mucosa (>10 mm in diameter); and
herpetiform ulceration, which consists of multiple small ulcers
(28). Some patients have continuous
oral ulcerations; in these cases, some ulcers heal as others
develop, with occasional genital ulcers. This corresponds to a
clinical state known as complex aphthosis (11). Complex aphthosis has an underlying
systemic cause, which does not correspond with the RAS
diagnosis.
5. Disease phases
The disease sequence comprises the following stages:
Premonition (24 h), comprising symptoms but no visible signs of
disease; pre-ulcerative (between 18 h and 3 days), comprising
erythema and mild edema; ulcerative (1-16 days), comprising active
ulceration; healing (4-35 days, usually #x003C;21 days), involving
a decrease in symptoms and progressive healing; and remission, in
which there is no evidence of ulcers (29). The ulcerative and remission phases are
those that can be evaluated with greater objectivity on dental
examination. Disease recurrence is established with the appearance
of new ulcers. Disease severity can be determined based on the
number, size and location of the lesions, pain, duration,
ulcer-free periods (30) and the
impact on patient quality of life (27,31).
6. Microscopic characteristics
The diagnosis of RAS is eminently clinical and is
based on careful examination. The incisional or excisional biopsy
of ulcers is recommended only in cases of uncertainty, when the
presence of an oral disease producing ulcers or a malignancy is
suspected (32). The microscopic
characteristics of RAS are nonspecific. The pre-ulcerative lesion
shows subepithelial inflammatory mononuclear cells with abundant
mast cells, edema of the connective tissues and neutrophils lining
the margins. Damage to the epithelium usually begins in the basal
layer and progresses through the superficial layers, ultimately
leading to ulceration and surface exudation (2,11).
7. Experimental models
At present, the only way to examine this disease has
been in those patients who suffer from it. In the English
literature, two models for the experimental evaluation of RAS have
been proposed, both using rabbits. One of the models induces ulcers
with 50% acetic acid (33,34) and the other by surgical incision in
the oral mucosa (35). Neither
registered methods are involved in the inflammatory processes
described in RAS. As RAS is an immunologically-mediated disease,
the chemical and mechanical induction of ulcers cannot be
considered valid models.
8. Treatment
Therapeutic alternatives focus on reducing painful
symptoms (36). Clinically, dental
surgeons at present can advise patients that the ulcers are likely
to heal in 2 weeks, and in more complex cases, treatment based on
topical corticosteroids can be implemented (37), which is the same approach used for
several diseases of unknown cause, including pemphigus, pemphigoid
and oral lichen planus. Despite the use of topical corticosteroids
over several years for RAS, there is a lack of high-quality
evidence for their efficacy (38) and
even less for systemic interventions (31). However, the recommended protocol is a
combination of a topical corticosteroid plus a topical anesthetic
and a buccal antiseptic (38). The
combination includes triamcinolone (0.1% paste, up to four times
daily) in addition to topical lidocaine (2% viscous solution,
maximum 8 doses/day) and oropharyngeal chlorhexidine (0.12%, 15 ml
as a mouthwash twice daily) as an adjuvant (4). Patients should be instructed to avoid
recognized trigger foods, and acidic foods and drinks (39).
9. Conclusions
The key concepts associated with RAS are as follows:
Its cause is unknown, it cannot be prevented, it is immunologically
mediated, diagnosis is clinical, there are no experimental models
for its investigation, and recommended treatment includes a
combination of corticosteroids and topical anesthesia plus an
antiseptic. Taking these key concepts into account, several
questions require further biomedical research. These include
determining what the molecular differences are between a healthy
individual and a patient with RAS, determining which molecules are
involved in the ulcerative phase of disease and the phase of
disease remission, and establishing whether there are molecules
that can predict the clinical course and the severity of ulcers.
Answering these questions can open up novel therapeutic and
preventive possibilities.
Acknowledgements
Not applicable.
Funding
Funding was provided by Fondo Nacional de Desarrollo
Científico y Tecnológico (Fondecyt; grant no. 11180170).
Availability of data and materials
Not applicable.
Authors' contributions
CR conceived the review and analyzed the relevant
literature. CR sourced the literature and wrote the manuscript. CR
critically revised the manuscript, produced the figures and have
read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Edgar NR, Saleh D and Miller RA: Recurrent
aphthous stomatitis: A review. J Clin Aesthet Dermatol. 10:26–36.
2017.PubMed/NCBI
|
|
2
|
Preeti L, Magesh K, Rajkumar K and Karthik
R: Recurrent aphthous stomatitis. J Oral Maxillofac Pathol.
15:252–256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Jin LJ, Lamster IB, Greenspan JS, Pitts
NB, Scully C and Warnakulasuriya S: Global burden of oral diseases:
Emerging concepts, management and interplay with systemic health.
Oral Dis. 22:609–619. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
BMJ Best Practice: Aphthous ulcers 2018.
https://bestpractice.bmj.com/topics/en-us/564/guidelines.
Accessed April 26, 2018.
|
|
5
|
Shulman JD, Beach MM and Rivera-Hidalgo F:
The prevalence of oral mucosal lesions in U.S. adults: data from
the Third National Health and Nutrition Examination Survey,
1988-1994. J Am Dent Assoc. 135:1279–86. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shulman JD: Prevalence of oral mucosal
lesions in children and youths in the USA. Int J Paediatr Dent.
15:89–97. 2005.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Davatchi F, Tehrani-Banihashemi A,
Jamshidi AR, Chams-Davatchi C, Gholami J, Moradi M, Akhlaghi M,
Foroozanfar MH, Barghamdi M, Noorolahzadeh E, et al: The prevalence
of oral aphthosis in a normal population in Iran: a WHO-ILAR
COPCORD study. Arch Iran Med. 11:207–209. 2008.PubMed/NCBI
|
|
8
|
Safadi RA: Prevalence of recurrent
aphthous ulceration in Jordanian dental patients. BMC Oral Health.
9(31)2009. View Article : Google Scholar
|
|
9
|
Patil S, Reddy SN, Maheshwari S,
Khandelwal S, Shruthi D and Doni B: Prevalence of recurrent
aphthous ulceration in the Indian Population. J Clin Exp Dent.
6:e36–e40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang H, He F, Xu C, Fang C and Peng J:
Clinical analysis for oral mucosal disease in 21 972 cases. Zhong
Nan Da Xue Xue Bao Yi Xue Ban. 43:779–783. 2018.(In Chinese).
PubMed/NCBI View Article : Google Scholar
|
|
11
|
Akintoye SO and Greenberg MS: Recurrent
aphthous stomatitis. Dent Clin North Am. 58:281–297.
2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Chavan M, Jain H, Diwan N, Khedkar S,
Shete A and Durkar S: Recurrent aphthous stomatitis: A review. J
Oral Pathol Med. 41:577–583. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Gallo Cde B, Mimura MA and Sugaya NN:
Psychological stress and recurrent aphthous stomatitis. Clinics
(Sao Paulo). 64:645–648. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Rivera C: Immune system and zinc are
associated with recurrent aphthous stomatitis. An assessment using
a network-based approach. J Oral Res. 6:245–251. 2017. View Article : Google Scholar
|
|
15
|
Wu J, Chen ZP, Shang AQ, Wang WW, Chen ZN,
Tao YJ, Zhou Y and Wang WX: Systemic bioinformatics analysis of
recurrent aphthous stomatitis gene expression profiles. Oncotarget.
8:111064–111072. 2017.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Mimura MAM, Borra RC, Hirata CHW and de
Oliveira Penido N: Immune response of patients with recurrent
aphthous stomatitis challenged with a symbiotic. J Oral Pathol Med.
46:821–828. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ślebioda Z, Krawiecka E, Szponar E and
Dorocka-Bobkowska B: Evaluation of serum zinc levels in patients
with recurrent aphthous stomatitis (RAS). BMC Oral Health.
17(158)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Slezakova S, Borilova Linhartova P,
Masopustova L, Bartova J, Petanova J, Kuklinek P, Fassmann A, Dusek
L and Izakovicova Holla L: Association of the NOD-like receptor 3
(NLRP3) gene variability with recurrent aphthous stomatitis in the
Czech population. J Oral Pathol Med. 47:434–439. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Karasneh J, Bani-Hani M, Alkhateeb A,
Hassan A, Alzoubi F and Thornhill M: TLR2, TLR4 and CD86 gene
polymorphisms in recurrent aphthous stomatitis. J Oral Pathol Med.
44:857–863. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Karakus N, Yigit S, Rustemoglu A, Kalkan G
and Bozkurt N: Effects of interleukin (IL)-6 gene polymorphisms on
recurrent aphthous stomatitis. Arch Dermatol Res. 306:173–180.
2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Alkhateeb A, Karasneh J, Abbadi H, Hassan
A and Thornhill M: Association of cell adhesion molecule gene
polymorphisms with recurrent aphthous stomatitis. J Oral Pathol
Med. 42:741–746. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Guimarães AL, Correia-Silva Jde F, Sá AR,
Victória JM, Diniz MG, Costa Fde O and Gomez RS: Investigation of
functional gene polymorphisms IL-1beta, IL-6, IL-10 and TNF-alpha
in individuals with recurrent aphthous stomatitis. Arch Oral Biol.
52:268–272. 2007.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Schemel-Suárez M, López-López J and
Chimenos-Küstner E: Oral ulcers: Differential diagnosis and
treatment. Med Clin (Barc). 145:499–503. 2015.(In Spanish).
PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cui RZ, Bruce AJ and Rogers RS III:
Recurrent aphthous stomatitis. Clin Dermatol. 34:475–481.
2016.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Rogers RS III: Recurrent aphthous
stomatitis: Clinical characteristics and associated systemic
disorders. Semin Cutan Med Surg. 16:278–283. 1997.PubMed/NCBI
|
|
26
|
Lalla RV, Choquette LE, Feinn RS,
Zawistowski H, Latortue MC, Kelly ET and Baccaglini L: Multivitamin
therapy for recurrent aphthous stomatitis: A randomized,
double-masked, placebo-controlled trial. J Am Dent Assoc.
143:370–376. 2012.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rajan B, Ahmed J, Shenoy N, Denny C,
Ongole R and Binnal A: Assessment of quality of life in patients
with chronic oral mucosal diseases: A questionnaire-based study.
Perm J. 18:e123–e127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Albrektson M, Hedström L and Bergh H:
Recurrent aphthous stomatitis and pain management with low-level
laser therapy: A randomized controlled trial. Oral Surg Oral Med
Oral Pathol Oral Radiol. 117:590–594. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Vucicevic Boras V and Savage NW: Recurrent
aphthous ulcerative disease: Presentation and management. Aust Dent
J. 52:10–15; quiz 73. 2007.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Tappuni AR, Kovacevic T, Shirlaw PJ and
Challacombe SJ: Clinical assessment of disease severity in
recurrent aphthous stomatitis. J Oral Pathol Med. 42:635–641.
2013.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Brocklehurst P, Tickle M, Glenny AM, Lewis
MA, Pemberton MN, Taylor J, Walsh T, Riley P and Yates JM: Systemic
interventions for recurrent aphthous stomatitis (mouth ulcers).
Cochrane Database Syst Rev. CD005411. 2012.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Belenguer-Guallar I, Jiménez-Soriano Y and
Claramunt-Lozano A: Treatment of recurrent aphthous stomatitis. A
literature review. J Clin Exp Dent. 6:e168–e174. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Karavana Hizarcioğlu SY, Sezer B, Güneri
P, Veral A, Boyacioğlu H, Ertan G and Epstein JB: Efficacy of
topical benzydamine hydrochloride gel on oral mucosal ulcers: An in
vivo animal study. Int J Oral Maxillofac Surg. 40:973–978.
2011.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Karavana SY, Gökçe EH, Rençber S, Özbal S,
Pekçetin C, Güneri P and Ertan G: A new approach to the treatment
of recurrent aphthous stomatitis with bioadhesive gels containing
cyclosporine A solid lipid nanoparticles: In vivo/in vitro
examinations. Int J Nanomedicine. 7:5693–5704. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Fernandes Teixeira FM, Figueiredo Pereira
Md, Gomes Ferreira NL, Miranda GM and Andrade Aguiar JL: Spongy
film of cellulosic polysaccharide as a dressing for aphthous
stomatitis treatment in rabbits. Acta Cir Bras. 29:231–236.
2014.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Dan S, Jinwei Z, Qiang Z, Jianwei S and
Weijun Z: Exploring the molecular mechanism and biomarker of
recurrent aphthous stomatitis based on gene expression microarray.
Clin Lab. 63:249–253. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Swain SK, Gupta S and Sahu MC: Recurrent
aphthous ulcers-still a challenging clinical entity. Apollo Med.
14:202–206. 2017. View Article : Google Scholar
|
|
38
|
Staines K and Greenwood M: Aphthous ulcers
(recurrent). BMJ Clin Evid. 1303:2015.PubMed/NCBI
|
|
39
|
Scully C: Clinical practice. Aphthous
ulceration. N Engl J Med. 355:165–172. 2006.PubMed/NCBI View Article : Google Scholar
|