Introduction
Nonalcoholic fatty liver disease (NAFLD) is defined
as hepatic steatosis without secondary hepatic fat accumulation,
including alcoholic consumption, steatogenic medication or
hereditary disorders (1).
Nonalcoholic fatty liver (NAFL) and nonalcoholic fatty
steatohepatitis (NASH) are the categories of NAFLD. With regards to
NASH, NAFL is not associated with injury of hepatocytes
(ballooning) (1). The survival rate
in patients with NAFLD is lower compared to the general population
standardized-mortality ratio due to the risk of cardiovascular
disease and hepatocellular carcinoma (2–4).
Therefore, it is recommended that NAFLD is diagnosed and treated
(5). Abdominal ultrasound (US) is
the simplest and most practical diagnostic imaging modality
(6,7). To treat NAFLD efficiently, the target
of metabolism for treatment should be known. Body mass index (BMI)
is a simple marker of obesity and the index is an independent
predictor of NAFLD (8). Weight loss
is known to improve NASH (9).
However, whether weight loss improves NAFL remains unclear. The
therapeutic targets, other than BMI, should be investigated.
NAFLD is associated with metabolic disturbances,
including diabetes and hyperlipidemia (1,10).
Diabetes is an independent predictor of NAFLD (11) and hyperlipidemia is prevalent among
patients with NAFLD (12).
Insulin-sensitizing agents have been applied to patients with
NAFLD. A large, randomized-controlled trial with metformin did not
effectively improve NASH (13).
Pioglitazone improves steatosis and inflammation (14), but did not improve fibrosis.
Steatosis and inflammation are improved with insulin-sensitizing
agents; however, it is difficult to treat fibrosis. Statins are
important for the treatment of hyperlipidemia. However, simvastatin
is not effective in the treatment of NASH (15). The metabolic disorder parameters are
not known with regards to NAFLD. Therefore, the present study aimed
to investigate the blood examination parameters that were closely
associated with NAFLD. Diabetes and hyperlipidemia were studied as
they were expected to indicate the therapeutic target of NAFLD.
Materials and methods
Inclusion criteria
The patients who underwent abdominal US between
April 2013 and November 2013, and had laboratory data that were
available on the date of the abdominal US, were included in the
study. The patient records were analyzed retrospectively, and were
divided into two groups: Patients with NAFLD and those without
NAFLD. The study was reviewed by the institutional Ethics Committee
and it was determined that the study was not a clinical trial as it
was performed as part of daily clinical practice. Patient anonymity
was preserved throughout the analysis.
Exclusion criteria
The patients whose laboratory data were not
available on the day of US were excluded. The patients who were
positive for hepatitis B surface antigen or hepatitis C antibody
were also excluded. Liver cirrhosis, primary biliary cirrhosis,
autoimmune hepatitis or high alcohol consumption were also criteria
for patient exclusion due to the possible elevation of their liver
enzymes (16,17). The patients with muscular dystrophy
or dermatomyositis were excluded due to the possibility of elevated
aspartate aminotransferase (AST) or lactate dehydrogenase (LDH)
levels. Additionally, patients were excluded from the study if
prednisolone was prescribed as it may cause NAFLD (18) and if methotrexate was prescribed as
it may cause liver toxicity (19).
Abdominal US
NAFLD was diagnosed using abdominal US, following
the standardized criteria (20,21).
Briefly, NAFLD was diagnosed when bright liver or hepatorenal echo
contrast was observed with abdominal US. Abdominal US was performed
by Senior Fellows of the Japan Society of Ultrasonics in Medicine
with SSA-700A (Toshiba Medical Systems Corporation, Ohtawara,
Japan) using a 3.5-MHz curved-array probe. Additionally, abdominal
US was performed by Board Certified Fellows of the Japan Society of
Ultrasonics in Medicine with SSA-700A using a 5.0-MHz curved-array
probe. The investigators were blinded to the clinical and
laboratory data.
Laboratory data
The analyzed laboratory data were the levels of
alkaline phosphatase (ALP), AST, alanine aminotransferase (ALT),
γ-glutamyl transpeptidase (γ-GTP), LDH, high-density lipoprotein
cholesterol (HDL), low-density lipoprotein cholesterol (LDL),
triglyceride (TG), blood glucose (BG) and hemoglobin A1c
(HbA1c).
Statistical analysis
One-way analysis of variance was performed for the
baseline characteristics. Wald analysis was performed to evaluate
the efficiency of each parameter by multivariate regression
analysis to predict NAFLD. The receiver operating characteristic
(ROC) curve was created to evaluate the predicting performance of
the regression model. The threshold value was determined as the
highest sensitivity and specificity, and was calculated
automatically by the software using the location where a line with
a slope of 45° came into contact with the ROC curve. Stepwise
analysis was performed to select variables to predict NAFLD. The
statistical software JMP 10.0.2 (SAS Institute, Inc., Cary, NC,
USA) was used. P<0.05 was considered to indicate a statistically
significant difference.
Results
Parameters associated with NAFLD
Table I demonstrates
the patient characteristics. Age and HDL were significantly lower
in patients with NAFLD compared to those without NAFLD (P<0.05).
ALP, AST, ALT, γ-GTP, LDL, TG, BG and HbA1c were significantly
higher in patients with NAFLD compared to those without NAFLD
(P<0.05). Table II demonstrates
the logistic regression analysis. TG was the parameter most
significantly associated with NAFLD (χ2=9.89, P=0.0017).
With the parameters in Table II,
the logistic curve was illustrated as an equation: ln(P/1-P) =
−3.8081 - 0.0104 × HDL + 0.0038 × LDL + 0.0087 × TG + 0.0081 × BG +
0.2626 × HbA1c (P<0.0001). P represents the possibility of
NAFLD.
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Characteristics | Total | Non-NAFLD | NAFLD | P-value |
|---|
| Patient, n | 293 | 125 | 168 | |
| Male/female, n | 140/153 | 62/63 | 78/90 | |
| Age, years | 67±13 | 63.4±12.9 | 69.4±12.8 | 0.0005 |
| ALP, IU/l | 241.7±91.4 | 225.1±81.9 | 253.0±63.9 | 0.0030 |
| AST, IU/l | 27.5±16.6 | 27.5±15.7 | 23.4±10.1 | <0.0001 |
| ALT, IU/l | 27.6±25.9 | 35.0±34.8 | 19.0±11.9 | <0.0001 |
| γ-GTP, IU/l | 48.2±66.6 | 51.1±28.7 | 39.5±56.0 | 0.0258 |
| HDL, mg/dl | 59.0±21.3 | 42.5±17.6 | 63.5±19.0 | 0.0002 |
| LDL, mg/dl | 115.7±28.5 | 114.2±35.5 | 112.7±23.9 | 0.0483 |
| TG, mg/dl | 128.2±75.2 | 160.8±129.9 | 104±56.8 | <0.0001 |
| BG, mg/dl | 122±47.5 | 133.2±40.8 | 112.6±42.7 | 0.0001 |
| HbA1c, % | 6.30±1.06 | 6.67±0.93 | 6.00±0.91 | <0.0001 |
 | Table IIMultivariate logistic regression
analysis. |
Table II
Multivariate logistic regression
analysis.
| Variables | χ2 | OR | 95% CI | P-value |
|---|
| HDL | 1.34 | 0.9896 | 0.9713–1.0066 | 0.2469 |
| LDL | 0.37 | 1.0038 | 1.0160–0.9963 | 0.5419 |
| TG | 9.89 | 1.0088 | 1.0036–1.0146 | 0.0017 |
| BG | 2.38 | 1.0081 | 0.9984–1.0194 | 0.1228 |
| HbA1c | 1.58 | 1.3004 | 0.8650–1.9720 | 0.2084 |
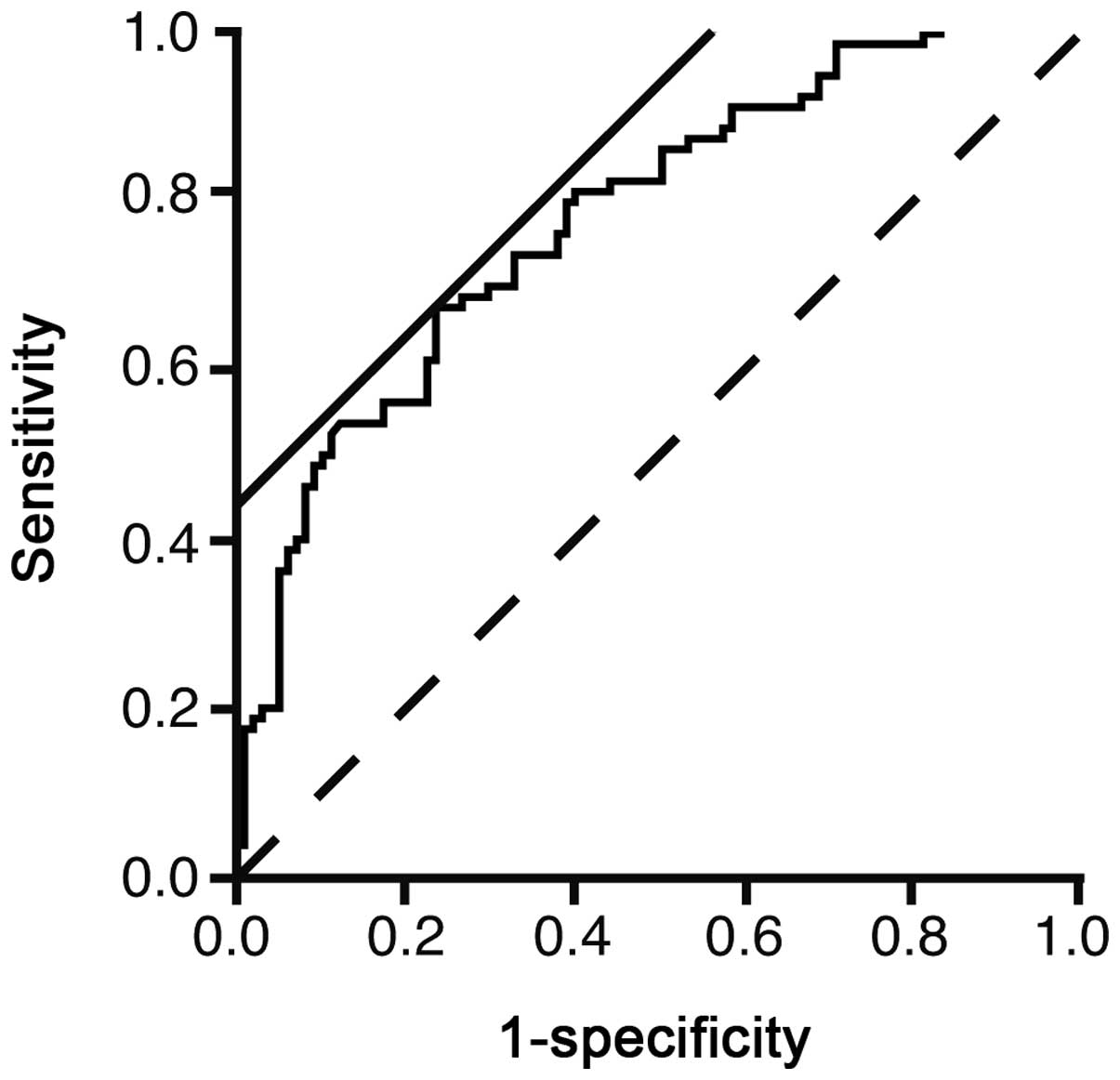
The ROC curve was illustrated to evaluate the
usefulness of the logistic model to predict NAFLD (Fig. 1). The area under the curve was
0.7739. The threshold value of ln(P/1-P) was 0.4218. The
sensitivity and specificity of the value were 66.7 and 76.5%,
respectively. However, the sensitivity and specificity did not
appear to be large enough for using in a clinical setting.
To select the parameters strongly associated with
NAFLD, stepwise analysis was performed (Table III). TG, BG and HbA1c were
strongly correlated with NAFLD.
 | Table IIIStepwise analysis of the
parameters. |
Table III
Stepwise analysis of the
parameters.
| Variables | Partial regression
coefficient | χ2 | P-value |
|---|
| HDL | 0 | 1.5603 | 0.21162 |
| LDL | 0 | 0.5523 | 0.45738 |
| TG | 0.0103 | 16.3461 | 5.28e-5 |
| BG | 0.0066 | 1.7043 | 0.19173 |
| HbA1c | 0.3189 | 2.4377 | 0.11845 |
Discussion
Hyperlipidemia is associated with NAFLD. The study
by Nakahara et al (22)
reported that hyper-LDL cholesterolemia and hypo-HDL
cholesterolemia were present in 37.5 and 19.5% of patients with
NAFLD in whom liver biopsy was performed. Hypertriglycemia is most
prevalent among patients with NAFLD. Ma et al (23) performed multivariate analysis of 949
retired elderly employees and reported that HbA1c and TG are
independent markers of NAFLD. Sung et al (24) followed healthy workers without NAFLD
for 4.4 years to observe the incidence of NAFLD. TG was
independently associated with incident NAFLD. These studies
indicate that hypertriglycemia is more associated with NAFLD
compared to hyper-LDL cholesterolemia and hypo-HDL cholesterolemia.
In the present study, TG had the largest χ2 and the
smallest P-value (Table II). The
data were consistent with the aforementioned previous studies. TG
was selected by stepwise analysis as the strongest predictor of
NAFLD in comparison with BG and HbA1c (Table III). The data indicated that TG
was more closely associated with NAFLD than BG and HbA1c.
Therefore, this may indicate that a diet high in carbohydrates may
result in increased TG accumulation in hepatocytes (25,26).
In conclusion, elevated TG was a marker of NAFLD.
References
|
1
|
Chalasani N, Younossi Z, Lavine JE, et al:
The diagnosis and management of non-alcoholic fatty liver disease:
practice Guideline by the American Association for the Study of
Liver Diseases, American College of Gastroenterology, and the
American Gastroenterological Association. Hepatology. 55:2005–2023.
2012. View Article : Google Scholar
|
|
2
|
Adams LA, Lymp JF, St Sauver J, et al: The
natural history of nonalcoholic fatty liver disease: a
population-based cohort study. Gastroenterology. 129:113–121. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dunn W, Xu R, Wingard DL, et al: Suspected
nonalcoholic fatty liver disease and mortality risk in a
population-based cohort study. Am J Gastroenterol. 103:2263–2271.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ascha MS, Hanouneh IA, Lopez R, Tamimi TA,
Feldstein AF and Zein NN: The incidence and risk factors of
hepatocellular carcinoma in patients with nonalcoholic
steatohepatitis. Hepatology. 51:1972–1978. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chatrath H, Vuppalanchi R and Chalasani N:
Dyslipidemia in patients with nonalcoholic fatty liver disease.
Semin Liver Dis. 32:22–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Musso G, Gambino R, Cassader M and Pagano
G: Meta-analysis: natural history of non-alcoholic fatty liver
disease (NAFLD) and diagnostic accuracy of non-invasive tests for
liver disease severity. Ann Med. 43:617–649. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Festi D, Schiumerini R, Marzi L, et al:
Review article: the diagnosis of non-alcoholic fatty liver disease
- availability and accuracy of non-invasive methods. Aliment
Pharmacol Ther. 37:392–400. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Miyake T, Kumagi T, Hirooka M, et al: Body
mass index is the most useful predictive factor for the onset of
nonalcoholic fatty liver disease: a community-based retrospective
longitudinal cohort study. J Gastroenterol. 48:413–422. 2013.
View Article : Google Scholar
|
|
9
|
Promrat K, Kleiner DE, Niemeier HM, et al:
Randomized controlled trial testing the effects of weight loss on
nonalcoholic steatohepatitis. Hepatology. 51:121–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abe N, Honda S and Jahng D: Evaluation of
waist circumference cut-off values as a marker for fatty liver
among Japanese workers. Saf Health Work. 3:287–293. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rafiq N, Bai C, Fang Y, et al: Long-term
follow-up of patients with nonalcoholic fatty liver. Clin
Gastroenterol Hepatol. 7:234–238. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Eguchi Y, Hyogo H, Ono M, et al:
Prevalence and associated metabolic factors of nonalcoholic fatty
liver disease in the general population from 2009 to 2010 in Japan:
a multicenter large retrospective study. J Gastroenterol.
47:586–595. 2012. View Article : Google Scholar
|
|
13
|
Haukeland JW, Konopski Z, Eggesbo HB, et
al: Metformin in patients with non-alcoholic fatty liver disease: a
randomized, controlled trial. Scand J Gastroenterol. 44:853–860.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vernon G, Baranova A and Younossi ZM:
Systematic review: the epidemiology and natural history of
non-alcoholic fatty liver disease and non-alcoholic steatohepatitis
in adults. Aliment Pharmacol Ther. 34:274–285. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nelson A, Torres DM, Morgan AE, Fincke C
and Harrison SA: A pilot study using simvastatin in the treatment
of nonalcoholic steatohepatitis: A randomized placebo-controlled
trial. J Clin Gastroenterol. 43:990–994. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alvarez F, Berg PA, Bianchi FB, et al:
International Autoimmune Hepatitis Group Report: review of criteria
for diagnosis of autoimmune hepatitis. J Hepatol. 31:929–938. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lindor KD, Gershwin ME, Poupon R, Kaplan
M, Bergasa NV and Heathcote EJ; American Associaiton for Study of
Liver Diseases. Primary biliary cirrhosis. Hepatology. 50:291–308.
2009. View Article : Google Scholar
|
|
18
|
Matsumoto T, Yamasaki S, Arakawa A, et al:
Exposure to a high total dosage of glucocorticoids produces
non-alcoholic steatohepatits. Pathol Int. 57:388–389. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khan N, Abbas AM, Whang N, Balart LA,
Bazzano LA and Kelly TN: Incidence of liver toxicity in
inflammatory bowel disease patients treated with methotrexate: a
meta-analysis of clinical trials. Inflamm Bowel Dis. 18:359–367.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sanyal AJ; American Gastroenterological
Association. AGA technical review on nonalcoholic fatty liver
disease. Gastroenterology. 123:1705–1725. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bedogni G, Miglioli L, Masutti F,
Tiribelli C, Marchesini G and Bellentani S: Prevalence of and risk
factors for nonalcoholic fatty liver disease: the Dionysos
nutrition and liver study. Hepatology. 42:44–52. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakahara T, Hyogo H, Yoneda M, et al;
Japan Study Group of Nonalcoholic Fatty Liver Disease (JSG-NAFLD).
Type 2 diabetes mellitus is associated with the fibrosis severity
in patients with nonalcoholic fatty liver disease in a large
retrospective cohort of Japanese patients. J Gastroenterol. Nov
26–2013.(Epub ahead of print).
|
|
23
|
Ma H, Xu C, Xu L, Yu C, Miao M and Li Y:
Independent association of HbA1c and nonalcoholic fatty liver
disease in an elderly Chinese population. BMC Gastroenterol.
13:32013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sung KC, Kim BS, Cho YK, et al: Predicting
incident fatty liver using simple cardio-metabolic risk factors at
baseline. BMC Gastroenterol. 12:842012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lottenberg AM, Afonso Mda S, Lavrador MS,
Machado RM and Nakandakare ER: The role of dietary fatty acids in
the pathology of metabolic syndrome. J Nutr Biochem. 23:1027–1040.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wiernsperger N: Hepatic function and the
cardiometabolic syndrome. Diabetes Metab Syndr Obes. 6:379–388.
2013. View Article : Google Scholar
|