Introduction
Ankylosing spondylitis (AS) is a chronic
inflammatory disease that primarily affects the axial skeleton,
peripheral joints, attachments of ligaments and entheses. The main
clinical characteristic of AS is inflammatory lower back pain and,
over time, certain patients develop spinal immobility and ankylosis
(1,2). AS exhibits a male predominance
(3).
Despite our longstanding knowledge of the familial
associations of AS, the underlying pathogenetic mechanism has not
been fully elucidated. A number of cytokines were shown to play a
critical role in the pathogenesis of AS. Interleukin (IL)-17 and
IL-23 are cytokines associated with inflammation, autoimmunity and
defense against bacteria. The elevated levels of IL-17 and IL-23 in
AS patients reflect their critical roles in the pathogenesis of AS
(4,5). It was previously demonstrated that the
carriers of single-nucleotide polymorphisms of the IL-23 receptor
in the Chinese Han population are susceptible to AS (6), whereas other studies reported
conflicting results (7,8). The pathogenetic mechanism underlying
the development of AS has not been fully elucidated. Genetic
findings may provide novel insight into the etiology of AS.
However, broader validation in different populations and further
investigation of the underlying mechanisms are required.
AS is significantly associated with the human
leukocyte antigen (HLA)-B27 gene (9); however, HLA-B27 accounts for only 16%
of the genetic variability in AS (10). Compared to healthy subjects, AS
patients exhibit increased expression of circulating
CD4+ and CD8+ T cells (11,12);
therefore, the abnormal expression of these T cells may also be
associated with AS. Furthermore, the pathogenesis of AS may be
correlated with CD4+CD25+CD127low
regulatory T cells (Tregs).
The Tripterygium glycosides tablet (TGT)
comprises wilforlide A (C30H46O3),
extracted from Tripterygium wilfordii, which is a
traditional medicinal plant that has been used in China for several
years for the long-term treatment of inflammatory conditions, such
as rheumatoid arthritis, various skin disorders, chronic nephritis
and AS. In the present study, 20 patients with active AS were
selected (AS group) and treated with TGT (20 mg, 3 times/day) for 6
weeks. A total of 20 healthy age- and gender-matched volunteers
were recruited as the control group. To investigate the possible
mechanisms of action of TGT in the treatment of AS, IL-17 and
CD4+CD25+CD127low Tregs were
measured in the peripheral blood of patients with AS. Our results
demonstrated that TGT was effective in improving the signs and
symptoms of patients with AS, possibly through the upregulation of
CD4+CD25+CD127low Treg and the
downregulation of IL-17 in the peripheral blood.
Materials and methods
Ethics and study populations
Ethical approval for this study was obtained from
the Human Research Ethics Committee of the Affiliated Hospital of
Nanjing University of Traditional Chinese Medicine (no.
2010NL_085_02) and the participants provided written informed
consent. TGT was purchased from Deeng Pharmaceutical Company
(product no. 0802102; Zhejiang DND Pharmaceutical Co., Ltd,
Shaoxing, China).
Between January, 2010 and January, 2013, a total of
20 AS patients and 20 healthy age- and gender-matched controls were
recruited in our hospital. All the patients and controls were Han
Chinese. The AS patients were treated with 20 mg TGT 3 times per
day for 6 weeks, without any additional treatment. The diagnosis of
AS was confirmed by experienced rheumatologists, according to the
modified New York criteria (13).
Subjects with rheumatoid arthritis, inflammatory bowel disease,
psoriasis or other autoimmune diseases, were excluded from the
study. Subjects under treatment with non-steroidal
anti-inflammatory drugs (NSAIDs) or any other medication were also
excluded from the study. The patients were followed up for 6 weeks.
Peripheral blood samples (2 ml) were obtained from the patients
prior to and after TGT treatment for 6 weeks. The samples were
collected in heparin-containing tubes and immediately stored at
−70°C until further processing.
Basic data acquisition
The Bath AS disease activity index (BASDAI), the
most widely used tool for the assessment of the AS functional
status and disease activity, was calculated for all the AS patients
using questionnaires (13). Serum
assays for erythrocyte sedimentation rate (ESR) and C-reactive
protein (CRP) levels were performed on AS patients prior to and
after TGT treatment (normal range of ESR, 0–15 mm/h; normal range
of CRP, 0–10 mg/l).
Serum IL-17 activity
The activity of serum IL-17 was measured using a
commercial human IL-17 ELISA kit (Bender Med Systems, San Diego,
CA, USA) according to the manufacturer’s instructions. The IL-17
levels in the serum of the AS patients prior to and after TGT
treatment were compared to those of the control group.
Determination of
CD4+CD25+CD127low Treg ratio by
flow cytometry
A total of 10 μl mouse anti-human CD4-fluorescein
isothiocyanate, 20 μl mouse anti-human phycoerythrin-labeled CD25
and 10 μl phycoerythrin and cyanine-5-labeled CD127 monoclonal
antibodies (all from Beckman Coulter, Miami, FL, USA) were added to
100 μl of peripheral blood. Following incubation at room
temperature in the dark for 15–30 min, 1 ml erythrocyte lysing
solution was added to the samples and incubated under the same
conditions for 15–20 min. The cells were washed with 2 ml
phosphate-buffered saline (PBS) and resuspended in 400 μl PBS. The
cells were then analyzed using a FACSCalibur flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA).
Statistical analysis
Statistical analyses were performed using the
statistical SPSS software, version 14.0 (SPSS Inc., Chicago, IL,
USA). The results are expressed as means ± SD. P<0.05 was
considered to indicate statistically significant differences. A
bivariate correlation analysis was used to determine the
association of IL-17 levels with BASDAI, ESR, CRP and
CD4+CD25+CD127low Treg in AS
patients.
Results
Clinical characteristics
BASDAI, ESR and CRP levels for the AS patients prior
to and after TGT treatment are presented in Table I. Prior to treatment, the BASDAI in
17 of the patients was >50 mm, with an even higher average score
in all these AS patients (51.94±11.57) compared to the normal
range. After 6 weeks of TGT treatment, the BASDAI score was
significantly decreased (24.47±13.01).
 | Table IBASDAI, ESR and CRP levels in AS
patients prior to and after TGT treatment (n=20). |
Table I
BASDAI, ESR and CRP levels in AS
patients prior to and after TGT treatment (n=20).
| Efficacy
measures | Prior to treatment
(mean ± SD) | After treatment (mean
± SD) |
|---|
| BASDAI | 51.94±11.57 | 24.47±13.01a |
| ESR (mm/h) | 31.18±15.63 | 15.87±14.65a |
| CRP (μg/ml) | 16.30±11.28 | 6.10±6.88a |
Prior to treatment, the ESR and CRP levels were
higher compared to those in healthy controls. After 6 weeks of TGT
treatment, the levels of ESR and CRP were significantly decreased
to within the normal range.
IL level
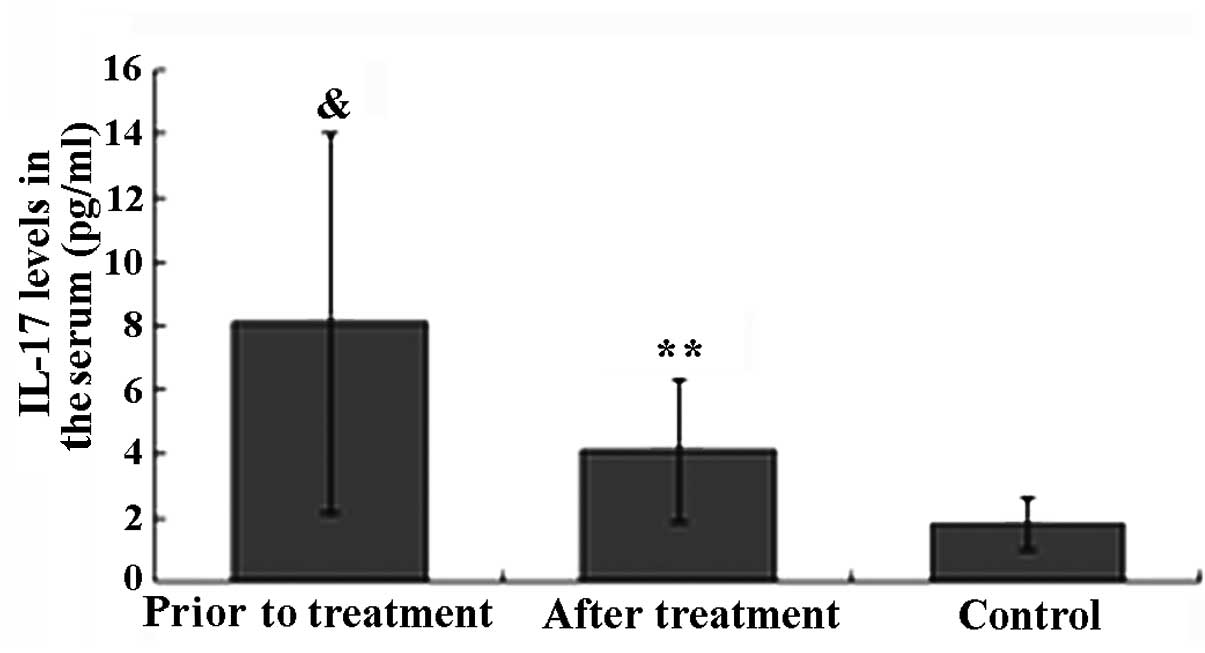
The IL-17 level in the healthy control group was
within the normal range (1.82±0.81 pg/ml). Prior to TGT treatment,
the IL-17 level in patients with active AS was significantly higher
compared to that in healthy controls (P<0.05). The IL-17 level
in 18 patients with active AS was higher than the normal range, and
in 5 of these patients, the level was significantly higher (>10
times the normal range). After 6 weeks of TGT treatment, the IL-17
level in 16 of the AS patients was decreased and in 5 of these
patients it returned within the normal range. The decrease in the
IL-17 level in AS patients after TGT treatment was found to be
statistically significant (P<0.01). There was no significant
difference in the IL-17 level between the AS patients after TGT
treatment and that in healthy controls (P>0.05; Fig. 1).
Ratio of
CD4+CD25+CD127low Tregs
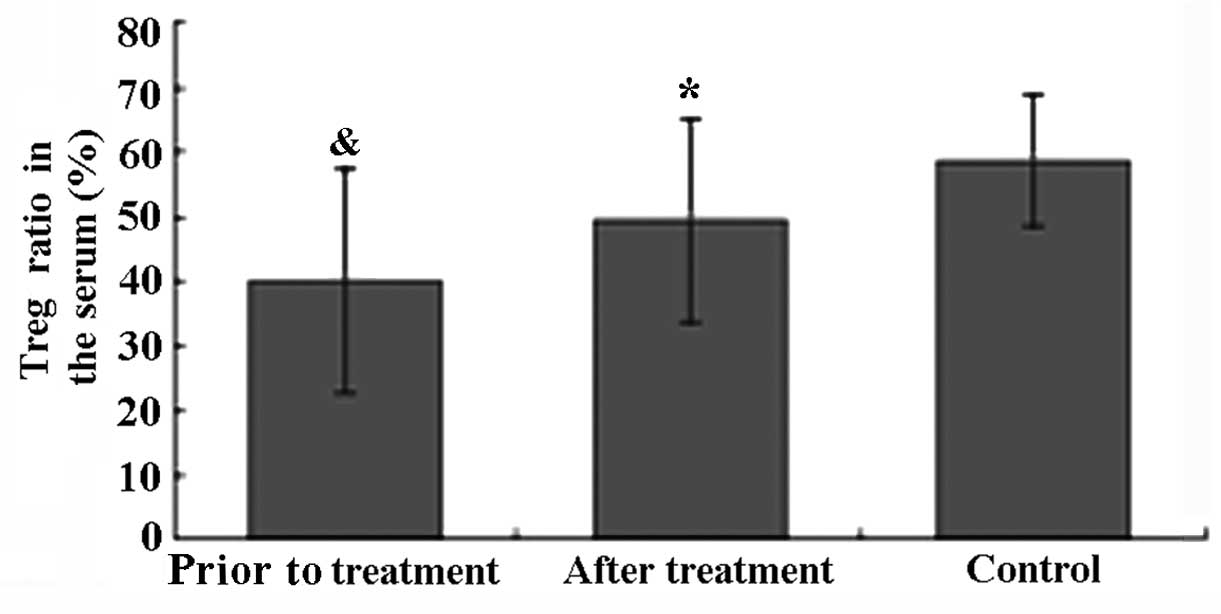
As shown in Fig. 2,
the ratio of CD4+CD25+CD127low
Tregs in the healthy control group was within the normal range
(58.6±10.2%). Prior to treatment, the ratio of
CD4+CD25+CD127low Tregs in
patients with active AS was significantly lower compared to that in
healthy controls (P<0.05). After TGT treatment, the ratio of
CD4+CD25+CD127low Tregs in the
serum of AS patients was significantly increased from 40.1±17.5 to
49.6±16.0% (P<0.05).
Association of IL-17 level with BASDAI,
ESR, CRP and CD4+CD25+CD127low
Tregs in AS patients
Prior to TGT treatment, the BASDAI and IL-17 levels
were higher compared to those in healthy individuals and were
decreased after 6 weeks of TGT treatment. The correlation analysis
revealed that 16 patients exhibiting a decrease in the IL-17 levels
also exhibited a significantly decreased BASDAI score (P<0.01).
By computing the Pearson’s correlation coefficient, we observed a
significantly positive correlation between BASDAI and IL-17
(r=0.960 and P<0.001).
Prior to TGT treatment, the ESR and CRP levels in AS
patients were higher compared to those in healthy controls and were
decreased after 6 weeks of TGT treatment. The Pearson’s correlation
coefficient demonstrated that ESR and CRP were significantly
correlated with IL-17 (r=0.826 and P<0.001; and r=0.754 and
P<0.01, respectively).
The ratio of
CD4+CD25+CD127low Tregs was
significantly increased following TGT treatment in AS patients and
the Pearson’s correlation coefficient demonstrated that
CD4+CD25+CD127low Tregs were
significantly negatively correlated with IL-17 (r=−0.739 and
P<0.01).
Discussion
AS is a chronic inflammation of the sacroiliac
joints, spine and peripheral joints. NSAIDs are currently the
first-line treatment for the pain and stiffness associated with AS.
However, there are significant side effects associated with NSAIDs,
such as injury to the gastrointestinal tract. When treatment with
NSAIDs is not sufficient, second-line medications, occasionally
refered to as disease-modifying antirheumatic drugs, including
sulfasalazine, methotrexate and corticosteroids, may be used.
Tumour necrosis factor (TNF) blockers, including Remicade, Enbrel
and Humira, recently emerged as promising medications for the
treatment of AS. However, TNF blockers are also associated with
severe side effects, including reactivation of latent tuberculosis
and neurological problems (14).
TGT, a product derived from traditional Chinese
medicinal plants, has been used to treat inflammatory conditions,
such as rheumatoid arthritis, various skin disorders, chronic
nephritis and AS in China. Over the last few years, TGT has been
used for the treatment of patients with active AS, with good
results. In the present study, the calculation of BASDAI and blood
tests for CRP and ESR in AS patients demonstrated the high
efficiency of TGT in the treatment of AS. However, the mechanism of
action of TGT has not been clearly determined. To elucidate the
mechanisms underlying the action of TGT in the treatment of AS,
IL-17 and CD4+CD25+CD127low Tregs
were measured in the peripheral blood of AS patients prior to and
after TGT treatment.
IL-17, as an inflammatory cytokine, is closely
correlated with the development of autoimmune diseases (15). Wang et al (16) reported significantly higher IL-17
levels in patients with active AS compared to those in healthy
individuals and a significantly positive association between IL-17
and BASDAI, Bath ankylosing spondylitis metrology index, Bath
ankylosing spondylitis functional index, ESR or CRP. We also
observed that the high expression of IL-17 in patients with active
AS is correlated with disease activity and the level of IL-17 in
the peripheral blood is closely correlated with ESR and CRP levels.
The level of IL-17 in AS patients was significantly decreased after
TGT treatment for 6 weeks (P<0.01). These results demonstrated
that TGT significantly inhibited IL-17 expression in patients with
active AS and was associated with an improvement in other
laboratory parameters.
Tregs, as major suppressors of the immune system,
exhibit a decreased prevalence in the blood of AS patients,
indicating that their absence may contribute to the pathogenesis of
AS (17). In the present study, we
also investigated the prevalence of
CD4+CD25+CD127low Tregs in the
peripheral blood of AS patients and found that their number was
significantly lower compared to that in healthy individuals,
whereas it was significantly increased after TGT treatment,
demonstrating the alteration of the body’s immune response through
T-cell activation. Furthermore, the enhanced immune inhibition was
correlated with the decrease in IL-17 levels.
Overall, these results suggest that TGT treatment
effectively improves the signs and symptoms of patients with AS,
possibly through the upregulation of
CD4+CD25+CD127low Tregs and the
downregulation of IL-17 in the peripheral blood. However, the
mechanism of action of TGT in AS patients has not been clearly
determined and requires further investigations. Our findings also
demonstrated that IL-17 may be a useful index for assessing the AS
activity and the treatment efficacy, which requires validation by
further clinical studies.
Acknowledgements
This study was supported by the Fund for the Talents
in Traditional Chinese Medicine of Jiangsu province (grant no.
LJ200907) and the Jiangsu Province Administration of Traditional
Chinese Medicine (grant no. LZ11014).
References
|
1
|
Rolle AS, Zimmermann B and Poon SH:
Etanercept-induced Henoch-Schönlein purpura in a patient with
ankylosing spondylitis. J Clin Rheumatol. 19:90–93. 2013.
|
|
2
|
Braun J and Sieper J: Ankylosing
spondylitis. Lancet. 369:1379–1390. 2007. View Article : Google Scholar
|
|
3
|
Calin A, Brophy S and Blake D: Impact of
sex on inheritance of ankylosing spondylitis: a cohort study.
Lancet. 354:1687–1690. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wendling D, Cedoz JP, Racadot E and
Dumoulin G: Serum IL-17, BMP-7, and bone turnover markers in
patients with ankylosing spondylitis. Joint Bone Spine. 74:304–305.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mei Y, Pan F, Gao J, et al: Increased
serum IL-17 and IL-23 in the patient with ankylosing spondylitis.
Clin Rheumatol. 30:269–273. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang X, Huang J, Lin Z, et al:
Single-nucleotide polymorphisms and expression of IL23R in Chinese
ankylosing spondylitis patients. Rheumatol Int. 30:955–959. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davidson SI, Wu X, Liu Y, et al:
Association of ERAP1, but not IL23R, with ankylosing spondylitis in
a Han Chinese population. Arthritis Rheum. 60:3263–3268. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen C, Zhang X, Li J and Wang Y:
Associations of IL-23R polymorphisms with ankylosing spondylitis in
East Asian population: a new case-control study and a
meta-analysis. Int J Immunogenet. 39:126–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Brewerton DA, Hart FD, Nicholls A, Caffrey
M, James DC and Sturrock RD: Ankylosing spondylitis and HL-A 27.
Lancet. 1:904–907. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Khan MA and Ball EJ: Genetic aspects of
ankylosing spondylitis. Best Pract Res Clin Rheumatol. 16:675–690.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Duftner C, Goldberger C, Falkenbach A, et
al: Prevalence, clinical relevance and characterization of
circulating cytotoxic CD4+CD28−T cells in
ankylosing spondylitis. Arthritis Res Ther. 5:R292–R300. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schirmer M, Goldberger C, Würzner R, et
al: Circulating cytotoxic CD8+CD28−T cells in
ankylosing spondylitis. Arthritis Res. 4:71–76. 2002. View Article : Google Scholar
|
|
13
|
van der Linden S, Valkenburg HA and Cats
A: Evaluation of diagnostic criteria for ankylosing spondylitis. A
proposal for modification of the New York criteria. Arthritis
Rheum. 27:361–368. 1984.
|
|
14
|
Murdaca G, Colombo BM and Puppo F:
Anti-TNF-alpha inhibitors: a new therapeutic approach for
inflammatory immune-mediated diseases: an update upon efficacy and
adverse events. Int J Immunopathol Pharmacol. 22:557–565.
2009.PubMed/NCBI
|
|
15
|
Zhu S and Qian Y: IL-17/IL-17 receptor
system in autoimmune disease: mechanisms and therapeutic potential.
Clin Sci (Lond). 122:487–511. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang XW, Lin ZM, Liao ZT, Wei QJ, Jiang YJ
and Gu JR: The study on expression of IL-23 and IL-17 in ankylosing
spondylitis. Chin J Immunol. 25:266–270. 2009.(In Chinese).
|
|
17
|
Wu Y, Ren M, Yang R, et al: Reduced
immunomodulation potential of bone marrow-derived mesenchymal stem
cells induced CCR4+CCR6+Th/Treg cell subset
imbalance in ankylosing spondylitis. Arthritis Res Ther.
13:R292011. View
Article : Google Scholar : PubMed/NCBI
|