Introduction
Pancreatic cancer is a globally lethal human
disease, with an overall 5-year survival rate of <4% (1), and a median survival period of 4–6
months (2,3). It is considered the fourth leading
cause of cancer mortality in males and females (4). The nucleoside analog of cytidine
5-fluorouracil (5-Fu) is widely used in the treatment of advanced
gastrointestinal cancer, including pancreatic cancer (5–7).
However, only few patients benefit from 5-Fu-based
chemotherapy. Intrinsic or acquired resistance to chemotherapy is a
leading cause of treatment failure and short survival time
(8,9). The reasons for the insensitivity of
pancreatic cancer cells to chemotherapy and the molecular
mechanisms that enable pancreatic cancer cells to escape the
cytotoxic effects have yet to be determined (10–12).
Abnormal biochemical characteristics associated with
pancreatic cancer cells include the increased utilization of
glucose (13). Increased
proliferation depends on abnormal glucose metabolism for the
generation of ATP as a main source of energy supply as most cancer
cells lack oxidative phosphorylation. This phenomenon is known as
the Warburg effect (14–18). This metabolic alteration is
frequently observed in cancer cells of various tissue origins, thus
targeting the glycolytic pathway may preferentially kill the
malignant cancer cells but spare normal cells.
Previously, we demonstrated that PI3K-Akt activated
by NGF-TrkA signaling was involved in the resistance to
chemotherapy (19). Akt may be
considered as the ‘Warberg gene’ (20), which is closely associated with
tumor glycolysis and glucose utilization. Since pancreatic cancer
cells demonstrate increased utilization of glucose, it is crucial
to target glycolysis metabolic pathway for the treatment of
pancreatic cancer.
To examine whether high glucose plays a role in the
resistance to 5-Fu and whether the inhibition of glycolysis using
glycolysis inhibitor 2-deoxy-D-glucose (2-DG) results in enhanced
sensitivity to 5-Fu, we investigated cell viability by 5-Fu
treatment on different concentrations of glucose in AsPC-1 and
Panc-1 pancreatic cancer cells. Additionally, we investigated
whether 2-DG is able to reverse high glucose-induced 5-Fu
resistance via PI3K-Akt signaling.
Materials and methods
Reagents
5-Fu, dimethyl sulfoxide (DMSO), 2-DG, glucose and
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
were purchased from Sigma-Aldrich (St. Louis, MO, USA). RIPA buffer
and PMSF were purchased from Beyotime (Haimen, Jiangsu, China).
Anti-p-Akt and PI3K inhibitor LY294002 were purchased from Abcam
(Cambridge, MA, USA). Anti-β-actin antibody was from Abnova
(Taiwan, China).
Cell culture
Human pancreatic cancer AsPC-1 and Panc-1 cells were
purchased from the Type Culture Collection of the Chinese Academy
of Sciences (Shanghai, China). The cells were grown in RPMI-1640
medium (Gibco, Carlsbad, CA, USA) supplemented with 10%
heat-inactivated FBS (Hyclone, Logan, UT, USA), penicillin 100 U/ml
and streptomycin 100 μg/ml (Gibco). The cultures were maintained at
37ºC in a 5% CO2 incubator.
Cell growth inhibition assay
Cell viability was assessed via an MTT assay. ASPC-1
and Panc-11 cells were seeded (3,000/well) in 96-well plates
for 24 h. Media containing 5-Fu, 2-DG, LY294002 or control medium
were added and incubated for the indicated times at 37ºC. MTT (0.5
mg/ml in PBS) was added to each well and incubated for 4 h
at 37ºC. The media were then discarded and 100 μl DMSO was added.
Following agitation for 10 min on an eppendorf shaker, absorbance
was read at 550 nm on a scanning microtiter. Data were expressed
relative to the untreated group, which was set as 100%.
Western blot analysis
Cells were lysed with modified RIPA buffer (50 mM
Tris, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 0.1%
SDS) containing 25 μg/ml leupeptin, 1 mM sodium orthovanadate, 2 mM
EDTA, and 1 mM PMSF. The protein concentration was determined using
a BCA method (Beyotime). Twenty micrograms of proteins per sample
were loaded onto 8% SDS-polyacrylamide gel, electrophoresed, and
blotted onto PVDF membrane. Proteins were probed with the primary
antibody overnight at 4ºC and secondary antibody at room
temperature for 1 h. Immunoreactivity was detected by the ECL
system (Xi’an Jiaotong University, China) and normalized to
β-actin.
Statistical analysis
Data were analyzed by SPSS 13.0 using t-test.
P<0.05 was considered statistically significant.
Results
High-glucose microenvironments alleviated
5-Fu-induced cell growth inhibition
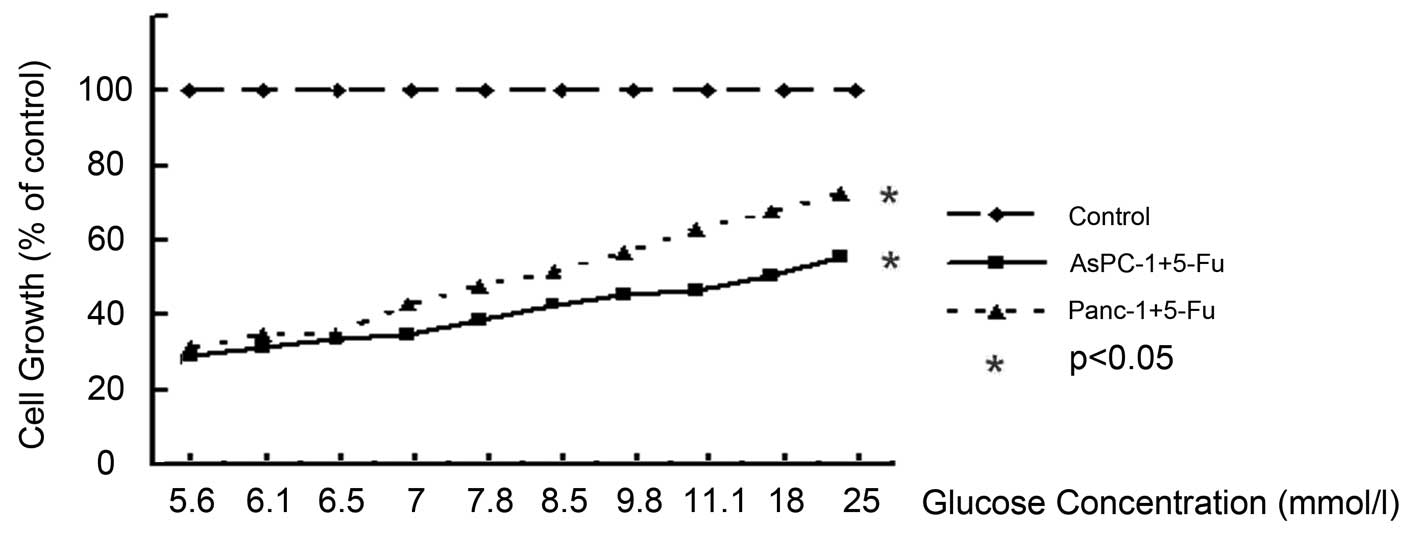
To investigate the influence of glucose levels on
resist to 5-Fu, cells were incubated in a series of gradually
increasing glucose concentrations for 72 h with 1 mM of 5-Fu.
Increased cell growth of AsPC-1 and Panc-1 cells
treated with 5-Fu was observed in response to the glucose
concentrations ranging from 5.6 to 25 mM. In AsPC-1 and Panc-1
cells, the cell viability rate was increased in a dose-dependent
manner at glucose concentrations of 5.6 mM (as a control) to 25 mM,
at 72 h, respectively (P<0.05) (Fig.
1). In comparison with parental ASPC-1 and Panc-1, incubation
with 5-Fu for 72 h at the glucose concentration of 5.6 mM decreased
the cell number to 28 and 31%, respectively (P<0.05).
High-glucose microenvironments showed a marked effect on the growth
of AsPC-1 and Panc-1 cells. At the glucose concentration of 25 mM,
5-Fu decreased the cell number to 55 and 72%, respectively
(P<0.05). The cytotoxic effect of 5-Fu reduced glucose in a
concentration-dependent manner.
2-DG enhanced cytotoxic effects of 5-Fu
in high-glucose microenvironments
Several studies demonstrated that 2-DG induces cell
growth inhibition and death in pancreatic cancer cells by
interfering with glucose metabolism. We hypothesized that the
enhanced resistance to 5-Fu in glucose microenvironments may be
blocked by the anti-glucose metabolism treatment of 2-DG. To test
this, 2-DG (10 mM) was used to interfere with cell glucose
metabolism and detect the sensitivity of the two cell responses to
5-Fu treatment at the glucose concentration of 25 mM. Growth of
AsPC-1 and Panc-1 was inhibited by incubation with 5-Fu or 2-DG
alone in a time-dependent manner (P<0.05). Treatment of tumor
cells with 0.5 mM 5-Fu combined with 5 mM 2-DG revealed a marked
decreased in cell growth compared with 5-Fu or 2-DG alone
(P<0.05), leading to a decrease of 54% in AsPC-1 and 52% in
Panc-1 at 72 h of incubation (Fig.
2).
2-DG enhanced 5-Fu cytotoxic effect
depending on PI3K signaling
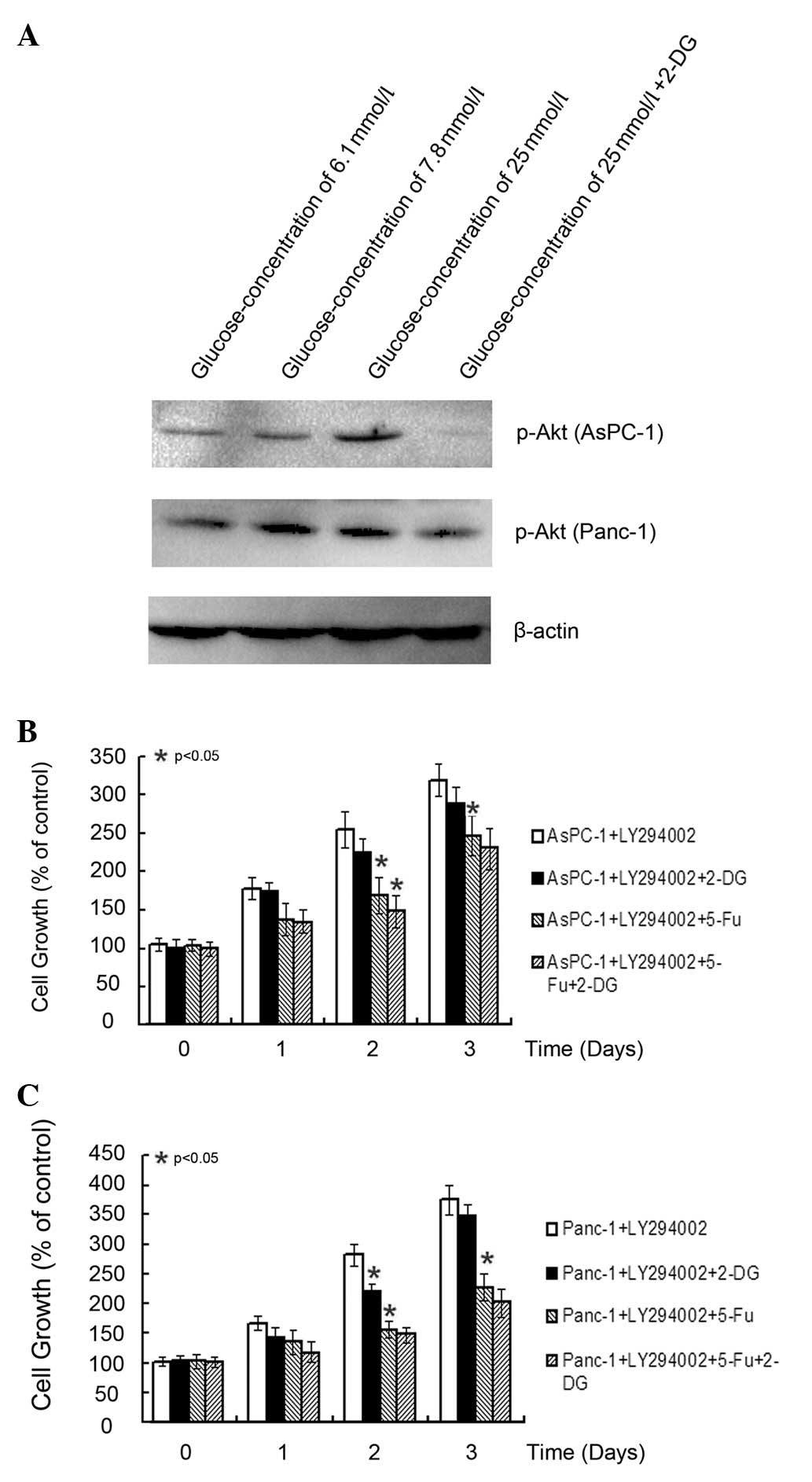
Treatment of AsPC-1 and Panc-1 cells with 10 mM 2-DG
resulted in ~38 and 41% inhibition of cell growth, respectively, in
a time-dependent manner. Three concentrations (6.1, 7.8 and 25 mM)
of glucose were selected to detect the expression of Akt, also
known as the Wurberg gene and to determine whether it can be
inhibited by 2-DG (Fig. 3A). As
cells incubated with higher concentrations of glucose exhibited
increased activity of p-Akt, we examined whether the upregulation
of Akt by glucose is important in 2-DG-induced cell growth.
Consequently, we treated cells with LY294002 and found that
LY294002 significantly attenuated the death of 2-DG-induced cells
(Fig. 3B and C). The combination of
5-Fu and 2-DG did not exhibit any significance compared with 5-Fu
alone. Although 2-DG reduced the expression of Akt, blocking
PI3K/Akt signaling using LY294002 did not enhance the inhibition of
5-Fu.
Discussion
2-DG is a glucose analog and acts as a competitive
inhibitor of glucose metabolism, causing a depletion of cellular
ATP (21), leading to blockage of
cell cycle progression (22), and
inducing cell death (23). In
addition, 2-DG inhibits protein glycosylation, and induces the
accumulation of misfolded proteins in the endoplasmic reticulum,
leading to endoplasmic reticulum stress response and constant cell
apoptosis (24). 2-DG has been
proven to be an anti-cancer drug for a variety of cancer cells and
increases the efficacy of radiotherapy and chemotherapy agents such
as adriamycin and paclitaxel (25,26).
In the present study, we used glucose as a model to
enhance cell resistance to the widely used anti-pancreatic cancer
agent 5-Fu in AsPC-1 and Panc-1 pancreatic cancer cells. The
results demonstrated that active PI3K-Akt by high-glucose
concentrations decreased the antitumor effect of 5-Fu, which is in
agreement with previous studies (19,27–31).
Of note, 2-DG significantly reversed the resistance to 5-Fu in 25
mM of glucose. Our data demonstrated that PI3K-Akt is required for
2-DG-induced cell growth inhibition. Similarly, enhanced
proliferation due to NGF-TrkA signaling or loss of PTEN makes cells
more sensitive to 2-DG (32,33).
Our results emphasize that an increase in blood
sugar as a result of diabetes, which is closely associated with
pancreatic cancer may increase the risk of cancer proliferation and
resistance to chemotherapy (34–37).
Controlling blood sugar levels is significant in the therapy of
pancreatic cancer. However, this resistance can be alleviated by
glycolysis inhibition using 2-DG. Our results suggest that
targeting glucose glycolysis is a viable approach for the
development of anti-cancer drugs, particularly for patients
experiencing difficulty in reducing blood sugar levels.
Activation of the PI3K/AKT pathway has been
implicated in a variety of tumors (38,39),
mediating tumor growth, and exhibiting resistance to apoptosis and
chemotherapy. PI3K/AKT pathway inhibition leads to a wide
spectrum of direct effects including cell cycle arrest, induction
of autophagy, sensitization to chemotherapeutics, inhibition of
metastasis as well as cell differentiation and death (40,41).
Results of the present study have shown that glucose and 2-DG were
involved in the regulation of PI3K/Akt signaling, and this may
explain the effect of aberrant glucose metabolism in pancreatic
cancer, and highlight the marked efficiency of 2-DG in high-glucose
microenvironments and the significance of controlling the blood
sugar of pancreatic cancer patients, particularly those with
diabetes. However, blocking PI3K/Akt did not provide an adequate
explanation for the entire mechanism of 2-DG, or the effect of 5-Fu
being greatly enhanced by using LY294002. Thus additional studies
should be conducted to further elucidate the mechanism
involved.
In conclusion, the results of the present study
demonstrated that the effect of 5-Fu-based chemotherapy on
pancreatic cancer is significantly reduced by high glucose,
although this effect can be reversed by 2-DG. It is therefore
crucial for pancreatic cancer patients to control blood sugar
levels in order to fully benefit from chemotherapy.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China, NSFC (grant no. 30973489).
References
|
1
|
Vincent A, Herman J, Schulick R, Hruban RH
and Goggins M: Pancreatic cancer. Lancet. 378:607–620. 2011.
View Article : Google Scholar
|
|
2
|
Dubecz A, Kohler J and Stein HJ:
Cholecystectomy in a trial of adjuvant chemotherapy after
pancreatic cancer resection. JAMA. 304:2590author reply 2590–2591.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torpy JM, Burke AE and Glass RM: JAMA
patient page. Pancreatic cancer. JAMA. 304:11402010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gu WJ and Liu HL: Induction of pancreatic
cancer cell apoptosis, invasion, migration, and enhancement of
chemotherapy sensitivity of gemcitabine, 5-FU, and oxaliplatin by
hnRNP A2/B1 siRNA. Anticancer Drugs. 24:566–576. 2013.PubMed/NCBI
|
|
6
|
Bhattacharyya M, Francis J, Eddouadi A,
Lemoine NR and Hallden G: An oncolytic adenovirus defective in
pRb-binding (dl922–947) can efficiently eliminate pancreatic cancer
cells and tumors in vivo in combination with 5-FU or gemcitabine.
Cancer Gene Ther. 18:734–743. 2011.
|
|
7
|
Oberic L, Viret F, Baey C, Ychou M,
Bennouna J, Adenis A, Peiffert D, Mornex F, Pignon JP, Celier P,
Berille J and Ducreux M: Docetaxel- and 5-FU-concurrent
radiotherapy in patients presenting unresectable locally advanced
pancreatic cancer: a FNCLCC-ACCORD/0201 randomized phase II trial’s
pre-planned analysis and case report of a 5.5-year disease-free
survival. Radiat Oncol. 6:1242011.PubMed/NCBI
|
|
8
|
Ischenko I, Seeliger H, Jauch KW and Bruns
CJ: Metastatic activity and chemotherapy resistance in human
pancreatic cancer - influence of cancer stem cells. Surgery.
146:430–434. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang C, Kolb A, Buchler P, Cato AC,
Mattern J, Rittgen W, Edler L, Debatin KM, Buchler MW, Friess H and
Herr I: Corticosteroid co-treatment induces resistance to
chemotherapy in surgical resections, xenografts and established
cell lines of pancreatic cancer. BMC Cancer. 6:612006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu QH, Zhang J, Zhao CY, Yu DH, Bu HJ,
Chen Y, Ni CY and Zhu MH: Surviving cells after treatment with
gemcitabine or 5-fluorouracil for the study of de novo resistance
of pancreatic cancer. Cancer Lett. 314:119–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tsujie M, Nakamori S, Nakahira S,
Takahashi Y, Hayashi N, Okami J, Nagano H, Dono K, Umeshita K,
Sakon M and Monden M: Human equilibrative nucleoside transporter 1,
as a predictor of 5-fluorouracil resistance in human pancreatic
cancer. Anticancer Res. 27:2241–2249. 2007.PubMed/NCBI
|
|
12
|
Shi X, Liu S, Kleeff J, Friess H and
Buchler MW: Acquired resistance of pancreatic cancer cells towards
5-fluorouracil and gemcitabine is associated with altered
expression of apoptosis-regulating genes. Oncology. 62:354–362.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coleman MC, Asbury CR, Daniels D, Du J,
Aykin-Burns N, Smith BJ, Li L, Spitz DR and Cullen JJ:
2-deoxy-D-glucose causes cytotoxicity, oxidative stress, and
radiosensitization in pancreatic cancer. Free Radic Biol Med.
44:322–331. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nam SO, Yotsumoto F, Miyata K, Shirasu N,
Miyamoto S and Kuroki M: Possible therapeutic targets among the
molecules involved in the Warburg effect in tumor cells. Anticancer
Res. 33:2855–2860. 2013.PubMed/NCBI
|
|
15
|
Dang CV: Rethinking the Warburg effect
with Myc micromanaging glutamine metabolism. Cancer Res.
70:859–862. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kim JW and Dang CV: Cancer’s molecular
sweet tooth and the Warburg effect. Cancer Res. 66:8927–8930.
2006.
|
|
17
|
Samudio I, Fiegl M and Andreeff M:
Mitochondrial uncoupling and the Warburg effect: molecular basis
for the reprogramming of cancer cell metabolism. Cancer Res.
69:2163–2166. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Samudio I, Fiegl M, McQueen T, Clise-Dwyer
K and Andreeff M: The Warburg effect in leukemia-stroma cocultures
is mediated by mitochondrial uncoupling associated with uncoupling
protein 2 activation. Cancer Res. 68:5198–5205. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu D, Zhang Y, Dang C, Ma Q, Lee W and
Chen W: siRNA directed against TrkA sensitizes human pancreatic
cancer cells to apoptosis induced by gemcitabine through an
inactivation of PI3K/Akt-dependent pathway. Oncol Rep. 18:673–677.
2007.PubMed/NCBI
|
|
20
|
Robey RB and Hay N: Is Akt the ‘Warburg
kinase’?-Akt-energy metabolism interactions and oncogenesis. Semin
Cancer Biol. 19:25–31. 2009.
|
|
21
|
Aft RL, Zhang FW and Gius D: Evaluation of
2-deoxy-D-glucose as a chemotherapeutic agent: mechanism of cell
death. Br J Cancer. 87:805–812. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Pelicano H, Martin DS, Xu RH and Huang P:
Glycolysis inhibition for anticancer treatment. Oncogene.
25:4633–4646. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ben Sahra I, Laurent K, Giuliano S,
Larbret F, Ponzio G, Gounon P, Le Marchand-Brustel Y,
Giorgetti-Peraldi S, Cormont M, Bertolotto C, Deckert M, Auberger
P, Tanti JF and Bost F: Targeting cancer cell metabolism: the
combination of metformin and 2-deoxyglucose induces p53-dependent
apoptosis in prostate cancer cells. Cancer Res. 70:2465–2475.
2010.
|
|
24
|
Kurtoglu M, Gao N, Shang J, Maher JC,
Lehrman MA, Wangpaichitr M, Savaraj N, Lane AN and Lampidis TJ:
Under normoxia, 2-deoxy-D-glucose elicits cell death in select
tumor types not by inhibition of glycolysis but by interfering with
N-linked glycosylation. Mol Cancer Ther. 6:3049–3058. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Aft RL, Lewis JS, Zhang F, Kim J and Welch
MJ: Enhancing targeted radiotherapy by copper(II)diacetyl-
bis(N4-methylthiosemicarbazone) using 2-deoxy-D-glucose. Cancer
Res. 63:5496–5504. 2003.PubMed/NCBI
|
|
26
|
Maschek G, Savaraj N, Priebe W,
Braunschweiger P, Hamilton K, Tidmarsh GF, De Young LR and Lampidis
TJ: 2-deoxy-D-glucose increases the efficacy of adriamycin and
paclitaxel in human osteosarcoma and non-small cell lung cancers in
vivo. Cancer Res. 64:31–34. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Y, Wang Z, Chang P, Xiang L, Pan F,
Li J, Jiang J, Zou L, Yang L, Bian Z and Liang H: The effect of
focal adhesion kinase gene silencing on 5-fluorouracil
chemosensitivity involves an Akt/NF-kappaB signaling pathway in
colorectal carcinomas. Int J Cancer. 127:195–206. 2010. View Article : Google Scholar
|
|
28
|
You F, Aoki K, Ito Y and Nakashima S: AKT
plays a pivotal role in the acquisition of resistance to
5-fluorouracil in human squamous carcinoma cells. Mol Med Rep.
2:609–613. 2009.PubMed/NCBI
|
|
29
|
Upadhyay AK, Singh S, Chhipa RR,
Vijayakumar MV, Ajay AK and Bhat MK: Methyl-beta-cyclodextrin
enhances the susceptibility of human breast cancer cells to
carboplatin and 5-fluorouracil: involvement of Akt, NF-kappaB and
Bcl-2. Toxicol Appl Pharmacol. 216:177–185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kodach LL, Bos CL, Duran N, Peppelenbosch
MP, Ferreira CV and Hardwick JC: Violacein synergistically
increases 5-fluorouracil cytotoxicity, induces apoptosis and
inhibits Akt-mediated signal transduction in human colorectal
cancer cells. Carcinogenesis. 27:508–516. 2006. View Article : Google Scholar
|
|
31
|
Yang H, Ni L, Ma YQ and Song YG: PI3K
p85alpha gene silencing by RNA interference promotes
5-fluorouracil-induced apoptosis of colorectal cancer LoVo cells.
Nan Fang Yi Ke Da Xue Xue Bao. 30:1085–1088. 2010.(In Chinese).
|
|
32
|
Cheng Y, Diao DM, Zhang H, Song YC and
Dang CX: Proliferation enhanced by NGF-NTRK1 signaling makes
pancreatic cancer cells more sensitive to 2DG-induced apoptosis.
Int J Med Sci. 10:634–640. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Blouin MJ, Zhao Y, Zakikhani M, Algire C,
Piura E and Pollak M: Loss of function of PTEN alters the
relationship between glucose concentration and cell proliferation,
increases glycolysis, and sensitizes cells to 2-deoxyglucose.
Cancer Lett. 289:246–253. 2010. View Article : Google Scholar
|
|
34
|
Mizuno S, Nakai Y, Isayama H, Takahara N,
Miyabayashi K, Yamamoto K, Kawakubo K, Mohri D, Kogure H, Sasaki T,
Yamamoto N, Sasahira N, Hirano K, Tsujino T, Ijichi H, Tateishi K,
Tada M and Koike K: Diabetes is a useful diagnostic clue to improve
the prognosis of pancreatic cancer. Pancreatology. 13:285–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McAuliffe JC and Christein JD: Type 2
diabetes mellitus and pancreatic cancer. Surg Clin North Am.
93:619–627. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Aggarwal G, Kamada P and Chari ST:
Prevalence of diabetes mellitus in pancreatic cancer compared to
common cancers. Pancreas. 42:198–201. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Mizuno S, Nakai Y, Isayama H, Yanai A,
Takahara N, Miyabayashi K, Yamamoto K, Kawakubo K, Mohri D, Kogure
H, Sasaki T, Yamamoto N, Sasahira N, Hirano K, Tsujino T, Ijichi H,
Tateishi K, Akanuma M, Tada M and Koike K: Risk factors and early
signs of pancreatic cancer in diabetes: screening strategy based on
diabetes onset age. J Gastroenterol. 48:238–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Florena AM, Tripodo C, Guarnotta C, Ingrao
S, Porcasi R, Martorana A, Lo Bosco G, Cabibi D and Franco V:
Associations between Notch-2, Akt-1 and HER2/neu expression in
invasive human breast cancer: a tissue microarray immunophenotypic
analysis on 98 patients. Pathobiology. 74:317–322. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cen B, Mahajan S, Wang W and Kraft AS:
Elevation of receptor tyrosine kinases by small molecule akt
inhibitors in prostate cancer is mediated by Pim-1. Cancer Res.
73:3402–3411. 2013. View Article : Google Scholar
|
|
40
|
Floc’h N, Kinkade CW, Kobayashi T, Aytes
A, Lefebvre C, Mitrofanova A, Cardiff RD, Califano A, Shen MM and
Abate-Shen C: Dual targeting of the Akt/mTOR signaling pathway
inhibits castration-resistant prostate cancer in a genetically
engineered mouse model. Cancer Res. 72:4483–4493. 2012.PubMed/NCBI
|
|
41
|
Dansen TB and Burgering BM: Unravelling
the tumor-suppressive functions of FOXO proteins. Trends Cell Biol.
18:421–429. 2008. View Article : Google Scholar : PubMed/NCBI
|