Introduction
When changing from the upright to the supine
position, an increase in preload leads to a higher stroke volume
(SV) according to the Frank-Starling law of the heart (1,2). For
non-invasive determination of hemodynamic parameters, numerous
techniques have been proposed including impedance cardiography
(ICG) and inert gas rebreathing (IGR). ICG has been demonstrated to
track changes in heart rate, diastolic blood pressure, total
peripheral resistance, stroke volume and cardiac output during tilt
table testing (3), while IGR is
considered a viable option due to its relatively high accuracy and
reproducibility (4-8).
In patients with heart failure, a missing compensation of
hemodynamic parameters may be expected. Ejection fraction (EF) is
an established surrogate parameter for the estimation of systolic
left ventricular function (LVF) in echocardiography.
Echocardiography may support non-invasive diagnosis of left
ventricular function by determining ventricle size, wall thickness
and ejection fraction, as well as identifying pericardial effusion
or a thrombus (9). Reduced systolic
left ventricular function is established in patients following
myocardial infarction, myocarditis or with congenital heart disease
(9). However, it is not possible to
adequately apply this method in patients with obesity or pronounced
emphysema (10).
Hemodynamic data including SV and cardiac output
(CO), however, may be more physiological measures. Furthermore, the
posture dependent measurement of these data may provide additional
information on LVF from pathophysiological considerations. The
present study aimed to evaluate the hemodynamic response to
postural stress using non-invasive IGR in patients with normal as
well as impaired LFV.
Materials and methods
Subjects
The total numbers of patients and patients with
normal EF enrolled was 91 patients undergoing IGR and CMR. Patients
were enrolled at the First Department of Medicine, University
Medical Center Mannheim (University of Heidelberg, Mannheim,
Germany) from August, 2006 to January, 2007. Inclusion criteria
were as follows: Arterial hypertension (36.3%), coronary heart
disease (19.8%) and cardiomyopathies (17.6%). The exclusion
criteria were inability to perform rebreathing manoeuvre,
claustrophobia and implanted foreign devices including a pacemaker
or cardioverter defibrillator. The criteria for valid IGR data were
complete mixing of the insoluble (indicated by a steady-state) and
reduction of the soluble test gas, measurement of respiratory rate
and absence of a relevant leak flow evaluated by oxygen consumption
curves. The study protocol was approved by the Medical Ethics
Commission II, Medical Faculty Mannheim of the University of
Heidelberg and conducted in accordance with the Declaration of
Helsinki. Written informed consent was obtained from all
patients.
Study protocol
Non-invasive hemodynamic measurements using IGR were
performed once, prior to or following cardiac magnetic resonance
imaging (CMR), in upright and supine position. An interval of 5 min
between the measurements was adhered to in order to guarantee
complete elimination of test gases according to previous
recommendations (11). Stabilization
of circulation was awaited and controlled by measurement of blood
pressure (BP) and heart rate (HR). Blood pressure and heart rate
were measured directly prior to the rebreathing maneuver and
implemented in the IGR system.
IGR
IGR is based on the Fick principle and has been
previously described in detail elsewhere (4). Nitrous oxide (N2O; 0.5%) as a
soluble gas and insoluble sulfur hexafluoride (SF6;
0.1%) were used as test gases. Concentrations were measured by an
online photo-magnetoacoustic gas analyzer (Innocor software version
5.01; Innovision ApS, Glamsbjerg, Denmark), and a valid shunt
correction was applied using the patient's individually measured
hemoglobin value; hemoglobin levels were measured during routine
laboratory testing within one week of IGR measurements.
CMR
Electrocardiogram-gated cine images were acquired
using a segmented steady-state free precession sequence (TrueFISP)
during repeated end-expiratory breath-holds on a 1.5 Tesla
whole-body imaging system (MAGNETOM Sonata; Siemens Healthineers,
Erlangen, Germany). Three long-axis views and 7 to 12 short-axis
views were obtained. Areas subtended by endocardial tracings were
determined in each end-diastolic and end-systolic slice. Total
end-diastolic and end-systolic cavity volumes (EDV and ESV,
respectively) were calculated using a modified Simpson's rule
equation (9) calculating EF as EF
(%)=[(EDV-ESV)/EDV] x100. EF was defined as normal (≥55%), mildly
abnormal (45-54%), moderately abnormal (30-44%) and severely
abnormal (<30%) according to European Association of
Echocardiography/American Society of Echocardiography
recommendations (9).
Statistical analysis
Data were presented as the mean ± standard
deviation. Statistical analyses were performed using
MedCalc® for Microsoft Windows®, version
12.3.0 (MedCalc Software bvba, Ostend, Belgium). Statistical
testing comprised Student's t-tests and one-way analysis of
variance with the post-hoc Student-Newman-Keuls test, and Pearson's
product-moment correlation coefficient, considering P<0.05 to
indicate statistical significance. All data was used for the
respective statistical tests.
Results
A total of 91 patients undergoing CMR were analyzed.
Information on their baseline characteristics is provided in
Table I. The most frequent
concomitant diseases were arterial hypertension (36.3%), coronary
heart disease (19.8%) and cardiomyopathies (17.6%), as listed in
Table II. All hemodynamic parameters
measured by IGR and CMR in upright and supine position are listed
in Table III.
 | Table IBaseline characteristics (n=91). |
Table I
Baseline characteristics (n=91).
| Parameter | Unit | Value | Range |
|---|
| Age | Years | 52±17 | 16-79 |
| Male gender | n (%) | 57 (62.6) | - |
| Weight | kg | 79±15 | 47-118 |
| Height | cm | 173±8 | 155-190 |
| cHb | g/dl | 14.0±1.6 | 9.8-17.2 |
 | Table IIConcomitant pathologies. |
Table II
Concomitant pathologies.
| Pathology | Total cases, n (%
total) |
|---|
| Arterial
hypertension | 33 (36.3) |
| Coronary heart
disease | 18 (19.8) |
| Cardiomyopathy,
thereof | 16 (17.6) |
|
-
Hypertrophic | 9 (9.9) |
|
-
Dilated | 6 (6.6) |
|
-
Restrictive | 1 (1.1) |
| Myocardial
hypertrophy | 13 (14.3) |
| Atrial
fibrillation | 11 (12.1) |
| Myocardial
infarction | 8 (8.8) |
| Pleural effusion | 8 (8.8) |
| Myocarditis | 7 (7.7) |
| Pericardial
effusion | 7 (7.7) |
| Obstructive lung
disease | 5 (5.5) |
| Brugada's
syndrome | 4 (4.4) |
| Takotsubo | 3 (3.3) |
 | Table IIIHemodynamic parameters. |
Table III
Hemodynamic parameters.
| | |
EF | |
|---|
| | | Overall (n=91) | Normal (n=42) | Mildly abnormal
(n=21) | Moderate abnormal
(n=16) | Severely abnormal
(n=12) | |
|---|
| Parameter | Unit | Value | Range | Value | Range | Value | Range | Value | Range | Value | Range | P-value |
|---|
| COCMR | l/min | 5.2±1.4 | 2.7-9.0 | 5.2±1.4 | 2.7-8.8 | 5.9±1.5 | 3.4-9.0 | 5.0±0.9 | 3.2-7.1 | 4.0±0.9 | 2.8-5.7 | 0.002 |
| SVCMR | ml | 79±22 | 32-147 | 84±21 | 45-147 | 82±23 | 41-127 | 76±18 | 35-113 | 62±23 | 32-113 | 0.02 |
| HRCMR | bpm | 67±13 | 36-109 | 63±9 | 36-76 | 75±16 | 42-109 | 68±12 | 52-91 | 69±16 | 45-96 | 0.004 |
| EF | % | 50±14 | 10-74 | 62±4 | 55-74 | 51±3 | 46-54 | 39±5 | 31-45 | 23±6 | 10-30 | <0.01 |
|
LVEDVCMR | ml | 169±62 | 73-336 | 136±35 | 73-239 | 159±46 | 85-267 | 197±50 | 101-278 | 269±58 | 152-336 | <0.01 |
| Upright |
|
COIGR | l/min | 4.4±1.3 | 1.2-8.8 | 4.3±1.3 | 1.4-6.6 | 4.9±1.3 | 3.1-8.0 | 4.1±1.3 | 1.2-7.2 | 3.9±1.0 | 2.5-5.7 | 0.09 |
|
SVIGR | ml | 60±19 | 18-122 | 60±17 | 18-97 | 63±17 | 36-101 | 57±18 | 25-85 | 59±29 | 33-122 | 0.84 |
|
HRIGR | bpm | 74±14 | 44-108 | 72±12 | 49-93 | 80±16 | 44-108 | 73±15 | 47-98 | 72±18 | 48-103 | 0.20 |
|
BPsystolic | mmHg | 129±15 | 91-167 | 126±13 | 97-147 | 127±12 | 102-148 | 135±21 | 91-165 | 132±18 | 102-167 | 0.22 |
|
BPdiastolic | mmHg | 78±11 | 52-111 | 76±10 | 52-96 | 78±8 | 58-91 | 81±12 | 56-101 | 82±14 | 58-111 | 0.44 |
| Supine |
|
COIGR | l/min | 5.0±1.2 | 2.2-7.6 | 5.1±1.3 | 2.8-7.6 | 5.5±1.0 | 3.8-7.3 | 4.5±1.0 | 2.2-6.1 | 4.4±1.3 | 2.7-6.1 | 0.04 |
|
SVIGR | ml | 75±23 | 25-148 | 79±22 | 50-148 | 74±18 | 45-112 | 66±18 | 25-101 | 70±36 | 36-134 | 0.21 |
|
HRIGR | bpm | 69±14 | 39-105 | 66±11 | 39-96 | 76±14 | 52-105 | 71±15 | 47-103 | 71±18 | 45-102 | 0.05 |
|
BPsystolic | mmHg | 127±16 | 96-161 | 125±17 | 96-160 | 123±10 | 108-150 | 133±18 | 102-159 | 132±15 | 104-161 | 0.15 |
|
BPdiastolic | mmHg | 75±11 | 41-111 | 72±11 | 42-100 | 74±8 | 54-86 | 78±10 | 49-96 | 80±15 | 54-111 | 0.08 |
IGR
Mean CO and SV measured by IGR were 4.4±1.3 l/min
and 60±19 ml in the upright position, which both significantly
increased to 5.0±1.2 l/min and 75±23 ml in the supine position,
respectively (P<0.01). Conversely, HR decreased significantly
from 74 to 69 bpm (P<0.01).
CMR
As determined by CMR, EF was normal (EF ≥55%) in 42
patients. In 21 patients it was mildly abnormal (45-54%), in 16
moderately abnormal (30-44%) and in 12 severely abnormal (<30%)
according to European Association of Echocardiography/American
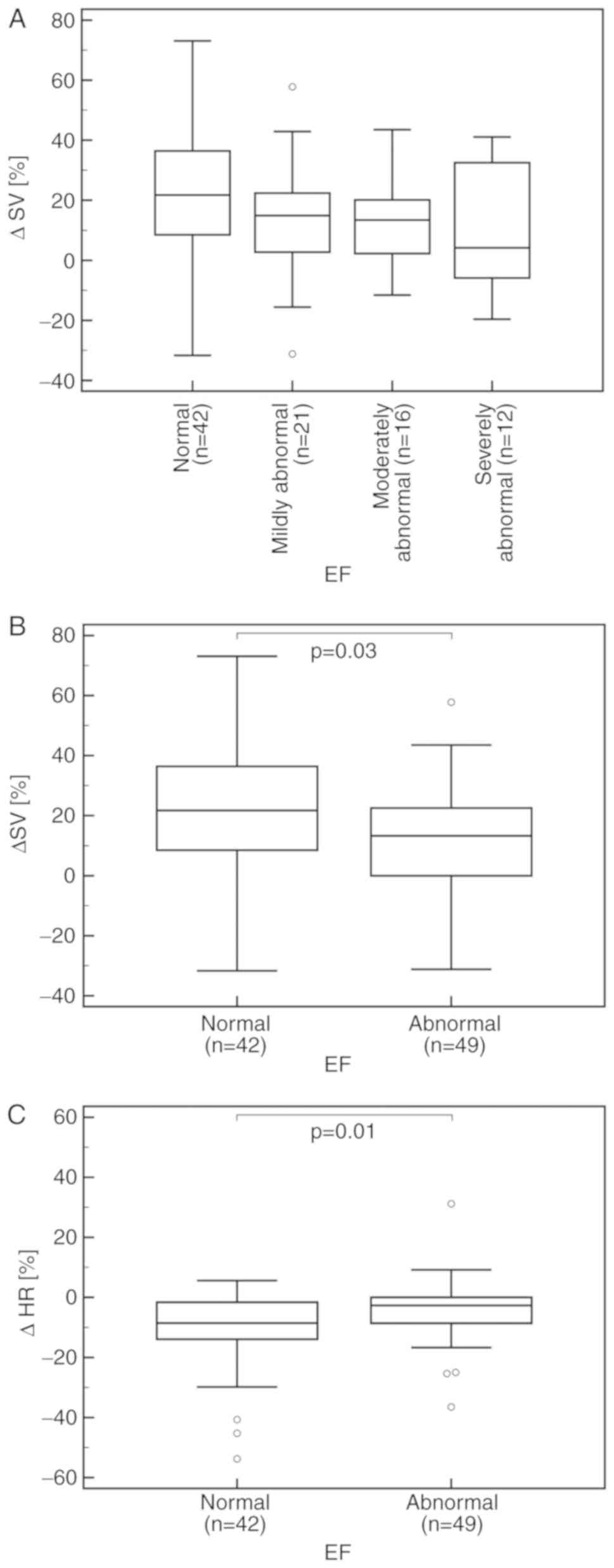
Society of Echocardiography recommendations (9). An overall trend for a lower percentage
change in SV (%ΔSV) was identified between the four EF classes in
the order of normal to severely abnormal values, respectively,
though this was deemed to be non-significant (P=0.17; Fig. 1A). When comparing patients with
abnormal EF values to those with normal values, there was a mild
yet significantly lower %ΔSV (P=0.03; Fig. 1B) and %ΔHR, of 13 and 4% vs. 22 and
11%, respectively (P=0.01; Fig. 1C).
No significant difference was identified in CO between the four EF
groups (P=0.69) nor between patients with normal and abnormal EF,
respectively (P=0.20; data not shown). There was a significant
negative pearson's product-moment correlation coefficient between
%ΔSV and %ΔHR (r=-0.48, P<0.01). By contrast, no association
between %ΔCO and %ΔHR was identified (r=-0.07, P=0.49; data not
shown).
Discussion
Postural changes of cardiac function may be tracked
non-invasively using IGR. The present study demonstrated an
increase in both CO and SV when changing from upright to supine
position as expected according to the Frank-Starling law of the
heart (1,2). In accordance with the previous findings
of Stefadouros et al (12),
this study identified a reduced ability to adapt stroke volume in
patients with systolic heart failure. This was indicated by a lower
percentage change in SV between EF classes, and a significant
difference between patients with abnormal and normal EF values. In
heart failure with preserved left ventricular ejection fraction
(HFpEF), a decrease in SV and CO has been previously reported,
which was associated with a reduced left ventricular distensibility
in response to postural change (13).
Accordingly, growth differentiation factor 15, as a novel marker of
HFpEF, was also associated with a reduced cardiac output response
in an orthostatic test (14).
Apart from IGR, numerous non-invasive techniques for
the determination of hemodynamic parameters including IGR have been
proposed in previous years. Hamm et al (15) examined IGR in patients with aortic
valve stenosis, and also Saur et al (16) in patients with lung diseases. While
IGR has been demonstrated to be relatively accurate (4), techniques not requiring active
collaboration including impedance cardiography (ICG), pulse contour
analysis and continuous wave Doppler have exhibited greater
reproducibility (17-19). This may be advantageous in serial
measurements. Shortly following its introduction in 1966, ICG was
used to track hemodynamic changes during tilt table testing
(3). Uncalibrated non-invasive
pulse-contour analyses were able to recognize CO changes induced by
fluid challenge and passive leg raise test (20). However, changes in thoracic water
content, arrhythmias and movement artifacts were demonstrated to
alter the measurement accuracy of ICG (21-23), as was hemoglobin
based pulmonary shunt flow correction (24). Further research is required to
identity the optimal non-invasive technique; with IGR representing
a promising technology.
Although the present cohort included a sufficient
number of patients, there are limitations to be considered. First,
there was no standardization of orthostatic testing of only single
measurements. Second, the assertions are restricted to systolic
heart failure while HFpEF may be of special interest due to a
reduced ventricular distensibility, this should be studied further
due to unique/different cardiac characteristics. Nevertheless,
non-invasive measurement of cardiac function during postural
changes using IGR is feasible, easy to perform and associated with
low costs. The maneuver may also be performed by trained nursing
staff and medical technical assistants. It therefore may be useful
in the evaluation of patients presenting with syncope, and during
the treatment of heart failure and arterial hypertension.
In conclusion, previous study our group demonstrated
that IGR measurements were easy to perform and exhibited agreement
with CMR (4). In the present study it
was demonstrated that use of IGR to estimate hemodynamic response
to postural changes may be feasible. Using IGR, a significant
difference in the %ΔSV was detected between patients with normal
and impaired EF. Several clinical scenarios affecting left
ventricular function as well as an evaluation of the ideal
non-invasive technique are worthy of further prospective
investigations.
Acknowledgements
Not applicable.
Funding
Not applicable.
Availability of data and materials
All data generated and/or analyzed during this study
are included in this published article.
Authors' contributions
KS and FT were responsible for study design and
writing the manuscript draft. TP and JDM were responsible for data
collection. CD was conducted data analysis. FT and JB were
responsible for the statistical analyses. JS, IA and MB critically
revised the manuscript for important intellectual content.
Ethics approval and consent to
participate
The study protocol was approved by the Medical
Ethics Commission II, Medical Faculty Mannheim of the University of
Heidelberg (Mannheim, Germany) and conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Frank O: Zur Dynamik des Herzmuskels. Z
Biol (Munich). 32:370–437. 1895.
|
|
2
|
Starling E: The linacre lecture on the law
of the heart. London, UK: Longmans, Green and Co. 1918. View Article : Google Scholar
|
|
3
|
Smith JJ, Bush JE, Wiedmeier VT and
Tristani FE: Application of impedance cardiography to study of
postural stress. J Appl Physiol. 29:133–137. 1970. View Article : Google Scholar
|
|
4
|
Saur J, Fluechter S, Trinkmann F,
Papavassiliu T, Schoenberg S, Weissmann J, Haghi D, Borggrefe M and
Kaden JJ: Noninvasive determination of cardiac output by the
inert-gas-rebreathing method-comparison with cardiovascular
magnetic resonance imaging. Cardiology. 114:247–254.
2009.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Agostoni P, Cattadori G, Apostolo A,
Contini M, Palermo P, Marenzi G and Wasserman K: Noninvasive
measurement of cardiac output during exercise by inert gas
rebreathing technique: A new tool for heart failure evaluation. J
Am Coll Cardiol. 46:1779–1781. 2005.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Christensen P, Clemensen P, Andersen PK
and Henneberg SW: Thermodilution versus inert gas rebreathing for
estimation of effective pulmonary blood flow. Crit Care Med.
28:51–56. 2000.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dong L, Wang JA and Jiang CY: Validation
of the use of foreign gas rebreathing method for non-invasive
determination of cardiac output in heart disease patients. J
Zhejiang Univ Sci B. 6:1157–1162. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gabrielsen A, Videbaek R, Schou M,
Damgaard M, Kastrup J and Norsk P: Non-invasive measurement of
cardiac output in heart failure patients using a new foreign gas
rebreathing technique. Clin Sci (Lond). 102:247–252.
2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Lang RM, Badano LP, Mor-Avi V, Afilalo J,
Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA,
Kuznetsova T, et al: Recommendations for cardiac chamber
quantification by echocardiography in adults: An update from the
American Society of Echocardiography and the European Association
of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging.
16:233–270. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Erbel R, Brennecke R, Goerge G,
Mohr-Kahaly S, Wittlich N, Zotz R and Meyer J: Possibilities and
limits of 2-dimensional echocardiography in quantitative image
analysis. Z Kardiol. 78 (Suppl 7):S131–S142. 1989.(In German).
PubMed/NCBI
|
|
11
|
Damgaard M and Norsk P: Effects of
ventilation on cardiac output determined by inert gas rebreathing.
Clin Physiol Funct Imaging. 25:142–147. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Stefadouros MA, El Shahawy M, Stefadouros
F and Witham AC: The effect of upright tilt on the volume of the
failing human left ventricle. Am Heart J. 90:735–743.
1975.PubMed/NCBI View Article : Google Scholar
|
|
13
|
John JM, Haykowsky M, Brubaker P, Stewart
K and Kitzman DW: Decreased left ventricular distensibility in
response to postural change in older patients with heart failure
and preserved ejection fraction. Am J Physiol Heart Circ Physiol.
299:H883–H889. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dinh W, Füth R, Lankisch M, Hess G, Zdunek
D, Scheffold T, Kramer F, Klein RM, Barroso MC and Nickl W:
Growth-differentiation factor-15: A novel biomarker in patients
with diastolic dysfunction? Arq Bras Cardiol. 97:65–75. 2011.(In
English, Portuguese, Spanish). PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hamm K, Trinkmann F, Heggemann F,
Gruettner J, Schmid-Bindert G, Borggrefe M, Haghi D and Saur J:
Evaluation of aortic valve stenosis using a hybrid approach of
Doppler echocardiography and inert gas rebreathing. In Vivo.
26:1027–1033. 2012.PubMed/NCBI
|
|
16
|
Saur J, Trinkmann F, Doesch C, Scherhag A,
Brade J, Schoenberg SO, Borggrefe M, Kaden JJ and Papavassiliu T:
The impact of pulmonary disease on noninvasive measurement of
cardiac output by the inert gas rebreathing method. Lung.
188:433–440. 2010.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Saur J, Trinkmann F, Weissmann J,
Borggrefe M and Kaden JJ: Non-invasive determination of cardiac
output: Comparison of a novel CW Doppler ultrasonic technique and
inert gas rebreathing. Int J Cardiol. 136:248–250. 2009.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Trinkmann F, Berger M, Hoffmann U,
Borggrefe M, Kaden JJ and Saur J: A comparative evaluation of
electrical velocimetry and inert gas rebreathing for the
non-invasive assessment of cardiac output. Clin Res Cardiol.
100:935–943. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Trinkmann F, Sampels M, Doesch C,
Papavassiliu T, Brade J, Schmid-Bindert G, Hoffmann U, Borggrefe M,
Kaden JJ and Saur J: Is arterial pulse contour analysis using
nexfin a new option in the noninvasive measurement of cardiac
output?-A pilot study. J Cardiothorac Vasc Anesth. 27:283–287.
2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Bubenek-Turconi SI, Craciun M, Miclea I
and Perel A: Noninvasive continuous cardiac output by the Nexfin
before and after preload-modifying maneuvers: A comparison with
intermittent thermodilution cardiac output. Anesth Analg.
117:366–372. 2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Appel PL, Kram HB, Mackabee J, Fleming AW
and Shoemaker WC: Comparison of measurements of cardiac output by
bioimpedance and thermodilution in severely ill surgical patients.
Crit Care Med. 14:933–935. 1986.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Franko E, Van De Water J and Wang X: Ideal
measurement of cardiac output: Is impedance cardiography the
answer? Vasc Endovascular Surg. 25:550–558. 1991. View Article : Google Scholar
|
|
23
|
Saur J, Trinkmann F, Doesch C, Weissmann
J, Hamm K, Schoenberg SO, Borggrefe M, Haghi D and Kaden JJ:
Non-invasive measurement of cardiac output during atrial
fibrillation: Comparison between cardiac magnetic resonance imaging
and inert gas rebreathing. Cardiology. 115:212–216. 2010.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Trinkmann F, Papavassiliu T, Kraus F,
Leweling H, Schoenberg SO, Borggrefe M, Kaden JJ and Saur J: Inert
gas rebreathing: The effect of haemoglobin based pulmonary shunt
flow correction on the accuracy of cardiac output measurements in
clinical practice. Clin Physiol Funct Imaging. 29:255–262.
2009.PubMed/NCBI View Article : Google Scholar
|