Introduction
Influenza is an acute respiratory disease, with high
morbidity and mortality rates in humans and animals (1). The influenza A virus is prone to
antigenic drifting or antigenic conversion and reorganization of
the genome, which results in the emergence of novel subtypes and a
lack of immunity in the majority of the population (2). The Western medicine currently
available for the prevention and treatment of influenza A virus
infection causes severe side-effects and drug resistance due to the
wide range of applications (3,4). To
date, the vaccines against the latest influenza A virus have been
ineffective. Therefore, traditional Chinese medicine is beginning
to play an important role against influenza A virus infection,
which could be mainly regulated by the immune system (5).
Baicalin is a flavonoid extracted from the root of
Scutellaria baicalensis that demonstrates a variety of
biological activities (6–8). Baicalin has been found to be an
inhibitor of the reverse transcriptase of the human
immunodeficiency virus in vitro (9). Baicalin also acts as a potent
inhibitor of the hepatitis B virus by reducing DNA synthesis
(10). In addition, baicalin has
been reported to have protective effects and inhibit death in mice
infected with influenza A virus (11). Currently, there are no available
studies on the antiviral mechanisms of baicalin against the
influenza A virus.
The aim of the present study was to investigate the
effects of baicalin on the mRNA expression of toll-like receptor 7
(TLR7) and myeloid differentiation primary response gene 88
(MYD88), and the effects on the protein expression of
phosphorylated nuclear factor κB (NF-κB)-P65 and c-jun/activator
protein 1 (AP-1), together with the expression of interleukin
(IL)-1β, tumor necrosis factor (TNF)-α and IL-6, in the lungs of
mice infected with influenza A/FM1/1/47 (H1N1), in order to
determine the mechanisms underlying the antiviral effects of
baicalin and to provide evidence in vitro that may result in
the development of novel anti-influenza drugs.
Materials and methods
Mice and H1N1
Two batches of imprinting control region (ICR) mice
(n=216), weighing 13–15 g, were treated according to the ‘Guide for
the Care and Use of Laboratory Animals’ prepared by the National
Institutes of Health. The first and second batches consisted of 120
(qualification no. 0213824) and 96 mice (qualification no.
0218801), respectively, and were purchased from the Vital River
Experimental Center (Beijing, China). H1N1 was provided by the
China Academy of Traditional Chinese Medicine (Beijing, China) and
was transplanted into the allantoic cavity of 9-day-old embryonated
hen eggs 3 times in succession. This study was approved by the
Ethics Committee of the Beijing University of Chinese Medicine
(Beijing, China).
Drugs
Baicalin was provided by Professor Zhengyun Chu from
the Liaoning Traditional Chinese Medicine University (Shenyang,
China). Ribavirin particles were purchased from Sichuan Bali
Pharmaceutical Co., Ltd. (Chengdu, China).
Reagents
An M-MLV reverse transcription kit (Takara Bio,
Inc., Shiga, Japan), TaqE (Takara), dNTP (Takara), DNA marker
(Takara), SYBR-Green mix (Bio-Rad, Hercules, CA, USA), agarose
(Promega Corporation, Madison, WI, USA), diethylpyrocarbonate
(Sigma, St. Louis, MO, USA) and TRIzol (Invitrogen Life
Technologies, Carlsbad, CA, USA) were used in this study. Primers
were synthesized by Sangon Co., Ltd. (Shanghai, China) as follows:
GAPDH (201 bp) forward, 5′-CTCATGACCACAGTCCATGC-3′ and reverse,
5′-CACATTGGGGGTAGGAACAC-3′; TLR7 (117 bp) forward,
5′-ACGCTTTCTTTGCAACTGTG-3′ and reverse, 5′-TTTGTGTGCTCCTGGACCTA-3′;
and MYD88 (136 bp) forward, 5′-TGGTGGTTGTTTCTGACGAT-3′ and reverse,
5′-GGAAAGTCCTTCTTCATCGC-3′. NF-κB-P65 and p-c-jun rabbit monoclonal
primary antibodies were purchased from Bioworld Technology Co., Ltd
(Nanjing, China), while IgG goat polyclonal secondary antibody was
purchased from the Beijing Zhongshan Golden Bridge Biotechnology
Co., Ltd. (Beijing, China) and cell lysis buffer was bought from
Beyotime (Nanjing, China). The western blot analysis was conducted
at the Key Laboratory of Antiviru of the Ministry of Education.
Enzyme-linked immunosorbent assay (ELISA) kits, IL-1β, TNF-α and
IL-6 were obtained from Bender MedSystems (Vienna, Austria).
Effects of baicalin on mouse
survival
The ICR mice (n=120) were randomly divided into 6
groups, as presented in Table I.
The mice were lightly anesthetized by the inhalation of diethyl
ether and intranasally infected with 10X LD50 of H1N1, except the
normal group who received sodium chloride. The mice were treated
with baicalin at various doses (93.75, 187.5 and 375 mg/kg/day);
the ribavirin group was used as a positive control and received
ribavirin (100 mg/kg/day), and the placebo and normal groups were
treated with sodium chloride (200 μl). All the mice underwent oral
gavage once daily for 7 days at the beginning of the study, 24 h
post-virus inoculation. Each group was observed for 14 days and the
number of deaths were recorded.
 | Table IProtective effects of baicalin on mice
with influenza A infection (n=120). |
Table I
Protective effects of baicalin on mice
with influenza A infection (n=120).
| | | | Lung parameters |
|---|
| | | |
|
|---|
| Group | Dose, mg/kg/day | Survival rate, % | MDD | Score | Lung index | Lung index
inhibition, % |
|---|
| Normal | - | 100.00 | 14.00±0.00 | 0.0±0.00 | 0.79±0.14 | - |
| Placebo | - | 5.00 | 7.25±2.82 | 3.2±0.71 | 1.42±0.31a | - |
| Ribavirin | 100.00 | 100.00b | 14.00±0.00b | 1.2±0.53c | 0.89±0.13c | 37.3 |
| Baicalin | 93.75 | 55.00b | 10.40±2.96d | 2.5±0.65d | 1.21±0.22d | 14.8 |
| 187.50 | 85.00b | 13.05±3.08c | 1.7±0.45c | 0.96±0.21c | 32.4 |
| 375.00 | 95.00b | 13.70±3.31b | 1.4±0.39c | 0.93±0.18c | 34.5 |
Effects of baicalin on mouse lung
parameter
The second batch of 96 ICR mice were randomly
divided into 6 groups and treated as aforementioned. After 5 days
of treatment, the mice were weighed and sacrificed by orbital
blood, and the lungs were removed and weighed. The lung index and
lung index inhibition were calculated as follows (12): Lung index = A / B × 100; and lung
index inhibition = (C - D) / C × 100, where A is the lung weight, B
is the body weight, C is the lung index of the placebo group and D
is the lung index of the drug-treated group. Four lungs from each
group were fixed in 10% formalin solution and then embedded in
paraffin for histological examination. The remaining lungs were cut
in half; one half was homogenized for ELISA and the other half was
stored in liquid nitrogen for protein and total RNA extraction.
mRNA assay of TLR7 and MYD88 by
fluorescence quantitative polymerase chain reaction (qPCR)
Total RNA of the lung tissue was extracted by TRIzol
reagent. The reverse transcription system (25 μl) was as follows: 3
μl RNA, 1 μl oligo(dT), 5 μl 5X buffer, 5 μl dNTP (10 mmol/l), 0.5
μl RNase inhibitor, 1 μl M-MLV and 9.5 μl ddH2O. The
mixture was incubated at 42°C for 60 min and then at 70°C for 10
min. The fluorescence qPCR system (20 μl) was as follows: 1.5 μl
cDNA, 0.5 μl primers F (10 pmol/μl), 0.5 μl primers R (10 pmol/μl),
10 μl SYBR-Green mix and 7.5 μl ddH2O. qPCR was
performed as follows: Predenaturation at 94°C for 15 min,
denaturation at 95°C for 15 sec, annealing at 62°C for 30 sec,
extension at 72°C for 15 sec for a total of 40 cycles and then
extension at 72°C for 10 min. The PCR products were assessed by
electrophoresis in 1.2% agarose gel, and the integral optical
density (IOD) value of GAPDH and the target bands were observed by
the gel imaging analysis system (Beijing Binta Instrument
Technology Co., Ltd, Beijing, China), with ethidium bromide
staining. The ratio of the IOD value of the target bands to the IOD
value of GAPDH was calculated as the relative expression of the
target gene.
Western blot analysis of NF-κB-P65 and
c-jun/AP-1
Lung tissue was ground into a powder in liquid
nitrogen, and protein was extracted using lysis buffer according to
the manufacturer’s instructions. The protein was quantified by the
Bradford protein assay. A total of 40 μg protein sample dissolved
in 10 μl phosphate-buffered saline was added to an equal volume of
1X sample buffer. Subsequent to being boiled for 5 min, the
proteins were separated by SDS-PAGE electrophoresis and transferred
to polyvinylidene fluoride membranes using a semi-electric switch
membrane machine at 30 mA for 90 min. The membranes were blocked by
anti-NF-κB-P65 and anti-p-c-jun antibodies at 4°C overnight and
then washed 3 times using Tris-buffered saline and Tween 20 buffer
for 10 min each. Horseradish peroxidase-conjugated secondary
antibodies were then added and oscillated at 37°C for 60 min.
Electrochemiluminescence liquid was added and the samples were
exposed to X-ray film after developing and fixing. The results were
scanned for use after the observation.
ELISA of TNF-α, IL-1β and IL-6
Lung tissue homogenates were prepared and ELISA was
conducted according to the manufacturer’s instructions.
Statistical analysis
The NF-κB-P65, c-jun/AP-1 and β-actin gray values
were measured by Image-Pro Plus 6.0 software (Media Cybernetics,
Inc., Rockville, MD, USA), and the ratio of the gray value was
associated with the protein expression level. The assay data was
analyzed by SPSS software, version 13.0 (SPSS Inc., Chicago, IL,
USA). The group data are presented as the mean ± standard error of
the mean. P≤0.05 was considered to indicate a statistically
significant difference.
Results
Protective effects of baicalin on
mice
Baicalin displayed a protective effect on mice with
influenza A infection (Table I).
All the doses of baicalin (93.75, 187.5 and 375 mg/kg/day)
significantly prolonged the survival time of the mice. A survival
rate of 55% was obtained at the lowest dose of 93.75 mg/kg/day.
Compared with the other 2 doses, baicalin at a dose of 375
mg/kg/day exerted the best effects with a survival rate of 95% and
a mean time to death (MDD) of 13.70±3.31 days. The lung parameter
data showed that baicalin provided dose-dependent protective
effects against viral pneumonia (Table
I). Inhibition of the lung index was detected at 14.8, 32.4 and
34.5% at doses of 93.75, 187.5 and 375 mg/kg/day baicalin,
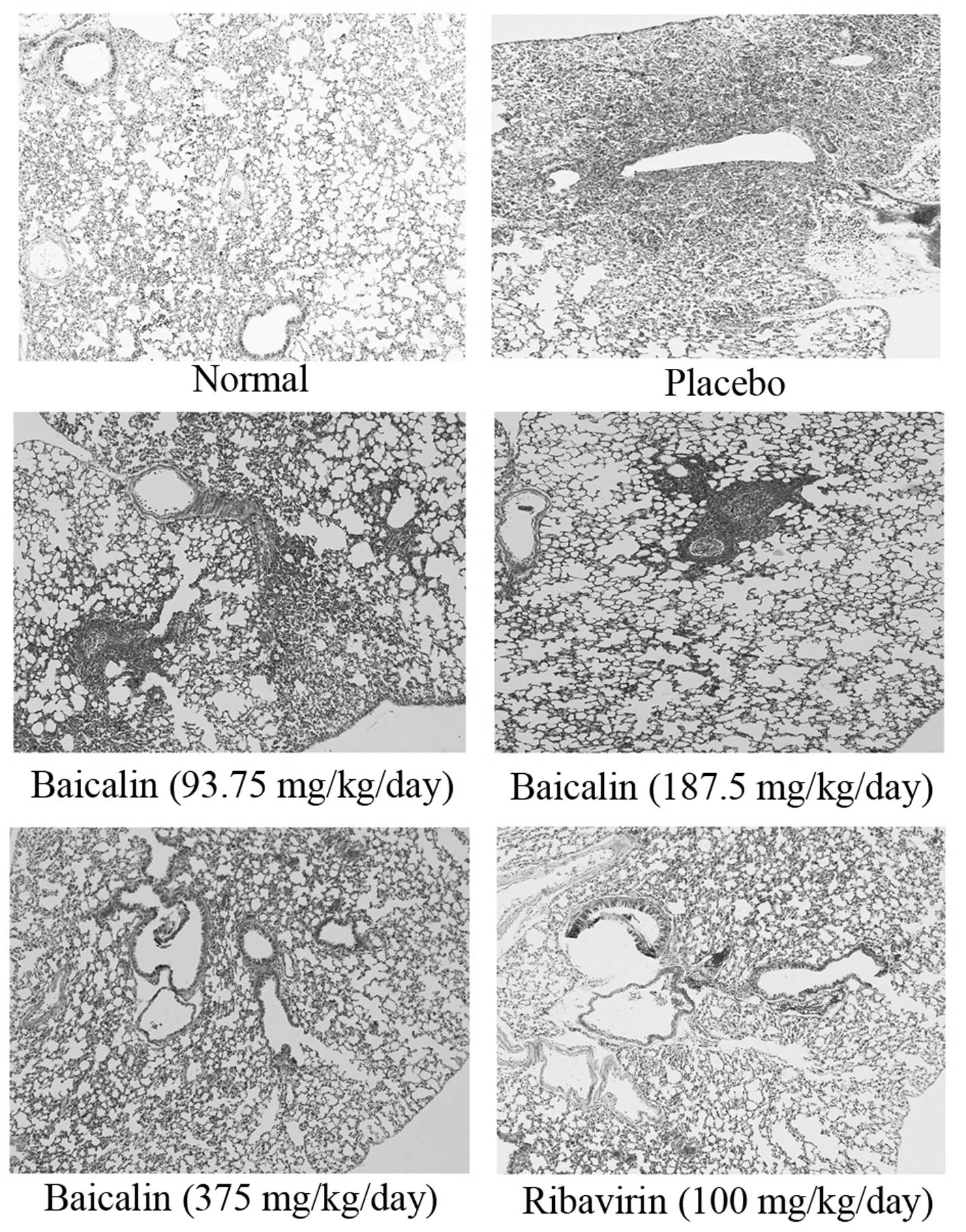
respectively. The findings of the histological examination were
consistent with the lung parameter data (Fig. 1). Protection from bronchitis and
interstitial pneumonia was found in all the groups that were
treated with baicalin, and the degrees of protection varied
depending on the dose.
Effects of baicalin on mRNA expression of
TLR7 and MYD88
The method described by Livak and Schmittgen
(13) was used to assess the
effects of baicalin on the mRNA expression of TLR7 and MYD88
(Table II). Compared with the
normal group, the mRNA expression levels of TLR7 and MYD88 were
higher in the placebo group (P<0.01). The mRNA expression levels
of TLR7 and MYD88 at doses of 187.5 and 375 mg/kg/day baicalin were
significantly lower (P<0.01) compared with the placebo
group.
 | Table IIEffects of baicalin on the mRNA
expression of TLR7 and MYD88 (mean ± standard error; n=8). |
Table II
Effects of baicalin on the mRNA
expression of TLR7 and MYD88 (mean ± standard error; n=8).
| |
2−ΔΔCT |
|---|
| |
|
|---|
| Group | Dose, mg/kg/day | TLR7 | MYD88 |
|---|
| Normal | - | 1.01±0.15 | 1.03±0.13 |
| Placebo | - | 4.66±0.65a | 3.27±0.53a |
| Ribavirin | 100.00 | 2.39±0.41b | 1.82±0.34b |
| Baicalin | 93.75 | 3.95±0.45c | 2.86±0.52c |
| 187.50 | 1.95±0.28b | 1.85±0.13b |
| 375.00 | 1.82±0.27b | 1.97±0.24b |
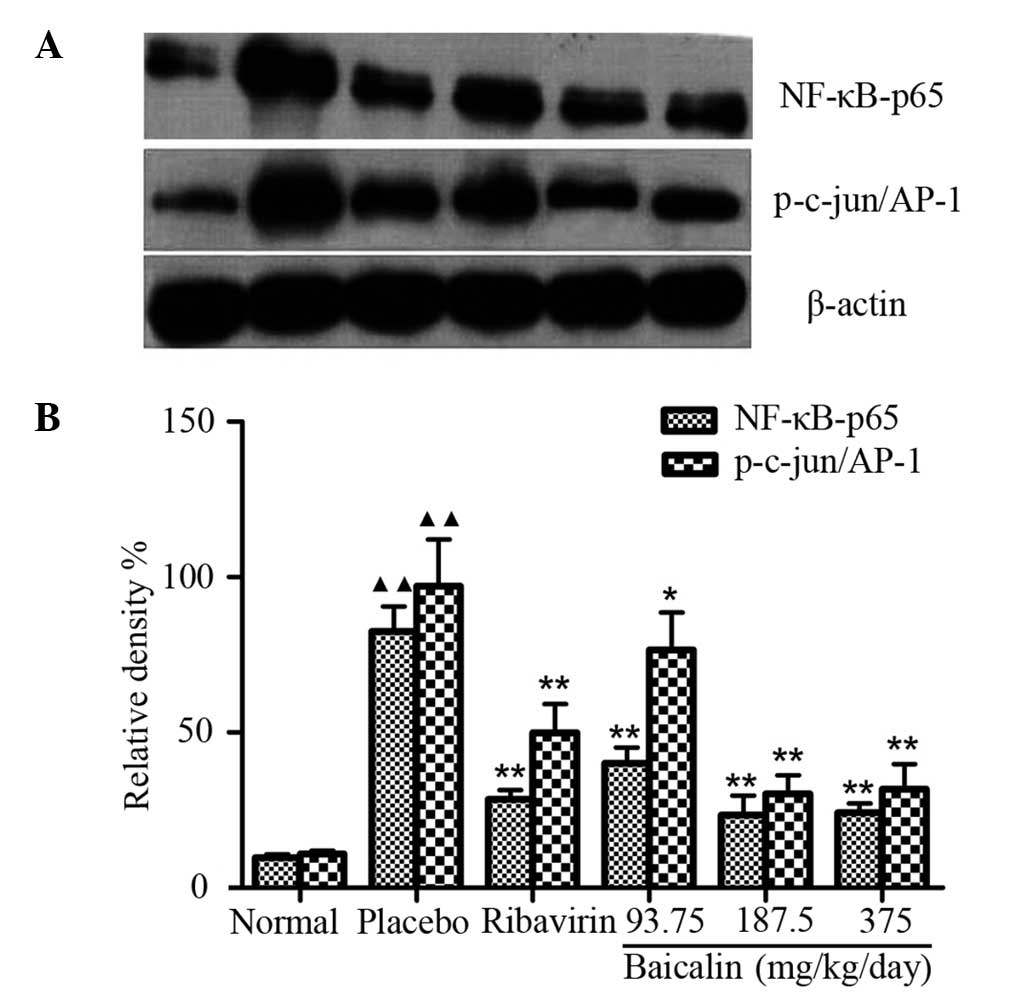
Effects of baicalin on the protein
expression of NF-κB-P65 and c-jun/AP-1
Compared with the normal group, the protein
expression levels of NF-κB-P65 and c-jun/AP-1 were higher in the
placebo group (P<0.01). The expression levels of NF-κB-P65 and
c-jun/AP-1 at doses of 187.5 and 375 mg/kg/day baicalin were
significantly lower (P<0.01) compared with the placebo group
(Fig. 2).
Effects of baicalin on the expression of
TNF-α, IL-1β and IL-6
The expression levels of TNF-α, IL-1β and IL-6 were
higher in the placebo group (P<0.01) compared with the normal
group. The expression of TNF-α, IL-1β and IL-6 at doses of 187.5
and 375 mg/kg/day baicalin were significantly lower (P<0.01)
compared with the placebo group (Table III).
 | Table IIIEffects of baicalin on the expression
of TNF-α, IL-1β and IL-6 (mean ± standard error; n=12). |
Table III
Effects of baicalin on the expression
of TNF-α, IL-1β and IL-6 (mean ± standard error; n=12).
| Groups | Dose,
mg/kg/day | TNF-α, pg/ml | IL-1β, pg/ml | IL-6, pg/ml |
|---|
| Normal | - | 256.23±44.45 | 653.69±94.15 | 5989.71±634.16 |
| Placebo | - |
765.85±95.47a |
1055.21±121.47a |
9048.64±1150.62a |
| Ribavirin | 100.00 |
281.62±42.16b |
740.5±103.29b |
7581.41±852.24b |
| Baicalin | 93.75 |
583.74±73.41c |
957.94±111.38c |
8476.21±958.56c |
| 187.50 |
310.23±47.58b |
766.19±102.56b |
6785.64±758.82b |
| 375.00 |
261.10±41.86b |
729.17±84.19b |
6550.96±657.47b |
Discussion
In the present study, the inhibitory activity of
baicalin against H1N1 in mice was examined and its mechanisms
investigated. The oral administration of baicalin showed positive
effects on the mice infected with H1N1, increasing the survival
rate, prolonging the MDD and inhibiting lung index and lung
consolidation.
The aim of the present study was to investigate the
mechanisms underlying the antiviral activity of baicalin. It has
been reported that the pathological injury caused by H1N1 infection
is due to host inflammatory responses to the virus rather than the
virus directly destroying respiratory epithelia (14). TLR, as a pattern recognition
receptor, plays a role in recognizing the pathogen of influenza
virus infection (15). TLR
identifies extracellular and intracellular pathogen associations
with the molecular pattern of the invading virus (16,17).
TLR7 is activated once the single stranded RNA of influenza A is
identified, and signals of the immune cells are transmitted through
the MYD88 pathway. The NF-κB and AP-1 signaling pathways are then
induced (18,19) resulting in the activation of
cytokines, such as interferon-α/β, TNF-α, IL-1 and IL-6 production
(20), which is a trigger of the
subsequent antiviral acquired immunity reaction (21). When these pathways are out of
control, a large number of pro-inflammatory mediators are produced
causing inflammatory injury. Previous studies have shown that
various levels of TNF-α, IL-1β and IL-6 are associated with the
degree of pathological damage (22).
Morphology is closely associated with intracellular
biochemical changes. In the present study, hematoxylin and eosin
staining revealed severe pneumonia in the placebo group, including
hyperemia, leukopedesis, bronchiole epithelium cell necrosis, lung
exudates, alveolus interstitial pneumonia and lung abscess.
Molecular biology showed that the mRNA expression of TLR7 and
MYD88, the protein expression of NF-κB and AP-1 and the secretion
of TNF-α, IL-1β and IL-6 were significantly increased in the
placebo group. Following treatment with various doses of baicalin,
the number of pulmonary lesions was reduced significantly, and the
mRNA expression of TLR7 and MYD88, the protein expression of NF-κB
and AP-1 and the secretion of TNF-α, IL-1β and IL-6 were
significantly decreased, indicating that baicalin inhibits the
activation of the TLR7/MYD88 signaling pathway to reduce the
secretion of inflammatory cytokines, thereby reducing the
inflammatory injury and restoring the stability and balance of
immune function.
The signal transduction pathway induced by influenza
virus infection is a complex network. Certain signal molecules play
multiple roles following the infection of influenza virus,
therefore it is difficult to determine a specific signal molecule
to facilitate or inhibit the proliferation of the influenza virus.
NF-κB is generally considered to be the key transcription factor
that affects the secretion of type I interferon and other antiviral
cytokines (23,24), but its moderate activation is a
prerequisite for the infection of the influenza virus (25). It is known that the NF-κB pathway
can regulate the synthesis of influenza virus RNA and that the
knockout of P65 reduces RNA synthesis (26). In a sense, when studying NF-κB-P65
it is difficult to grasp the interactions between the influenza
virus and its host as a whole. Therefore, the systematic methods
based on the genome, the transcriptome, the proteome and
bioinformatics may contribute to our understanding of the
interpretation of influenza virus infection and antiviral
drugs.
Acknowledgements
The authors would like to thank Professor Zhengyun
Chu of Liaoning Traditional Chinese Medicine University for
donating the baicalin. This study was sponsored by the Provincial
Project from Ningxia Hui Autonomous Region (grant no. NGY
2012067).
References
|
1
|
Romanowska M, Stefanska I, Donevski S and
Brydak LB: Infections with A(H1N1)2009 influenza virus in Poland
during the last pandemic: experience of the National Influenza
Center. Adv Exp Med Biol. 756:271–283. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wan QF, Wang H, Lin Y, et al: Effects of
quercetin on CDK4 mRNA and protein expression in A549 cells
infected by H1N1. Biomed Rep. 1:766–770. 2013.PubMed/NCBI
|
|
3
|
Le QM, Kiso M, Someya K, et al: Avian flu:
isolation of drug-resistant H5N1 virus. Nature (London).
437:11082005. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Saito R, Li D, Suzuki H, et al: High
prevalence of amantadine-resistance influenza a (H3N2) in six
prefectures, Japan, in the 2005–2006 season. J Med Virol.
79:1569–1576. 2007.PubMed/NCBI
|
|
5
|
Sharma U, Bala M, Saini R, et al:
Polysaccharide enriched immunomodulatory fractions from
Tinospora cordifolia(Willd) miers ax hook. f. & Thoms.
Indian J Exp Biol. 50:612–617. 2012.PubMed/NCBI
|
|
6
|
Evers DL, Chao CF, Wang X, et al: Human
cytomegalovirus-inhibitory flavonoids: studies on antiviral
activity and mechanism of action. Antiviral Res. 68:124–134. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wan QF, Gu LG, Yin SJ, et al: Protection
effect of baicalin on lung injury of mice infected with influenza
FM1. Chin J Tradit Chin Med Pharm. 2848–2851. 2011.
|
|
8
|
Wan QF, Gu LG, Yin SJ, et al: Effect of
baicalin on cell apoptosis FAS/FAS-L system of pneumonia mice lung
tissue infected with FM1. Chin Pharmacol Bull. 27:1555–1559.
2011.
|
|
9
|
Kitamura K, Honda M, Yoshizaki H, et al:
Baicalin, an inhibitor of HIV-1 production in vitro. Antiviral Res.
37:131–140. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Romero MR, Efferth T, Serrano MA, et al:
Effect of artemisinin/artesunate as inhibitors of hepatitis B virus
production in an ‘in vitro’ replicative system. Antiviral Res.
68:75–83. 2005.PubMed/NCBI
|
|
11
|
Chu ZY, Chu M and Teng Y: Effect of
baicalin on in vivo anti-virus. Zhongguo Zhong Yao Za Zhi.
32:2413–2415. 2007.(In Chinese).
|
|
12
|
Xu G, Dou J, Zhang L, et al: Inhibitory
effects of baicalein on the influenza virus in vivo is determined
by baicalin in the serum. Biol Pharm Bull. 33:238–243. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cook DN, Beck MA, Coffman TM, et al:
Requirement of MIP-1α for an inflammatory response to viral
infection. Science. 269:1583–1585. 1995.
|
|
15
|
Kawai T and Akira S: Toll-like receptor
and RIG-I-like receptor signaling. Ann NY Acad Sci. 1143:1–20.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MacLeod H and Wetzler LM: T cell
activation by TLRs: A role for TLRs in the adaptive immune
response. Sci STKE. 2007:pe482007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Walsh KB, Teijaro JR, Zuniga EI, et al:
Toll-like receptor 7 is required for effective adaptive immune
responses that prevent persistent virus infection. Cell Host
Microbe. 11:643–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rahman I, Gilmour PS, Jimenez LA, et al:
Oxidative stress and TNF-α induce histone acetylation and
NF-κB/AP-1 activation in alveolar epithelial cells: potential
mechanism in gene transcription in lung inflammation. Mol Cell
Biochem. 234–235:239–248. 2002.
|
|
19
|
Ludwig S, Ehrhardt C, Neumeier ER, et al:
Influenza virus-induced AP-1-dependent gene expression requires
activation of the JNK signaling pathway. J Biol Chem.
276:10990–10998. 2001. View Article : Google Scholar
|
|
20
|
Adachi M, Matsukura S, Tokunaga H, et al:
Expression of cytokines on human bronchial epithelial cells induced
by influenza virus A. Int Arch Allergy Immunol. 113:307–311. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qian C and Cao X: Regulation of toll-like
receptor signaling pathways in innate immune responses. Ann NY Acad
Sci. 1283:67–74. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Aldridge JR Jr, Moselev CE, Boltz DA, et
al: TNF/iNOS-producing dendritic cells are the necessary evil of
lethal influenza virus infection. Proc Natl Acad Sci USA.
106:5306–5311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Severa M and Fitzgerald KA: TLR-mediated
activation of type I IFN during antiviral immune responses:
fighting the battle to win the war. Curr Top Microbiol Immunol.
316:167–192. 2007.PubMed/NCBI
|
|
24
|
Tenoever BR, Ng SL, Chua MA, et al:
Multiple functions of the IKK-related kinase IKKɛ in
interferon-mediated antiviral immunity. Science. 315:1274–1278.
2007.PubMed/NCBI
|
|
25
|
Nimmerjahn F, Dudziak D, Dirmeier U, et
al: Active NF-κB signaling is a prerequisite for influenza virus
infection. J Gen Virol. 85:2347–2356. 2004.
|
|
26
|
Kumar N, Xin ZT, Liang Y, et al: NF-κB
signaling differentially regulates influenza virus RNA synthesis. J
Virol. 82:9880–9889. 2008.
|