Introduction
Tuberculosis (TB) is the result of infection with
Mycobacterium tuberculosis (M. tuberculosis) and is a
significant cause of morbidity and mortality worldwide. Each year
>9 million people are infected by TB and >1.7 million succumb
to TB annually (1). The incidence
of TB in Iran has been reported as 13.7 per 100,000 in 2009;
however, its incidence was higher in the Sistan-Balouchestan
province, southeastern Iran. The higher incidence is due to
bordering with Afghanistan and Pakistan; two countries with a high
TB prevalence (2). Cell-mediated
immunity is essential for suppression of Mycobacterial
infection as it is an intracellular parasite (3). The fact that only 10% of those
infected with M. tuberculosis progress to clinical disease
revealed that genetic factors, as well as environmental factors are
involved in the pathophysiology of TB (4).
In addition, the host genetic basis of TB has been
confirmed by twin studies that indicated a two times higher risk of
disease in identical twins compared to non-identical twins
(5).
Several genes have been found to play a role in TB
susceptibility and the relative significance of these genes in
disease progression or various forms of disease is often modified
by the ethnicity in different populations (6).
The active form of vitamin D, 1-25-dihydroxyvitamin
D3, is an important hormone that modulates the activity
of different defense and immune cells, including lymphocytes,
monocytes, macrophages and epithelial cells (7). Since vitamin D3 increases
phagocytosis via the activation of macrophages and affects immune
response, it is potentially involved in the development of several
diseases (8). Vitamin D3
may limit the growth of M. tuberculosis in macrophages
(7). Vitamin D3 exerts
its effects through the vitamin D receptor (VDR) and regulates
numerous target genes by binding to its nuclear receptor. Active
VDR binds to vitamin D response elements that are located in the
promoter region of target genes and controls the transcription of
these genes (9). The VDR
gene is located in chromosome 12cen-q12, including at least five
promoter regions, eight exons that code proteins and six
untranslated exons, which are alternatively spliced. Since there
are several polymorphisms in the VDR gene that may affect
VDR activity, those polymorphisms have been known as potential
candidates for genetic susceptibility to TB (10, 11).
The Fok1 polymorphism (rs2228570) of the
VDR gene, which is located in the translation initiation
start site, produces two versions of the VDR protein with different
lengths (three amino acids). The short protein, which is encoded by
the ‘F’ allele, is more active than the longer one.
Additionally, other studies have presented several polymorphisms in
strong linkage disequilibrium (LD) in the 3′ untranslated region
(3′UTR) of the VDR gene, including Taq1 (rs731236),
Bsm1(rs154410) and Apa1(rs7975232). Polymorphisms can
be detected by restriction fragment length polymorphism (RFLP).
This region of the VDR gene regulates gene expression.
Therefore, the polymorphisms that are located in this region may
influence VDR activity (12). Thus,
the present study was designed to evaluate the possible role of the
VDR Fok1, Taq1 and Bsm1 polymorphisms and haplotypes
on pulmonary TB (PTB) susceptibility in a local population of
southeastern Iran.
Materials and methods
Patient selection
The case-control study was conducted prospectively
at a university-affiliated hospital (Boo-Ali Hospital, Zahedan,
southeastern Iran). The hospital is a referral center for TB. The
study was conducted between March 2010 and May 2011 and a total of
120 patients were selected. Diagnosis of pulmonary TB was made by
clinical findings; positive sputum smear for acid-fast bacilli and
the results of chest X-ray, but only patients who were confirmed by
culture were included in the study. Patients affected with other
diseases or conditions, such as myocardial infarction, cirrhosis,
acute pancreatitis and septic shock, were excluded from the study.
A total of 131 normal healthy subjects who underwent the physical
examination at Boo-Ali Hospital were recruited during the study
period and were matched for age, gender, ethnicity and geographical
origin to patients. The inclusion criteria for normal healthy
subjects were absence of clinical symptoms and signs suggestive of
active PTB and had a normal chest X-ray. No medical history of TB
or other infectious diseases, autoimmune diseases, cancer or other
diseases that affect host immunity were observed in the control
group. C reactive protein (CRP) was measured for the control group
and only negative CRP results were used in the final analysis. The
Dean for research affairs of the University Ethics Committee
approved the protocol prior to commencing the study.
DNA extraction
Genomic DNA was extracted from 200 µ1 of peripheral
blood in EDTA using the DNA extraction kit (Roche Diagnostics,
Mannheim, Germany).
Genotyping of VDR Fok1, Taq1 and Bsm1
polymorphisms
Genotypes were detected using a polymerase chain
reaction-restriction fragment length polymorphism (PCR-RFLP). The
primer sequences, annealing temperature, restriction enzymes and
fragments sizes are shown in Table
I. PCR was performed in a 25 µ1 final volume containing 25 pmol
of each primer, 0.1 mmol/1 dNTP (Fermentas, Lithuania), 0.3 µg
genomic DNA, 1.5 mmol/1 MgCl2, 2.5 µ1 10X PCR buffer and
1.5 units Taq DNA polymerase (Fermentas), according to the
following protocol: Initial denaturation at 94˚C for 4 min; 30
cycles of denaturation at 94˚C for 45 sec, annealing for 30 sec and
extension at 72˚C for 45 sec; and final extension at 72˚C for 5
min. The PCR products were digested overnight with Fok1,
Taq1 and Bsm1 restriction endonucleases (Fermentas)
and visualized in 2.5% agarose gel electrophoresis.
 | Table I.Primer sequences, annealing
temperature, restriction enzymes and fragment sizes of the
vitaminD receptor gene polymorphisms. |
Table I.
Primer sequences, annealing
temperature, restriction enzymes and fragment sizes of the
vitaminD receptor gene polymorphisms.
| Target
sequence | Primer
sequence | Annealing
temperature, °C | PCR product | RFLP fragments | Refs |
|---|
| Fok1 | F:
5′-AGCTGGCCCTGGCACTGACTCTGGCT-3′ | 57 | 267 | F(C):
305 | |
| R:
5′-ATGGAAACACCTTGCTTCTTCTCCCTC-3′ | | | f(T): 115,
190 | 11 |
| Taq1 | F:
5′-GGGACGATGAGGGATGGACAGAGC-3′ | 61 | 716 | T(T): 512,
204 | |
| R:
5′-GGAAAGGGGTTAGGTTGGACAGGA-3′ | | | t(C): 311,
201, 204 | 11 |
| Bsm1 | F:
5′-AACTTGCATGAGGAGGAGCATGTC-3′ | 61 | 813 | B(A):
813 | |
| R:
5′-GGAGAGGAGCCTGTGTCCCATTTG-3′ | | | b(G): 335,
478 | 11 |
The presence and absence of a restriction site were
assigned a lowercase and uppercase letter, respectively (a
and A for Apa1, t and T for Taq1, f and
F for Fok1, b and B for Bsm1).
Statistical analysis
The statistical analysis of the data was performed
using SPSS software for Windows, version 20 (SPSS, Inc., Chicago,
IL, USA). The differences between the groups were analyzed by
independent sample t-test, χ2 test or Fisher's exact
test, as appropriate. The χ2 test was used for deviation
of genotype distribution from the Hardy-Weinberg equilibrium.
Allele frequencies were calculated by the gene counting method. The
odds ratio (OR) and 95% confidence interval (CI) for each variable
were also estimated. The frequency of haplotypes was calculated
using PHASE software, version 2.1 (13). Logistic regression analysis was used
to assess the independent effect of each risk polymorphism and
haplotypes on TB. Bonferroni's post hoc correction was applied to
confirm the association of haplotypes with the disease. A two-sided
significance level of 0.05 was considered to indicate a
statistically significant difference. The computation of LD between
single-nucleotide polymorphisms (SNPs) was estimated using the
normalized measure of allelic association D' and the
characterization of these patterns was determined using Haploview
software, version 4.2 (http://www.broad.mit.edu/mpg/haploview).
Results
Patient characteristics
The demographic and clinical characteristics of PTB
patients and controls are shown in Table II. There was no statistically
significant difference in gender, age and ethnic characteristics in
the patients compared to the control subjects. The frequency of
smokers was significantly higher in the PTB patients compared to
controls (47 vs. 35; P=0.0001).
 | Table II.Demographic characteristics of
pulmonary tuberculosis (PTB) patients and controls. |
Table II.
Demographic characteristics of
pulmonary tuberculosis (PTB) patients and controls.
| Variables | PTB n=120 | Controls n=131 | P-value | OR (95%CI) |
|---|
| Age, years | 51.5±19.7 | 48.1±12.2 | 0.1 | |
| Gender (M/F) | 45/75 | 38/93 | 0.09 | |
| Smoking, n (%) | 47 (39) | 35 (27) | 0.0001 | 3.2 (1.8-5.8) |
| Race, n (%) | | | 0.1 | |
|
Persian | 46 (38) | 34 (26) | | |
|
Balouch | 70 (59) | 90 (69) | | |
|
Afghan | 4 (3) | 7 (5) | | |
Genotype frequencies
The genotype and allele frequencies of VDR
polymorphisms in PTB patients and healthy controls are shown in
Table III. All loci conformed to
the Hardy-Weinberg equilibrium in the patient and control groups
(P>0.05).
 | Table III.Genotype and allele frequencies of
vitaminD receptor (VDR) gene polymorphisms in pulmonary
tuberculosis (PTB) patients and healthy controls. |
Table III.
Genotype and allele frequencies of
vitaminD receptor (VDR) gene polymorphisms in pulmonary
tuberculosis (PTB) patients and healthy controls.
| VDR
polymorphisms | PTB, n=120 (%) | Controls, n=131
(%) | P-value | OR (95% CI) | P-value | ORa (95% CI) |
|---|
| Fok1 |
|
FF | 65 (54) | 93 (71) | | 1 | | |
|
Ff | 44 (37) | 31 (24) | 0.013 | 2.0 (1.2-3.6) | 0.015 | 2.0 (1.2-3.6) |
|
ff | 11 (9) | 7 (5) | 0.1 | 1.5 (0.9-2.5) | 0.08 | 1.6 (1-2.6) |
| Ff +
ff | 55 (46) | 38 (29) | 0.006 | 2.1 (1.2-3.5) | 0.006 | 2.1 (1.2-3.6) |
|
F | 174 (73) | 217 (83) | | 1 | | − |
|
f | 66 (27) | 45 (17) | 0.006 | 1.8 (1.2–2.8) | | |
|
Taq1 |
|
TT | 52 (43) | 67 (51) | | 1 | | |
|
Tt | 54 (45) | 50 (38) | 0.2 | 1.4 (0.8-2.4) | 0.17 | 1.5 (0.9-2.5) |
|
tt | 14 (12) | 14 (11) | 0.6 | 1.1 (0.8-1.7) | 0.5 | 1.2 (0.8-1.8) |
|
Tt+tt | 68 (57) | 64 (49) | 0.2 | 1.4 (0.8-2.3) | 0.2 | 1.4 (0.9-2.3) |
|
T | 158 (66) | 184 (70) | | 1 | | − |
|
t | 82 (34) | 78 (30) | 0.3 | 1.2 (0.8-1.8) | | − |
|
Bsm1 |
|
BB | 31 (26) | 39 (30) | | 1 | | |
|
Bb | 66 (55) | 70 (53) | 0.6 | 1.2 (0.7-2.1) | 0.7 | 1.1 (0.6-2) |
|
bb | 23 (19) | 22 (17) | 0.5 | 1.2 (0.8-1.7) | 0.4 | 1.2 (0.8-1.7) |
|
Bb+bb | 89 (74) | 92 (70) | 0.5 | 1.2 (0.7-2.1) | 0.6 | 1.2 (0.7-2.1) |
|
B | 128 (53) | 148 (57) | | 1 | | |
|
b | 112 (47) | 114 (43) | 0.5 | 1.1 (0.8-1.6) | | |
The frequency of the VDR Ff genotype was
significantly higher in PTB patients compared to controls and the
PTB risk was two times higher in individuals with Ff
genotype prior and subsequent to adjustment for age, gender,
smoking and ethnicity.
However, the frequency of the ff genotype was
not different between the two groups prior and subsequent to
adjustment for age, gender, smoking and ethnicity. A higher
frequency of the f allele was observed in TB patients and
the f allele may be a risk factor for PTB predisposition
(OR, 1.8; 95% CR, 1.2–2.8; P=0.006). These findings showed that
there were no significant difference regarding VDR Bsm1 and
Taq1 polymorphisms among the PTB patients and control
group.
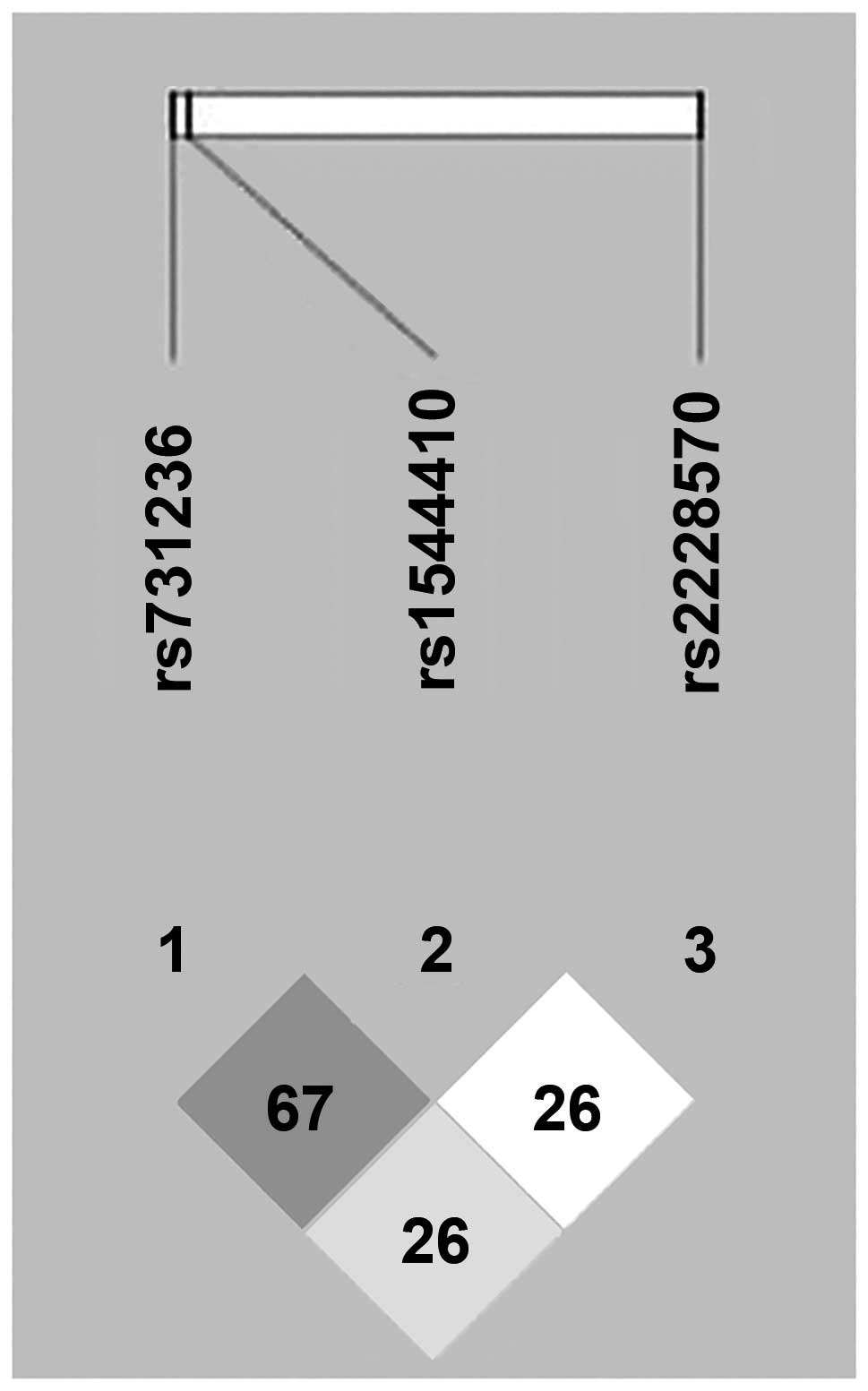
The LD patterns of the three VDR SNPs are
shown in Fig. 1. The frequency of
seven common haplotypes of the three VDR SNPs
[Fok1(C/T), Taq1(T/C) and
Bsm1(A/G)] are shown in Table IV. The frequency of f-T-B
and f-t-b haplotypes was significantly higher in PTB
patients compared to controls and haplotype-based association
analysis revealed that the f-T-B and f-t-b haplotypes
may have the potential to increase PTB susceptibility (OR, 1.3; 95%
CR, 1.1-1.5; P=0.014 and OR, 1.1; 95% CR, 1–1.2; P=0.012
respectively). The association was also statistically significant
following post hoc Bonferroni's correction.
 | Table IV.Haplotypes frequency of vitaminD
receptor gene polymorphisms in pulmonary tuberculosis (PTB)
patients and controls. |
Table IV.
Haplotypes frequency of vitaminD
receptor gene polymorphisms in pulmonary tuberculosis (PTB)
patients and controls.
| Fok1 | Taq1 | Bsm1 | PTB % | Control % | P-value | OR (95% CI) |
|---|
| F | T | B | 39.2 | 47.7 | | 1 |
| F | T | b | 15.8 | 14.9 | 0.3 | 1.3 (0.8-2.2) |
| F | t | B | 4.2 | 4.2 | 0.7 | 1.1 (0.7-1.7) |
| F | t | b | 13.3 | 16 | 0.96 | 1.0 (0.8-1.2) |
| f | T | B | 9.6 | 4.6 | 0.014 | 1.3 (1.1-1.5) |
| f | T | b | 1.2 | 3.1 | 0.3 | 0.9 (0.7-1.1) |
| f | t | b | 16.2 | 9.5 | 0.012 | 1.1 (1-1.2) |
Discussion
TB is a global health problem and its incidence is
not the same in different countries, ethnic groups and populations.
Much evidence supports an important role for host genetic
variations in the predisposition to TB, therefore, the combination
effect of genetic and environmental factors may influence the
development of TB (14). In
addition, there is other evidence that emphasizes the variations in
ethnicity for the susceptibility to TB (15). Different candidate genes have been
examined in associated studies to evaluate the identity of the TB
‘susceptibility factors,’ including human leukocyte antigen
(16, 17), natural-resistance-associated
macrophage protein 1 (16, 17), VDR (11, 16),
cluster of differentiation 14 (18), interleukins (19) and Toll-like receptors (20).
Several studies have reported a higher frequency of
vitamin D deficiency among TB patients and high doses of vitamin D
were extensively used for TB treatment (11, 21).
In vivo studies showed that vitamin D suppressed
intracellular growth of M. tuberculosis (22). Cathelicidin expression, which is the
first line of defense in patients, is induced by vitamin D
(23).
The active form of vitamin D can lead to macrophage
activation and subsequently limit the intracellular growth of M.
tuberculosis. This vitamin exerts its effect via binding to VDR
in the monocytes, therefore the VDR gene polymorphisms are
suggested to be involved in genetic susceptibility to TB (24). The association between the VDR
polymorphisms and TB susceptibility has been studied in different
populations and the results were contradictory (10, 25–27).
In the present study, a higher frequency of the
VDR Ff genotype of the Fok1 polymorphism was observed
in the patients compared to the controls. Therefore, this genotype
may be considered as a genetic risk factor for the PTB
susceptibility. Additionally, the presence of the Fok1
mutated allele (f allele), either in the heterozygous or
homozygous state, increased the disease risk.
There were no associations between the VDR
Taq1 and Bsm1 polymorphisms and PTB. The frequency of
the f-T-B and f-t-b haplotypes of the VDR
Fok1(C/T), Taq1(T/C) and
Bsm1(A/G) polymorphisms were significantly higher in
PTB patients.
An association between 25-hydroxycholecalciferol
deficiency and occurrence of TB among the Gujarati Asian population
in west London has been reported previously. In addition, a
significant interaction between the vitamin D status and
Fok1 and Taq1 polymorphisms and TB was observed
(11).
There was an association between ff genotype of
Fok1 but not Taq1 polymorphism and susceptibility to
PTB in Chinese Han population (25).
Although there has not been any reported association
between the VDR Taq1 and Fok1 polymorphisms and PTB
susceptibility in Peru, an association between the VDR gene
polymorphism and response to treatment of PTB has been observed
(26). In a case control study in
West Africa, no association between TB and the VDR Fok1, Bsm1,
Apa1 and Taq1 variants was reported; however, the
FA haplotype of the Fok1 and Apa1
polymorphisms was correlated with TB susceptibility (10). Although the study by Lombard et
al (28) did not report any
correlation between the VDR Fok1, Bsm1, Apa1 and Taq1
polymorphisms and TB, the F-b-A-T haplotype was observed as
a protective factor for TB in South Africa.
Similar to the results of the present study, the
association between the Fok1 polymorphism, but not the
Taq1 polymorphism, of the VDR gene with PTB has been
observed in the Chinese Tibetan population (29).
The results of Alagarasu et al (30) indicated that the b-A-T
haplotype of the 3′UTR VDR gene played a protective role
against human immunodeficiency virus (HIV) infection, whereas the
B-A-t haplotype may be associated with susceptibility to the
development of TB in HIV-1-infected patients.
In contrast to the results of the present study,
Banoei et al (31) revealed
that the tt and bb genotypes of the VDR Taq1
and Bsm1 polymorphisms are associated with the
predisposition to PTB in an Iranian population. In another study,
Merza et al (32) also
confirmed the association of the VDR Bsm1 (Bb + bb)
polymorphism and PTB in a local Iranian population.
In a meta-analysis that was performed on 23 studies
in 2010, an association between the Fok1 ff genotype and TB
has been observed among the Asian population (OR, 2.0; 95% CR,
1.3-3.2). Additionally a significant inverse association was
observed for the Bsm1 bb genotype (OR, 0.5; 95% CR,
0.4-0.8). There were no associations between these polymorphisms
and TB among the African or South American populations (27). The association between the VDR
Fok1 polymorphism and extra-PTB and spinal TB has been reported
in American and Chinese Han populations, respectively (33, 34).
In another study, no correlation between the
Taq1, Bsm1 and Fok1 polymorphisms were found for host
susceptibility to human TB in the Korean population (35).
Consistent with the findings of the present study, a
higher frequency of the Fok1 Ff and ff genotypes in
TB patients has been reported in the Chinese Kazak population.
There were no significant differences of the Taq1-Tt and
tt genotype frequencies between TB patients and healthy
controls (16).
Although the reason for this discrepancy remains
unclear, these different results in the association studies are
common and may be due to the different genetic background of
various populations, different selection criteria adopted for
patients and controls in particular clinical presentation and
environmental risk factors.
There were certain limitations in the present study,
such as a small sample size and different ethnic groups (Fars and
Balouch) existing in southeast Iran. Therefore, further
investigations using a larger sample size and different ethnic
groups are necessary to confirm the present results.
In conclusion, the results showed that the VDR
Ff genotype and f allele of the Fok1 polymorphism
was associated with PTB susceptibility. There were no associations
between the VDR Taq1 and Bsm1 polymorphisms and PTB.
In addition, the frequency of the f-T-B and f-t-b
haplotypes was significantly higher in the PTB patients.
Acknowledgements
The present study was extracted from the Master of
Science thesis (Comparison of VDR gene polymorphisms frequency in
PTB patients and healthy controls. Zahedan University of Medical
Sciences. Registered no. 1312) at Zahedan University of Medical
Sciences. The authors would like to thank the Deputy of Research
Affairs at the University for funding this project.
References
|
1
|
Global tuberculosis control, . WHO report
2010. World Health Organization Geneva, Switzerland: 2010,
(WHO/HTM/TB/2010.7).
|
|
2
|
Centers for Disease Control and
Prevention, . Reported tuberculosis in Sistan and Baluchestan.
Iran. CDC; Atlanta, GA: 2009
|
|
3
|
Flynn JL and Chan J: Immunology of
tuberculosis. Annu Rev Immunol. 19:93–129. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Casanova JL and Abel L: Genetic dissection
of immunity to mycobacteria: the human model. Annu Rev Immunol.
20:581–620. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Comstock GW: Tuberculosis in twins: a
re-analysis of the Prophit survey. Am Rev Respir Dis. 117:621–624.
1978.PubMed/NCBI
|
|
6
|
Blackwell JM: Genetics and genomics in
infectious disease susceptibility. Trends Mol Med. 7:521–526. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bellamy R, Ruwende C, Corrah T, et al:
Tuberculosis and chronic hepatitis B virus infection in Africans
and variation in the vitamin D receptor gene. J Infect Dis.
179:721–724. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bar-Shavit Z, Noff D, Edelstein S, Meyer
M, Shibolet S and Goldman R: 1,25-dihydroxyvitamin D3 and the
regulation of macrophage function. Calcif Tissue Int. 33:673–676.
1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Haussler MR, Haussler CA, Bartik L, et al:
Vitamin D receptor: molecular signaling and actions of nutritional
ligands in disease prevention. Nutr Rev. 66 (Suppl 2):S98–S112.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bornman L, Campbell SJ, Fielding K, et al:
Vitamin D receptor polymorphisms and susceptibility to tuberculosis
in West Africa: a case-control and family study. J Infect Dis.
190:1631–1641. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wilkinson RJ, Llewelyn M, Toossi Z, et al:
Influence of vitamin D deficiency and vitamin D receptor
polymorphisms on tuberculosis among Gujarati Asians in west London:
a case-control study. Lancet. 355:618–621. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Uitterlinden AG, Fang Y, Van Meurs JB,
Pols HA and Van Leeuwen JP: Genetics and biology of vitamin D
receptor polymorphisms. Gene. 338:143–156. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scheet P and Stephens M: A fast and
flexible statistical model for large-scale population genotype
data: applications to inferring missing genotypes and haplotypic
phase. Am J Hum Genet. 78:629–644. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mathema B, Kurepina NE, Bifani PJ and
Kreiswirth BN: Molecular epidemiology of tuberculosis: current
insights. Clin Microbiol Rev. 19:658–685. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hoal EG: Human genetic susceptibility to
tuberculosis and other mycobacterial diseases. IUBMB Life.
53:225–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu F, Zhang W, Zhang L, et al: NRAMP 1,
VDR, HLA-DRB 1, and HLA-DQB1 gene polymorphisms in susceptibility
to tuberculosis among the Chinese Kazakh population: a case-control
study. Biomed Res Int. 2013:4845352013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bellamy R, Ruwende C, Corrah T, McAdam KP,
Whittle HC and Hill AV: Variations in the NRAMP1 gene and
susceptibility to tuberculosis in West Africans. N Engl J Med.
338:640–644. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Alavi-Naini R, Salimi S, Sharifi-Mood B,
Davoodikia AA, Moody B and Naghavi A: Association between the CD14
gene C-159T polymorphism and serum soluble CD14 with pulmonary
tuberculosis. Int J Tuberc Lung Dis. 16:1383–1387. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Trajkov D, Trajchevska M, Arsov T, et al:
Association of 22 cytokine gene polymorphisms with tuberculosis in
Macedonians. Indian J Tuberc. 56:117–131. 2009.PubMed/NCBI
|
|
20
|
Jahantigh D, Salimi S, Alavi-Naini R,
Emamdadi A, Owaysee Osquee H and Farajian Mashhadi F: Association
between TLR4 and TLR9 gene polymorphisms with development of
pulmonary tuberculosis in Zahedan, southeastern Iran. Scientific
World Journal. 2013:5340532013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nursyam EW, Amin Z and Rumende CM: The
effect of vitamin D as supplementary treatment in patients with
moderately advanced pulmonary tuberculous lesion. Acta Med Indones.
38:3–5. 2006.PubMed/NCBI
|
|
22
|
Rockett KA, Brookes R, Udalova I, Vidal V,
Hill AV and Kwiatkowski D: 1,25-Dihydroxyvitamin D3 induces nitric
oxide synthase and suppresses growth of Mycobacterium tuberculosis
in a human macrophage-like cell line. Infect Immun. 66:5314–5321.
1998.PubMed/NCBI
|
|
23
|
Gombart AF: The vitamin D-antimicrobial
peptide pathway and its role in protection against infection.
Future Microbiol. 4:1151–1165. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Herr C, Greulich T, Koczulla RA, et al:
The role of vitamin D in pulmonary disease: COPD, asthma,
infection, and cancer. Respir Res. 12:312011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu W, Cao WC, Zhang CY, et al: VDR and
NRAMP1 gene polymorphisms in susceptibility to pulmonary
tuberculosis among the Chinese Han population: a case-control
study. Int J Tuberc Lung Dis. 8:428–434. 2004.PubMed/NCBI
|
|
26
|
Roth DE, Soto G, Arenas F, et al:
Association between vitamin D receptor gene polymorphisms and
response to treatment of pulmonary tuberculosis. J Infect Dis.
190:920–927. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gao L, Tao Y, Zhang L and Jin Q: Vitamin D
receptor genetic polymorphisms and tuberculosis: updated systematic
review and meta-analysis. Int J Tuberc Lung Dis. 14:15–23.
2010.PubMed/NCBI
|
|
28
|
Lombard Z, Dalton DL, Venter PA, Williams
RC and Bornman L: Association of HLA-DR, -DQ, and vitamin D
receptor alleles and haplotypes with tuberculosis in the Venda of
South Africa. Hum Immunol. 67:643–654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen XR, Feng YL, Ma Y, et al: Study on
the association of two polymorphisms of the vitamin D receptor
(VDR) gene with the susceptibility to pulmonary tuberculosis (PTB)
in Chinese Tibetans. Sichuan Da Xue Xue Bao Yi Xue Ban. 37:847–851.
2006.(In Chinese). PubMed/NCBI
|
|
30
|
Alagarasu K, Selvaraj P, Swaminathan S,
Narendran G and Narayanan PR: 5′ regulatory and 3′ untranslated
region polymorphisms of vitamin D receptor gene in south Indian HIV
and HIV-TB patients. J Clin Immunol. 29:196–204. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banoei MM, Mirsaeidi MS, Houshmand M, et
al: Vitamin D receptor homozygote mutant tt and bb are associated
with susceptibility to pulmonary tuberculosis in the Iranian
population. Int J Infect Dis. 14:e84–e85. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Merza M, Farnia P, Anoosheh S, et al: The
NRAMPI, VDR and TNF-alpha gene polymorphisms in Iranian
tuberculosis patients: the study on host susceptibility. Braz J
Infect Dis. 13:252–256. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Motsinger-Reif AA, Antas PR, Oki NO, Levy
S, Holland SM and Sterling TR: Polymorphisms in IL-1beta, vitamin D
receptor Fok1, and Toll-like receptor 2 are associated with
extrapulmonary tuberculosis. BMC Med Genet. 11:372010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang HQ, Deng A, Guo CF, et al:
Association between FokI polymorphism in vitamin D receptor gene
and susceptibility to spinal tuberculosis in Chinese Han
population. Arch Med Res. 41:46–49. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kang TJ, Jin SH, Yeum CE, et al: Vitamin D
receptor gene TaqI, BsmI and FokI polymorphisms in Korean patients
with tuberculosis. Immune Netw. 11:253–257. 2011. View Article : Google Scholar : PubMed/NCBI
|