Introduction
The database from the American Cancer Society (ACS)
provides a summary of the current ACS cancer screening rates. Aside
from skin cancer, the major incidence of cancer in 2015 was
reported as cancer of the female breast, prostate cancers in males,
and colorectal and lung cancer (1),
and according to the study of Bray et al (2) reported in Lancet Oncology, the incidence
of the total cancer cases will increase from 12.7 million new cases
in 2008 to 22.2 million by 2030. Therefore, cancer specialists have
determined that cancer is a life-threatening disease for future
generations (3). Therefore, it is
necessary to implement national cancer control plans to reduce the
burden of cancer risk factors. Increasing attention has been paid
to improve the palliative care and pain relief available to
patients. Even though the skills of surgery, radiotherapy and
chemotherapy, targeted therapy and immunotherapy have significantly
improved, an increasing number of patients are selecting
naturopathic therapy with traditional Chinese medicine (TCM), as
they are considered to be multicomponents and multitarget agents
exerting their therapeutic function in a more holistic way to the
whole body system via restoration of the normal balance and flow in
the body, which is believed to strengthen and enhance the
endogenous resistance of the body to disease and individualization
of therapy. In addition, the approval of three new herbal
medicine-derived drugs (ixabepilone, trabectedin and temsirolimus)
(4) in 2007 instigated the
consideration of herbal medicines as a source for innovative
antitumor-targeted therapeutic agents. Worldwide, including Western
countries, TCM has been more accepted for its efficacy in
preventing and treating cancer. Investigations of targeted therapy
with herbal medicines will potentially provide future discoveries
in oncological research. It is estimated that the United States
National Cancer Institute spends ~120 million USD each year on
TCM-related research (5). While, based
on the foundation of the system research of ‘omics’ approach
(6), the occurrence of cancer is known
to be based on various factors, several phases, multigenes and
complicated signaling pathways (7,8). Cancer is
considered a chronic health problem, similar to hypertension and
diabetes by the World Health Organization, and therefore, the
optimal effects of treatment involve improving life quality,
prolonging survival time and alleviating side effects. Therefore,
the concept of ‘survival with cancer’ has been suggested (9). The following is the evidence reported in
preclinical or clinical trials that demonstrates the mechanism and
efficacy of medicinal plants used as adjuvant treatment for cancer
patients (also shown in Table I).
 | Table I.Chinese medicines commonly used in
cancer treatment. |
Table I.
Chinese medicines commonly used in
cancer treatment.
| Active
components | Tumor name | Efficacy according
to bio-molecular levels | Preclinical or
clinical evidence of anticancer activity |
|---|
| Extract of
Hedyotis Diffuse Willd. | Human colon
carcinoma cell | Induced cell
apoptosis | Induced cell
morphological changes, reduced cell viability, caused DNA
fragmentation, loss of plasma membrane asymmetry, collapse of the
mitochondrial membrane, activated caspase-9 and caspase-3 |
| Oxymatrine (extract
from Sephora Flavescent Ait) | Human pancreatic
cancer, (PANC-1 cell), human gastric cancer cell | Induced cell
apoptosis, inhibited tumor metastasis | Inhibited cell
viability, induced cell apoptosis, downregulated the survivin
genes, upregulated the ratio of Bax/Bcl-2, released cytochrome
c and activated caspase-3 proteins. Suppressed cell
proliferation, decreased phosphorylation of EGFR (Tyr845), Cofilin
(Ser3) and LIMK1 (Thr508) |
| Tubeimu of TCM | Human breast cancer
cell (MDA-MB-453) | Induced cell
apoptosis | Induced cell
apoptosis and DNA fragmentation |
| Paris
saponin VII extracted from Trillium tschonoskii
Maxim | MCF-7/ADR
cells | Reversed multidrug
resistance, induced cell apoptosis | Suppressed cell
viability, as well as triggered apoptosis and drug resistance of
MCF-7/ADR cells, increased the expression of TNFR1, TRAILR1/DR4,
TRAIL R2/DR5 and FADD, and activated PARP, caspase-8 and caspase-3.
Reduced P-glycoprotein (P-gp) expression |
| Emodin azide
methylanthraguinone derivative | Human breast cancer
cells | Induced cell
apoptosis, inhibited angiogenesis, tumor metastasis, reduced
toxicity | Arrested cell
cycle, induced cell apoptosis, promoted hypoxia-inducible
factor-1α, glutathione-S-transferase P and N-acetyltransferase
expression, induced glutathione phase I and II detoxification
enzymes to inhibit angiogenesis, invasion, migration and
chemical-induced carcinogen-DNA adduct formation and inhibit the
activity of HER2/neu, CK2 kinase and p34cdc2 kinase |
| Capsaicin and
gingerol | KB-C2 cells | Reversed multidrug
resistance | Enhanced P-gp to
anti-drug efflux transporter and increased the cytotoxicity of
vinblastine |
| Germacrone (the
main component of Rhizma Curcuma) | Human breast cancer
cells (MCF-7/ADR) | Reversed multidrug
resistance, induced cell apoptosis | Germacrane and ADR
increased the cytotoxicity compared to ADR alone and promoted cell
apoptosis, downregulated the expression of anti-apoptotic proteins,
p53 and Bax, inhibited the expression of P-gp by the inhibition of
the multidrug resistance 1 (MDR1) gene promoter |
| Five tanshinones
(including cryptotanshine and dihydrotanshine) isolated from
Salvia miltiorrhiza (danshen) | SW620 AD300 colon
cancer cells | Reversed multidrug
resistance, enhanced efficacy | Decreased the
digoxin efflux ratio, enhanced the cytotoxicity of doxorubicin and
irinotecan in P-gp overexpressing SW620 AD300 colon cancer cells
via increasing the intracellular accumulation of the P-gp substrate
anticancer drugs, downregulated P-gp mRNA and protein expression
and inhibited P-gp ATPase activity |
| Quinolones,
indoloquinazoline alkaloids derived from Evodia
ruaecarpa | CEM/ADR5000
leukemia, porcine brain capillary endothelial cells | Reversed multidrug
resistance | Alleviated the
activity of P-gp modulators |
| Total extract of
Carthami Flos | MDR KB-V1
cells | Reversed multidrug
resistance and enhanced efficacy | Showed a function
of drug resistance, enhanced their chemosensitivities and produced
a general synergism in the cytotoxic effect |
| Ethanol extract of
Scutellaria barbata | Colon cancer cells,
CRC mouse xenograft model | Induced cell
apoptosis, inhibited angiogenesis and a signaling pathway | Induced cell
apoptosis, inhibited angiogenesis in vivo, reduced tumor
size without affecting body weight, suppressed the expression of
key mediators of the pathway in tumor tissues, inhibited the
expression of the target gene of the SHH signaling pathway,
vascular endothelial growth A (VEGF-A) |
| Xiaotansanjie
decoction | GCSCS | Inhibited
angiogenesis and tumor growth, induced cell apoptosis | Regulated the
expression of Notch-1, Hes1, VEGF and ki-67, inhibited the cell
viability in vivo, inhibited the expression of i Notch-1 and
Hes1 to inhibit tumor growth |
| Baicalin | MDA-MB −231
cells | Inhibited
metastasis and a signaling pathway | Inhibited cell
migration and invasion, suppressed tumor growth and the pulmonary
metastasis via blocking the p38 mitogen-activated protein kinase
(MAPK) activity and activated the inhibitor of p38 MAPK to decrease
the expression of matrix metalloproteinase (MMP)-2, MMP-9, uPA and
uPAR through the P38-MAPK signaling pathway |
| Pien Tze Huang | Mouse model of
colorectal cancer | Inhibited tumor and
angiogenesis, suppressed signaling pathways | Reduced the
expression of angiogenic factors, including inducible nitric oxide
synthase (iNOS), endothelial NOS, VEGF-A, basic fibroblast growth
factor (bFGF), as well as their specific receptors, VEGFR2 and
bFGFR, to inhibit tumor angiogenesis via suppression of multiple
signaling pathways, such as STAT3, AKT and MAPKs |
| Ganoderic acid | Breast cancer
cells | Induced cell
apoptosis, inhibited tumor angiogenesis and metastasis to suppress
a signaling pathway | Suppressed cell
growth, tumor angiogenesis and invasion, stimulated Me (GA-Me) the
production of tumor necrosis factor-α (TNF-α), downregulated the
expression of various nuclear factor-κB (NF-κB) regulated genes
[C-myc, cyclinD, Bcl-2, MMP-9, VEGF, interleukin (IL)-6 and IL-8]
to modulate the NF-κB signaling pathway |
| TPA and
saikosaponin α | HepG2 cell | Suppressed
signaling pathway to inhibit angiogenesis | Induced the
phosphorylation of ERK, JNK and c-jun by activating the downstream
transcriptional factors of the MAPK cascade (ATF2, c-jun, jun-B and
c-fos), decreased the efficacy of P15(INK4b)/p16(INK4a) RNAs, c-fos
RNA protein and AP-1-related DNA-binding activity |
| Nobiletin | Human gastric
adenocarcinoma AGS cells, | Inhibited
angiogenesis by suppressing signaling pathways to inhibit tumor
metastasis | Inhibited FAK and
Pi3K/Akt activation to downregulate the enzyme activities, protein
expression and messenger RNA levels of MMP-2 and MMP-9, Ras, C-Raf,
Rac-1, Cdc42 and RhoA, increased the protein level of RhodB,
decreased the phosphorylation and degradation of IκΒα and NF-κΒ to
inhibit the cell adhesion and invasion |
| Gegen Quinlian
decoction | Human renal
carcinoma cells | Inhibited
angiogenesis and metastasis | Suppressed the
enzyme activity of MMP2 to inhibit the expansion and
neoangiogenesis of cancer |
| Astragalus
polysaccharides | S180
sarcoma-bearing mice | Enhanced
immunomodulatory activities | Increased the
IFN-α, FasL and GrB mRNA levels, improved the activity of
intestinal intraepithelial γδT cytotoxicity of γδT cells and TNF-β
and IFN-α levels |
| Wogonin | SGC-7901 and MFC
cells | Enhanced
immunomodulatory activities | Exerted
cytotoxicity to induce apoptosis of cancer cells, direct or
indirect with DC, promoted the recruitment of DC, T and NK cells
into tumor tissues to invert the expression levels of VEGF, B7H1
and RAE-1ε, dephosphorylate STAT3 to inhibit its activation on
tyrosine 705 to decrease the expression of B7H1 and MHC class I
chain-related protein A and enhance calreticulin on the cell
membrane |
| Mistletoe
lectin-55 | Colon cancer cell
and colon cancer-bearing BALB/C mice | Enhanced
immunomodulatory activities | Enhanced
Ag-specific activation and proliferation of CD4+ and
CD8+ T cells and increased the number of tumor
Ag-specific CD8+ T cells with increasing production of
IFN-γ, increased activation of NK and γδT cells to delay colon
cancer development |
| Alocasia
cucullata | Mouse tumor models
and mammalian cancer cells | Enhanced
immunomodulatory activities | Enhanced the key
cytokines in vivo, such as IL-2, IFN-γ and TNF-α. In
vitro, activated the differentiation of THP-1 into
macrophage-like cells with specific macrophage surface markers,
such as CD11b and CD14, and increased the induction of TNF-α and
IL-1β |
| Ganoderma
lucidum (lucid ganoderma) | Cancer patients in
a clinical trial, in mouse models in vivo and in cells in
vitro | bio-Enhanced
immunomodulatory activities, induced cell apoptosis, antimetastasis
antiangiogenesis | Increased the mean
concentration of plasma IL-2, IL-6 and IFN-γ and decreased the
levels of IL-1 and TNF-α in clinical trials, increased
CD56+ (NK cells) and CD3+ (T lymphocyte),
CD4+ (T helper cells) and CD8+ (T suppressor
cells) in vivo and in vitro, cell cycle arrest,
induced cell apoptosis, anti-invasion, antimigration,
immunomodulation and antiangiogenesis |
| LQ (a mixture of
Chinese medicinal herbs, comprising Sinapisalba,
Atractylodes macrocephala, Coix lacryma-jobi and
Polyporus adusta) | Lung cancer in
mouse models | Induced cell
apoptosis, antiangiogenesis, antimetastasis, enhanced efficacy and
reduced toxicity | Inhibited tumor
size and weight, exhibited antiangiogenic activity and prolonged
the survival of tumor-bearing mice compared with DOX, inhibited
lung cancer metastasis without weight loss and organ toxicity
compared to CTX and DOX. In vitro, induced cell death on
cancer cell lines compared to normal cell lines |
| Astragalus,
turmeric, ginseng, Huachensu injection and Kanglaite
injiection | Clinical trials on
patients and H22 cells | Enhanced the immune
function, enhanced efficacy, reduced toxicity side effects and
improved life quality | Suppressed tumor
progression, increased the sensitivity of chemo- and
ratiotherapeutic treatment accompanied by lessening the damage and
enhanced the immune system function from the preclinical and
clinical studies, reduced cancer-related fatigue pain and the
symptoms of cachexia, as well as resistance to respiratory tract
infections and alleviation of gastrointestinal side effects
(diarrhea, nausea and vomiting) and protection of the liver
function, decreased the expression of survivin, increased the
expression of caspase-9 in H22 cells |
| Curcumin | MCF-7 cells, animal
cancer, models, colorectal cancer cells (CRC) | Induced cell
apoptosis, tumor metastasis, enhanced efficacy, improved life
quality | Enhanced cell
apoptosis, enhanced Bcl-2/Bax expression, improved the kidney
function of creatinine/blood urea nitrogen and glutamic oxalacetic
transaminase/glutamic pyruvic transaminase in the serum of animal
trials with mitomycin C treatment, chemosensitized the activity of
5-FU-based chemotherapy in vitro, inhibited cell
proliferation, metastasis and downregulated NF-κB-regulated gene
activation |
| Scutellaria
baicalensis and Qing-Shu-Yi-Qi-Tang | Lewis lung
carcinoma (LLC) in vivo and in vitro | Enhanced immunity,
reduced toxicity | In vivo,
investigated in chemotherapy with cachectic symptoms, this
combination increased the Th1/Th2 ratio and NK cytotoxicity and
increased the monocyte chemoattractant protein-1 symptoms. In
vitro, decreased the expression levels of NF-κB and muscle RING
finger protein-1, decreased tumor masses and losses of carcass
and/or gastrocnemius muscle |
| Chinese herbal
medicine (CHM) (Radix Astragalus, Radix Adenophorae,
Radix Ophiopogonis, Radix Glycyrrhizae Poria and
Oldenlandia diffusa) | Advanced non-small
cell lung cancer patients | Enhanced efficacy,
reduced toxicity, improved life quality | CHM combined with
conventional chemotherapy reduced nausea and vomiting at toxicity
grades of III–IV, prevented the decline of hemoglobin and platelets
in patients under chemotherapy at toxicity grades of I–V, reduced
chemotherapy toxicity, prolonged survival time, enhanced immediate
tumor response and improved the Karnofsky performance status in
advanced non-small cell lung cancer patients |
| Liu-Jun-Zi-Tang,
PHY906, coumarin and aescine, ginseng, Huangqi, Ban Zhi Lian,
TJ-48, Huachansu, Shenqi, fuzheng and Kanglaite injections | Patients following
surgery and with chemo- or radiotherapy, improved life quality | Enhanced efficacy,
reduced toxicity, | Improved the
postoperation symptoms, such as fatigue, pain, appetite, diarrhea,
nausea, vomiting and lymphedema in clinical trials, CHM in
combination with chemo- or radiotherapy enhanced the efficacy and
reduced the side effects and complications caused by chemo- and
radiotherapy, including gastrointestinal side effects, liver
function damage and bone marrow suppression |
Inducing cellular apoptosis
Cell apoptosis is a form of programmed cell death
that induces changes in cellular morphology and intracellular
processes without inflammatory reactions. The procedures of
apoptosis are formed as follows: Receiving apoptotic signals leads
to cytomorphological alterations, the interaction between the
intermolecular apoptotic proteins, activation of the caspase enzyme
that acts upon cytoskeleton proteins, mitochondria, nuclear
membrane or chromatin to cleave proteins and substrates, causing
the DNA to fragment, releasing the cytoplasm enclosed by the plasma
membrane, eventually leading to cell death (10–14).
Currently, it is known that the extrinsic or death receptor
pathway, the intrinsic or mitochondrial pathway and the
perforin/granzyme pathway are the major apoptotic pathways to
activate caspases (12,15–17). The
apoptotic process is involved in the maintenance of tissue
homeostasis, which can be triggered by a variety of stimuli,
including cytokines, hormones, toxic insults and viruses. Once
these stimuli seriously affect the self-homeostasis of the cells,
the genes, including oncogenes, tumor-suppressor genes, suicide
genes and DNA-repair genes, are activated and become cancerous
resulting in the continuous division of the faulty cells. However,
the herbal medicines have an important role in the prevention and
treatment of cancer and execute their multiple therapeutic effects
by inhibiting cancer-activating enzymes, stimulating a DNA repair
mechanism and promoting production of protective enzymes, thus,
showing anticancer effects.
Lin et al (18)
investigated the cellular effects of the ethane extract of
Hedyotis Diffusa Willd. (EEHDW) in the HT-29 human colon
carcinoma cell. The study reported that EEHDW inhibited the growth
of HT-29 cells by inducing cell morphological changes, which reduce
the cell viability due to DNA fragmentation and loss of plasma
membrane asymmetry, and also the collapse of mitochondrial
membranes, the activation of caspase-9 and caspase-3 and the
increase of the pro-apoptotic Bax to anti-apoptotic Bcl-2 ratio.
Oxymatrine (19) is an isolated
extract from the traditional Chinese herb Sephora flavescent
Ait, and its anticancer effect was examined on human pancreatic
cancer PANC-1 cells. The results showed its efficacy by releasing
cytochrome c and activating caspase-3 proteins.
Additionally, it downregulated the expression of living and
surviving genes and the Bax/Bcl-2 ratio was unregulated. The study
by Guo et al (20) demonstrated
that oxymatrine effectively suppressed the cell proliferation and
phosphorylation of EGFR (Tyr845), decreased phospho-cofilin (Ser3)
and phospho-LIMK1 (Thr508) without changing the total cofilin and
LIMK1 expression to inhibit the migration and invasion of human
gastric cancer cells. Hu et al (21) studied the efficacy of the TCM tubeimu
on the MDA-MB-453 human breast cancer cell line. The study reported
that the fragmentation of DNA could be observed by a fluorescence
microscope at 72 h and apoptosis frequently increased when the
cells were in a three-dimensional compared to a two-dimensional
culture. The research regarding the emodin azide methyl
anthraquinone derivative (22)
demonstrates that cytotoxic effects (such as cell death) are
through the arrest of the cell cycle and the induction of apoptosis
in cancer cells. The overall molecular mechanisms of emodin include
cell cycle arrest and apoptosis, promote the expression of
hypoxia-inducible factor 1α, glutathione S-transferase P and
N-acetyltransferase, and induce glutathione phase I and II
detoxification enzymes to inhibit angiogenesis, invasion, migration
and chemical-induced carcinogen-DNA adduct formation. HER2/neu, CK2
kinase and p34cdc2 kinase were also inhibited in the human breast
cancer cells.
Reversing multidrug resistance
Multidrug resistance (MDR) represents a prominent
obstacle in cancer chemotherapy. Therefore, with the elucidation of
the MDR mechanism, MDR has been associated with an adenosine
triphosphate (ATP)-dependent decrease in cellular drug
accumulation, which is attributed to the overexpression of certain
ATP-binding cassette (ABC) transporter proteins (23). There are 9 human MDR proteins that are
generally members of subfamily C in the ABC superfamily (23). Although their structures are different,
they share a similar ATP-driven transport mechanism (24). However, due to their different
locations, expression levels and activities, the drug resistance to
chemotherapy is varied. Using the method for identifying the MDR
mechanisms, there are three major mechanisms of drug resistance
known in cells: i) Decreased water-soluble drug absorption,
including folate antagonists and nucleoside analogues, which
require transporters to enter cells; ii) various changes in cells
occur that affect the capacity of cytotoxic drugs to kill cells,
such as alterations in cell cycle, increased repair of DNA damage,
reduced apoptosis and altered metabolism of drugs; and iii)
increased energy-dependent efflux of hydrophobic drugs that can
easily enter the cells by diffusion through the plasma membrane
(25). P-glycoprotein (P-gp), which
belongs to the first identified member of the ABC transporter
superfamily of membrane transport proteins (26), represents the most common capability of
resistance to hydrophobic anticancer drugs by actively extruding
the drugs from the cells, including vinblastine, daunorubicin and
paclitaxel. Therefore, patients with MDR tumor types often only
have limited options, but can withstand significantly high doses
(27). Thus, with the evidence that
more new drugs are deriving from herbal medicines, quercetin,
epigallocatechin gallate, curcumin, capsaicin and gingerol have
become promising candidates (28) as
they overcome MDR potency in antitumor treatment. They act as MDR
inhibitors, MDR modulators, MDR reversal agents or chemosensitizers
to reverse the resistance against anticancer drug (25–30).
Therefore, increasing studies are focusing on exploiting MDR
reversal compounds from medicinal plants, as they have low or no
side effects for use in cancer treatment (29), to bypass drug resistance in treating
cancer, and eventually medicinal herbs may be applied in the future
in cancer adjuvant therapy.
Capsaicin (8) and
gingerol (8) have been reported to
exhibit inhibitory effects on human P-gp. They increased the
intracellular concentration of P-gp substrates by inhibiting this
anticancer drug efflux transporter. MDR carcinoma KB-C2 cells were
more susceptible to the cytotoxicity of vinblastine, as compared
with vinblastine alone, when simultaneously treated with 50 µM
capsaicin or gingerol (31).
Therefore, capsaicin and gingerol can partially reverse MDR in
cells that express P-gp. Germacrone (32), the main component of Rhizma
curcuma, was tested on MCF-7/adriamycin (ADR) MDR human breast
cancer cells. The combination of germacrane and ADR increased the
cytotoxicity when compared with ADR alone, and promoted cell
apoptosis in a dose-dependent manner. The apoptotic effect was
enhanced with ADR, as analyzed by downregulating the expression of
anti-apoptotic proteins p53 and Bax. In addition, germacrone
inhibited the expression of P-gp by inhibiting the MDR1 gene
promoter. Tao et al (33)
investigated the reversal of P-gp in colon cancer cells by five
tanshinones isolated from Salvia miltiorrhiza (danshen), the
result demonstrated that only cryptotanshinone and
dihydrotanshinone decreased the digoxin efflux ratio in a
dose-dependent manner and they enhanced the cytotoxicity of
doxorubicin and irinotecan in P-gp overexpressing SW620 AD300 colon
cancer cells by increasing the intracellular accumulation of the
P-gp substrate anticancer drugs, which possibly downregulate the
P-gp mRNA and protein expression and inhibit P-gp ATPase activity.
Paris saponin VII (27), a type
of saponin extracted from Trillium tschonoskii Maxim,
dose-dependently suppressed cell viability, as well as triggered
apoptosis and drug resistance of MCF-7/ADR cells. Furthermore, it
also increased the expression of TNFR1, TRAILR1/DR4, TRAILR2/DR5
and FADD, and activated PARP, caspase-8 and caspase-3. By contrast,
the P-gp expression and activity were reduced. Quinolones,
indoloquinazoline alkaloids that are derived from the plant
medicine Evodia ruaecarpa, were tested on CEM/ADR5000
leukemia and porcine brain capillary endothelial cells. The results
showed that the quinolones are moderate modulators of P-gp activity
(34,35). Additionally, the drug resistance index
of the total extract of Carthami Flos (CF) in MDR KB-V1
cells and its synergistic effects with other chemotherapeutic
agents were studied. The results revealed that CF showed a drug
resistance index, according to the classic isobologram equation.
However, in combination with other chemotherapeutic agents, it
enhanced their chemosensitivities and provided a general synergism
of the cytotoxic effect (36).
Comprehensive efficacy on angiogenesis,
cellular signaling pathway and metastasis
Angiogenesis is the formation of new blood vessels
and is a complex and regulated process that involves a number of
intercellular pro- and antiangiogenic signaling pathways. These
pathways control the activity of blood vessel-associated cells,
including endothelial cells (ECs) and pericytes (37), which is formed by the initiation of
cell proliferation and migration in response to angiogenic
stimulators: Vascular endothelial growth factor (VEGF), fibroblast
growth factor-2 (FGF-2), interleukin-8 (IL-8), placental growth
factor (PlGF), transforming growth factor-β (TGF-β),
platelet-derived growth factor (PDGF), nitric oxide (NO), ephrins,
angiopoietins, endothelins, integrins, cadherins, chemokines and
Notch (37–39). The crosstalk among the overexpression
of growth factors, hypoxia-inducible factor and the dysregulation
of tyrosine kinase receptors is controlled by the
phosphoinositide-3-kinase (PI3K)/Akt/mTOR (40), Ras/Raf/ERK/mitogen-activated protein
kinase (MAPK) and hypoxia-inducible factor (HIF) regulatory
pathways (41,42). The complicated formation of
angiogenesis has provided a platform for tumor metastasis. The
tumor hypoxia-induced epithelial-to-mesenchymal transition (EMT)
also increased the metastatic phenotype of tumors (43). When oxygen and nutrients are supplied
to the tumor cells, angiogenesis has an important role in the
growth, invasion and metastasis for the tumor. Therefore, when the
tumor microenvironment has been converted by angiogenesis,
antiangiogenesis becomes a challenge for antitumor treatment.
Therefore, numerous studies have inhibited tumor angiogenesis from
four aspects (37,38,42): One is
restraining the inhibitor of tumor angiogenesis degraded by the
basilar membrane, and the second is inhibiting the inhibitor of
tumor angiogenesis for increasing endotheliocytes. The third is
preventing the activation of the growth factor that is associated
with tumor angiogenesis. The final aspect is inhibiting the
signaling pathways associated with tumor angiogenesis. However, the
efficacy is unilateral, which may be insufficient and possibly
generate drug resistance (44).
Therefore, these problems highlighted the necessity for the
exploitation of novel anticancer agents. However, there is numerous
evidence (41) that has proved that
plant-derived medicinal compounds may, in a number of cases, be at
least as effective in blocking angiogenesis as the currently used
synthetic drugs, which exhibit only a fraction of the side effects.
The following are examples of the plant-derived medicinal
compounds.
The TCM Scutellaria barbata is commonly used
in cancer treatment. The ethanol extract of Scutellaria
barbata (45) was able to induce
apoptosis of the colon cancer cells and inhibit angiogenesis in a
chick embryo chorioallantoxic membrane model. In a colorectal
cancer cell (CRC) mouse xenograft model in vivo, it could
reduce tumor size without affecting the body weight and suppress
the expression of key mediators of the Sonic hedgehog (SHH) pathway
in tumor tissues. The expression of the important target gene of
the SHH signaling pathway, vascular endothelial growth factor A
(VEGF-A), was also inhibited, which has an important role in
stimulating tumor angiogenesis. The study of the xiaotansanjie
decoction (46) in regulating the
expression of Notch-1, Hes1, VEGF and Ki-67 was measured by western
blotting and reverse transcription-polymerase chain reaction and
the cell viability was measured by the MTT assay. The data revealed
that cluster of differentiation (CD) 44+ gastric cancer
stem cells (GCSCS) showed more cell proliferation and VEGF
secretion compared to CD44− cells in vitro,
whereas in vivo, the CD44+ GCSCS had a high
expression of Notch-1 and Hes1, which was positively associated
with tumor growth. The study by Wang et al (47) reported that baicalin not only
dose-dependently inhibited MDA-MB-231 cell migration and in
vitro invasion, but also suppressed the growth of the tumor and
the pulmonary metastasis via blocking p38 MAPK activity and
activated the inhibitor of p38 MAPK which decreased the expression
of matrix metalloproteinase-2 (MMP-2), MMP-9, urokinase plasminogen
activator (uPA) and uPA receptor through the p38-MAPK signaling
pathway. Pien Tze Huang (48) was
tested on a mouse model of colorectal cancer and the treatment
reduced the expression of angiogenic factors, including inducible
NO synthase (NOS), endothelial NOS, VEGF-A, basic FGF (bFGF), as
well as their specific receptors VEGFR2 and bFGFR, to inhibit the
tumor angiogenesis via suppressing multiple signaling pathways,
such as STAT3, AKT and MAPKs. In addition, based on the
investigation of ganoderic acid Me (GA-Me) (49), the growth, angiogenesis and invasion of
breast cancer cells were suppressed. By contrast, tumor necrosis
factor-α (TNF-α) was stimulated. Furthermore, GA-Me downregulated
the expression of various nuclear factor-κB (NF-κB)-regulated genes
(C-myc, cyclin D, BCL-2, MMP-9, VEGF, IL-6 and IL-8) to modulate
the NF-κB signaling pathway. Saikosaponin (50) was investigated on the HepG2 cell line
and the results revealed that phosphorylation of ERK was
significantly induced by TPA and saikosaponin α, whereas the
phosphorylation of JNK was induced only by TPA and the
phosphorylation of P38 was not induced by either. This reaction was
derived from the activation of the downstream transcriptional
factors of the MAPK cascade (ATF2, c-jun, jun-B and c-fos).
However, the inhibitor could decrease the efficacy of p15
(INK4b)/p16 (INK4a) RNAs, c-fos RNA protein and AP-1-related
DNA-binding activity by TPA and saikosapanin, and c-jun
phosphorylation was only reduced by TPA. A study of nobiletin
(51) treatment of human gastric
adenocarcinoma AGS cells reported an inhibition of the activation
of focal adhesion kinase and PI3K/Akt, which are involved in the
downregulation of the enzyme activities, and protein and messenger
RNA levels of MMP-2, MMP-9, Ras, c-Raf, Rac-1, Cdc42 and RhoA. The
protein level of RhoB was progressively increased, whereas
treatment decreased the phosphorylation and degradation of
inhibitor of κBa (IκBa) and the nuclear response element of NF-κB
also exerted inhibitory effects on the cell adhesion and invasion.
The TCM Gegen Qinlian decoction (52)
significantly suppressed the enzyme activity of MMP-2 in human
renal carcinoma cells to inhibit the expansion and neoangiogenesis
of cancer.
Regulating the immunomodulatory
activities
To an extent, tumors produce a number of tumor
antigens, which are the primary triggers of the immune response.
They are activated via a major histocompatibility complex (MHC) and
the T cell response. In the process of immunosurveillance, they
trigger T-cell activation to recruit other immune effector cells
with the expression of co-stimulatory molecules and secretion of
chemokines and cytokines to regulate different aspects of the
immune response, including Th1 CD4 T cells that activate CD8 T
cells, and subsequently CD8 T cells are activated by direct antigen
presentation, via MHC class I or via CD4 T-cell-mediated
activation. The tumor cell is ultimately destroyed by direct
cell-mediated cytotoxicity, as well as an indirect antibody
complement-mediated cytotoxicity (53). However, the majority of tumors have
been demonstrated to lose the expression of MHC molecules,
resulting in the loss of presenting tumor antigens, thus evading
T-cell recognition (40,53). In addition, when the balance of the
immune checkpoint was destroyed, certain tumors upregulated
inhibitory molecules to evade the immune response (40). Due to the strong heterogeneity of
cancer, exploring novel effective drug targets and therapeutic
strategies to overcome the problem is required. TCM, with a long
history of treating various diseases through modulating the human
immune response, offers a potential to be a remedy of the new
immunomodulating regimen for cancer treatment. They exert broad
immunostimulatory activities by recruiting and activating different
types of immune cells, such as dendritic cells (DC), natural killer
(NK) T-cells, T cells, macrophage, monocytes (54–56) and
adjusting the production of cytokines (IFN-α, IL-2, IL-6 and IFN-γ)
(54,57,58) to fight
against the tumor cells.
In the study of astragalus polysaccharides (59) conducted on S180 sarcoma-bearing mice,
the IFN-α FasL and GrB mRNA levels were increased and the activity
of intestinal intraepithelial γδT cells in vivo was also
improved. The cytokine production and cytotoxicity of γδT cells and
the levels of TNF-β and IFN-α were all markedly improved following
treatment. The study of wogonin (54)
treatment revealed that it exerted cytotoxicity, directly or
indirectly with DC, to induce apoptosis of cancer cells (SGC-7901
and MFC cells). The treatment also promoted the recruitment of DC,
T and NK cells into tumor tissues to invert the expression levels
of vascular endothelial growth factor, B7H1, and RAE-1ε in tumor
tissues. Additionally, the treatment also dephosphorylated STAT3 to
inhibit its activation on tyrosine 705 in tumor cells, which
contributed to a decrease in the expression of B7H1 and MHC class I
chain-related protein A and the enhancement of calreticulin on the
cell membrane. Notably, these data indicated that wogonin could be
used in collaboration with a DC vaccine or activated lymphocytes
for tumor therapy. In addition, active chinese mistletoe lectin-55
was tested on colon cancer, and the results demonstrated that it
enhanced antigen (Ag)-specific activation and proliferation of
CD4+ and CD8+ T cells and increased the
number of tumor Ag-specific CD8+ T cells with increasing
production of IFN-γ. Of note, the treatment with ACML-55 (58) also showed increased activation of
innate lymphocytes, such as NK and γδT cells. These immune
responses significantly delayed colon cancer development in colon
cancer-bearing BALB/C mice. Alocasia cucullata (AC)
(57), a Chinese medicine, was
investigated on mouse tumor models cultured in mammalian cells, and
the results showed that its antitumor effect was closely associated
with a strong induction of key cytokines in vivo, such as
IL-2, IFN-γ and TNF-α. AC activated the differentiation of THP-1, a
human monocytic cell line, into macrophage-like cells in
vitro, accompanied with specific macrophage surface markers,
such as CD11b and CD14, and increased the induction of TNF-α and
IL-1β. All these data verified the potential of AC to be an
alternative immunomodulating herbal remedy for anticancer
treatment. Ganoderma lucidum (lucid ganoderma) (60), a traditional Chinese herb, has been
used extensively in East Asia for one thousand years. Compounds of
the extraction of Ganoderma lucidum mixed in PC-SPES
(61,62)
were administered to cancer patients in a clinical trial, in mouse
models in vivo and in cells in vitro. In the prostate
cancer patients, it improved the immunostimulating response with an
increased concentration of plasma IL-2, IL-6 and interferon-γ, and
it enhanced the natural killer cell activity and decreased the
production of plasma IL-1 and tumor necrosis factor-α.
Simultaneously, in patients with advanced-stage cancer in a
clinical trial, the statistical results showed that following
surgery, the patients undergoing oral administration of lucid
ganoderma extract for 12 weeks exhibited a significantly increased
mean concentration of plasma IL-2, IL-6 and IFN-γ, and
significantly decreased levels of IL-1 and TNF-α. There was also a
significant increase of CD56+ (NK cells) and a small
increase of CD3+ (T lymphocyte), CD4+
(T-helper cells) and CD8+ (T-suppressor cells) with
unchanged CD4:CD8 T-cell ratios, which were induced by the lucid
ganoderma extract as compared to the baseline levels (63). With regards to the in vitro and
in vivo studies, the anticancer mechanism of lucid ganoderma
treatment against the growth of different cancer cells appeared to
be mediated by cell cycle arrest, apoptosis, anti-invasion,
antimigration, immunomodulation and antiangiogenesis (60). This evidence greatly supported the
anticancer potential of lucid ganoderma.
Enhancing the efficacy, reducing toxicity
and improving the quality of life for patients
Over a few decades, systemic chemotherapy and
radiotherapy have been the optimal treatment options for the tumor
patients owing to the limitations and drawbacks (9) of surgery, including patients that are
diagnosed too late to undergo surgery and a number of complications
occurring following surgery. However, the major problem in cancer
chemoradiotherapy is the prolonged toxicity of the well-established
chemical drugs or the radioactive rays that resulted in numerous
side effects and complications, including pain, diarrhea, nausea,
vomiting, hair loss and hepatotoxicity. Even in advanced stages,
certain patients show a syndrome of cachexia (64), which leads to a poor quality of life.
Thus, the complementary and alternative medicines have become a
preference for patients. Consequently, there is certain evidence in
pre-clinical and clinical trials that has proved that TCM can
enhance the efficacy and reduce toxicity with chemoradiotherapy and
improve the quality of life and functional status of patients.
The study by Zhang et al (65) compared the efficacy of the TCM herbal
mixture LQ (66) (a mixture of Chinese
medicinal herbs, comprising Sinapis alba, Atractylodes
macrocephala, Coix lacryma-jobi and Polyporus
adusta) against lung cancer in mouse models using doxorubicin
(DOX) and cyclophosphamide (CTX). LQ inhibited the tumor size and
weight measured directly, as well as by fluorescent-protein imaging
in subcutaneous and orthotropic experimental metastasis and
angiogenesis lung cancer mouse models. Spontaneously, LQ had
antiangiogenic activity and prolonged the survival of tumor-bearing
mice compared with DOX. LQ was efficacious against primary and
metastatic lung cancer without weight loss and organ toxicity,
whereas CTX and DOX had opposing outcomes. In vitro, the
study proved that LQ had the potential to induce cell death on
cancer cell lines compared to normal cell lines. With regards to a
review of Chinese herbal medicines (CHMs) (67) (astragalus, turmeric, ginseng, Huachansu
injection and Kanglaite injection), data revealed that they have
significant advantages in suppressing tumor progression, increasing
the sensitivity of chemo- and ratiotherapeutics accompanied by
lessening the damage of the treatment and enhancing the immune
system function of the organism from the preclinical and clinical
studies. Additionally, they reduce cancer-related fatigue and pain
and the symptoms of cachexia, as well as resistance to respiratory
tract infections, alleviation of gastrointestinal side effects
(diarrhea, nausea and vomiting) and protection of the liver
function. The expression of survivin was also decreased. However,
the expression of caspase-9 increased in H22 cells. Furthermore, in
the study of Zhou et al (68),
mitomycin C (MMC) and curcumin synergistically enhanced apoptosis
in MCF-7 cells by activating the caspases and enhancing the
expression of Bcl-2/Bax. Additionally, curcumin significantly
improved the kidney function of creatinine/blood urea nitrogen and
glutamic oxalacetic transaminase/glutamic pyruvic transaminase in
the serum of animals undergoing MMC treatment. Furthermore, the
study by Shakibaei et al (69)
demonstrated that curcumin chemosensitized the activity of
5-FU-based chemotherapy on CRC accompanied with inhibition of cell
proliferation, metastasis and downregulation of NF-κB-regulated
gene activation. Scutellaria baicalensis (70) and Qing-Shu-Yi-Qi-Tang (70) were investigated on tumor-bearing mice
with cachectic symptoms undergoing chemotherapy, and this
combination increased the Th1/Th2 ratio and NK cytotoxicity, and
the expression of monocyte chemoattractant protein-1 was also
increased, whilst the expression levels of NF-κB and muscle RING
finger protein-1 were decreased. Evidently the tumor masses and
losses of carcass and/or gastrocnemius muscle were significantly
decreased. In addition, data from a meta-analysis regarding CHM as
an adjunctive therapy for advanced non-small cell lung cancer
reported that CHM combined with conventional chemotherapy markedly
reduced the nausea and vomiting at toxicity grade of III–IV and
prevented the decline of hemoglobin and platelets in patients under
chemotherapy at a toxicity grade of I–V (71). A systematic review suggested that CHM,
as an adjuvant therapy, can reduce chemotherapy toxicity, prolong
survival time, enhance immediate tumor response and improve the
Karnofsky performance status in advanced non-small cell lung cancer
patients. The review concluded that Liu-Jun-Zi-Tang, PHY906,
coumarin and aescine were capable of improving the postoperation
symptoms, such as fatigue, pain, appetite, diarrhea, nausea,
vomiting and lymphedema in clinical trials (9). Additionally, ginseng, Huangqi, Ban Zhi
Lian, TJ-48, Huachansu injection, Shenqi fuzheng injection and
Kanglaite injection in combination with chemo- or radiotherapy were
capable of enhancing the efficacy and diminishing the side effects
and complications caused by chemo- and radiotherapy, including
gastrointestinal side effects, liver function damage and even bone
marrow suppression.
Conclusion and future perspectives
Cancer is a multifactorial and multistep disease
that can change the tumor microenvironment, leading to alterations
in tumor metabolism. The circulation of energy regarding glucose,
lipid and amino acid metabolism and the formed metabolites
(7) (such as lipids, amino acids,
nucleotides, certain inflammatory cytokines and HIF) affected the
cancer cell proliferation, autophagy, survival and
invasion/metastasis and cellular signaling by blocking their
required nutrients, their associated pathways (72) and inhibiting the activity of the
regulated immune cells (73). However,
the above evidence has proved that TCM has comprehensive advantages
in treating cancer with regards to these aspects, which also
presented a close association with each other (Fig. 1). Therefore, it may be advantageous to
identify and develop novel natural compounds, active ingredients,
single herbs and combination formulas or prescriptions in TCM with
its multitargets and multisignaling pathways on the tumor, without
significant toxicity compared to single utilization with other
therapies. Even though the theory of TCM in treating cancer is in
the early stages, in recent years, with the enhanced knowledge of
translational science and molecular biology, herbs and combination
formulas have been reported to treat cancer as an effective therapy
in preclinical or clinical trials. The database regarding the
pharmacological activities and mechanisms of natural compounds
revealed that tumors carried diverse driver gene mutations and
crosstalked with different intracellular signals in the tumor
microenvironment, leading to carcinogenesis (74). Therefore, it is imperative to explore
the mechanism of TCM at the molecular levels to clarify the role of
more TCM in curing disease, and additionally, to make a scientific
foundation for internationalization of TCM. Based on the main
principles of TCM regarding dialectic approaches, individualization
and patients with different syndromes require treatment with
different approaches. Therefore, TCM with regards to treating
different syndromes in various individuals requires clarification.
In recent years, there have been numerous studies of the Chinese
medicinal compound monomer in the field of medical science,
however, there are various components in the herbal medicines, and
whether there is an interaction of the functional impacts between
themselves by one approach or several ways remains to be
elucidated. Therefore, further validation of the biodiversity of
the herbal medicines in preclinical or clinical trials, either
alone or in combination with existing therapy, is required to
benefit more patients with cancer.
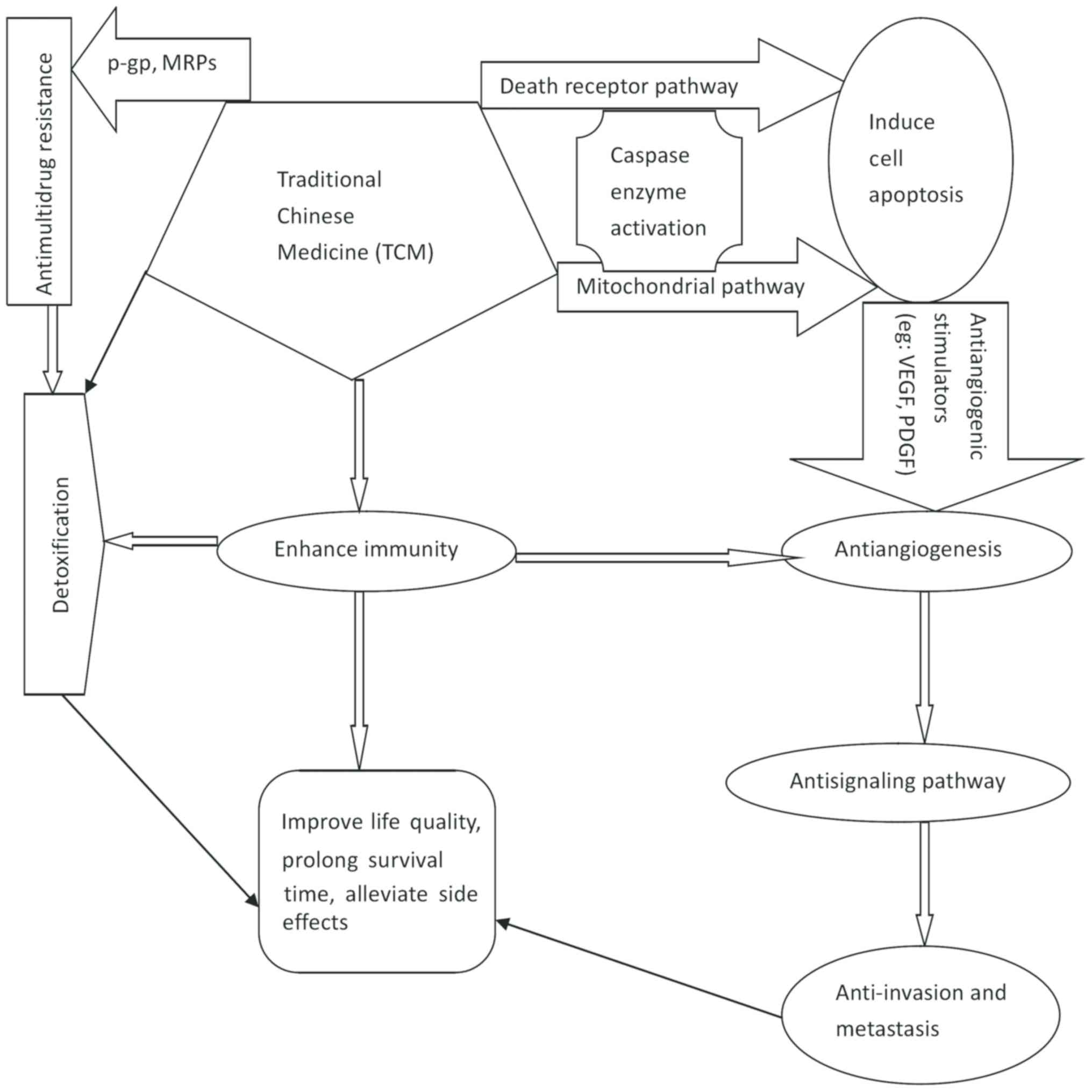 | Figure 1.Association of the anticancer mode of
action with TCM in the tumor microenvironment. TCM anticancer
activity involves targeting various steps (cell proliferation,
angiogenesis, cell signaling pathways, invasion, metastasis and
multidrug resistance) to inhibit tumor development, reduce the
toxicity of other therapies to tumors in the microenvironments, and
accordingly, to improve the quality of life for patients. TCM,
traditional chinese medicine; MRP, multidrug resistance protein;
VEGF, vascular endothelial growth factor; PDGF, platelet-derived
growth factor; P-gp, P-glycoprotein. |
References
|
1
|
Smith RA, Manassaram-Baptiste D, Brooks D,
Doroshenk M, Fedewa S, Saslow D, Brawley OW and Wender R: Cancer
screening in the United States, 2015: A review of current American
cancer society guidelines and current issues in cancer screening.
CA Cancer J Clin. 65:30–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bray F, Jemal A, Grey N, Ferlay J and
Forman D: Global cancer transitions according to the Human
Development Index (2008–2030): A population-based study. Lancet
Oncol. 13:790–801. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Adams C, Grey N, Magrath I, Miller A and
Torode J: The World Cancer Declaration: Is the world catching up?
Lancet Oncol. 11:1018–1020. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bailly C: Ready for a comeback of natural
products in oncology. Biochem Pharmacol. 77:1447–1457. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li X, Yang G, Li X, Zhang Y, Yang J, Chang
J, Sun X, Zhou X, Guo Y, Xu Y, et al: Traditional Chinese medicine
in cancer care: A review of controlled clinical studies published
in chinese. PLoS One. 8:e603382013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sen T and Samanta SK: Medicinal plants,
human health and biodiversity: A broad review. Adv Biochem Eng
Biotechnol. 147:59–110. 2015.PubMed/NCBI
|
|
7
|
Casey SC, Amedei A, Aquilano K, Azmi AS,
Benencia F, Bhakta D, Bilsland AE, Boosani CS, Chen S, Ciriolo MR,
et al: Cancer prevention and therapy through the modulation of the
tumor microenvironment. Semin Cancer Biol. Apr 10–2015.(Epub ahead
of print) doi: 10.1016/j.semcancer.2015.02.007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aggarwal BB and Shishodia S: Molecular
targets of dietary agents for prevention and therapy of cancer.
Biochem Pharmacol. 71:1397–1421. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Qi F, Zhao L, Zhou A, Zhang B, Li A, Wang
Z and Han J: The advantages of using traditional Chinese medicine
as an adjunctive therapy in the whole course of cancer treatment
instead of only terminal stage of cancer. Biosci Trends. 9:16–34.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jenkins VK, Timmons AK and McCall K:
Diversity of cell death pathways: Insight from the fly ovary.
Trends Cell Biol. 23:567–574. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Elmore S: Apoptosis: A review of
programmed cell death. Toxicol Pathol. 35:495–516. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boatright KM and Salvesen GS: Mechanisms
of caspase activation. Curr Opin Cell Biol. 15:725–731. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Romero A, Novoa B and Figueras A: The
complexity of apoptotic cell death in mollusks: An update. Fish
Shellfish Immunol. 46:79–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 12:770–776. 2000. View
Article : Google Scholar
|
|
15
|
Hensley P, Mishra M and Kyprianou N:
Targeting caspases in cancer therapeutics. Biol Chem. 394:831–843.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
de Poot SA, Lai KW, van der Wal L, Plasman
K, Van Damme P, Porter AC, Gevaert K and Bovenschen N: Granzyme M
targets topoisomerase II alpha to trigger cell cycle arrest and
caspase-dependent apoptosis. Cell Death Differ. 21:416–426. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chuang CH, Wang WJ, Li CF, Ko CY, Chou YH,
Chuu CP, Cheng TL and Wang JM: The combination of the prodrugs
perforin-CEBPD and perforin-granzyme B efficiently enhances the
activation of caspase signaling and kills prostate cancer. Cell
Death Dis. 5:e12202014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lin J, Chen Y, Wei L, Chen X, Xu W, Hong
Z, Sferra TJ and Peng J: Hedyotis Diffusa Willd extract
induces apoptosis via activation of the mitochondrion-dependent
pathway in human colon carcinoma cells. Int J Oncol. 37:1331–1338.
2010.PubMed/NCBI
|
|
19
|
Ling Q, Xu X, Wei X, Wang W, Zhou B, Wang
B and Zheng S: Oxymatrine induces human pancreatic cancer PANC-1
cells apoptosis via regulating expression of Bcl-2 and IAP
families, and releasing of cytochrome c. J Exp Clin Cancer Res.
30:662011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo B, Zhang T, Su J, Wang K and Li X:
Oxymatrine targets EGFR(p-Tyr845) and inhibits EGFR-related
signaling pathways to suppress the proliferation and invasion of
gastric cancer cells. Cancer Chemother Pharmacol. 75:353–363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hu M, Zhao M, An C, Yang M, Li Q, Zhang Y,
Suetsugu A, Tome Y, Yano S, Fu Y, et al: Real-time imaging of
apoptosis induction of human breast cancer cells by the traditional
Chinese medicinal herb tubeimu. Anticancer Res. 32:2509–2514.
2012.PubMed/NCBI
|
|
22
|
Hsu SC and Chung JG: Anticancer potential
of emodin. BioMedicine. 2:108–116. 2012. View Article : Google Scholar
|
|
23
|
Zhang YK, Wang YJ, Gupta P and Chen ZS:
Multidrug resistance proteins (MRPs) and cancer therapy. AAPS J.
17:802–812. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wilkens S: Structure and mechanism of ABC
transporters. F1000 Prime Rep. 7:142015. View Article : Google Scholar
|
|
25
|
Szakács G, Paterson JK, Ludwig JA,
Booth-Genthe C and Gottesman MM: Targeting multidrug resistance in
cancer. Nat Rev Drug Discov. 5:219–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Fan L, Sun Y, Miao X, Zhang F, Meng
J, Han J, Zhang D, Zhang R, Yue Z, et al: Paris saponin VII from
Trillium tschonoskii reverses multidrug resistance of
adriamycin-resistant MCF-7/ADR cells via P-glycoprotein inhibition
and apoptosis augmentation. J Ethnopharmacol. 154:728–734. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cort A and Ozben T: Natural product
modulators to overcome multidrug resistance in cancer. Nutr Cancer.
67:411–423. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Eichhorn T and Efferth T: P-glycoprotein
and its inhibition in tumors by phytochemicals derived from Chinese
herbs. J Ethnopharmacol. 141:557–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ugocsai K, Varga A, Molnár P, Antus S and
Molnár J: Effects of selected flavonoids and carotenoids on drug
accumulation and apoptosis induction in multidrug-resistant colon
cancer cells expressing MDR1/LRP. Vivo. 19:433–438. 2005.
|
|
31
|
Nabekura T, Kamiyama S and Kitagawa S:
Effects of dietary chemopreventive phytochemicals on P-glycoprotein
function. Biochem Biophys Res Commun. 327:866–870. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xie XH, Zhao H, Hu YY and Gu XD:
Germacrone reverses Adriamycin resistance through cell apoptosis in
multidrug-resistant breast cancer cells. Exp Ther Med. 8:1611–1615.
2014.PubMed/NCBI
|
|
33
|
Hu T, To KK, Wang L, Zhang L, Lu L, Shen
J, Chan RL, Li M, Yeung JH and Cho CH: Reversal of P-glycoprotein
(P-gp) mediated multidrug resistance in colon cancer cells by
cryptotanshinone and dihydrotanshinone of Salvia
miltiorrhiza. Phytomedicine. 21:1264–1272. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Adams M, Mahringer A, Kunert O, Fricker G,
Efferth T and Bauer R: Cytotoxicity and p-glycoprotein modulating
effects of quinolones and indoloquinazolines from the Chinese herb
Evodia rutaecarpa. Planta Med. 73:1554–1557. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Adams M, Mahringer A, Bauer R, Fricker G
and Efferth T: In vitro cytotoxicity and P-glycoprotein modulating
effects of geranylated furocoumarins from Tetradium
daniellii. Planta Med. 73:1475–1478. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu JY, Yu ZL, Fong WF and Shi YQ:
Chemotherapeutic activities of Carthami Flos and its
reversal effect on multidrug resistance in cancer cells. Afr J
Tradit Complement Altern Med. 10:36–40. 2013.PubMed/NCBI
|
|
37
|
Gordon MS, Mendelson DS and Kato G: Tumor
angiogenesis and novel antiangiogenic strategies. Int J Cancer.
126:1777–1787. 2010.PubMed/NCBI
|
|
38
|
Bikfalvi A, Moenner M, Javerzat S, North S
and Hagedorn M: Inhibition of angiogenesis and the
angiogenesis/invasion shift. Biochem Soc Trans. 39:1560–1564. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ziche M and Morbidelli L: Molecular
regulation of tumour angiogenesis by nitric oxide. Eur Cytokine
Netw. 20:164–170. 2009.PubMed/NCBI
|
|
40
|
Jinushi M: Immune regulation of
therapy-resistant niches: Emerging targets for improving anticancer
drug responses. Cancer Metastasis Rev. 33:737–745. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Z, Dabrosin C, Yin X, Fuster MM,
Arreola A, Rathmell WK, Generali D, Nagaraju GP, El-Rayes B,
Ribatti D, et al: Broad targeting of angiogenesis for cancer
prevention and therapy. Semin Cancer Biol. Jan 15–2015.(Epub ahead
of print) doi: 10.1016/j.semcancer.2015.01.001. View Article : Google Scholar
|
|
42
|
Cook KM and Figg WD: Angiogenesis
inhibitors: Current strategies and future prospects. CA Cancer J
Clin. 60:222–243. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Lu KV, Chang JP, Parachoniak CA, Pandika
MM, Aghi MK, Meyronet D, Isachenko N, Fouse SD, Phillips JJ,
Cheresh DA, et al: VEGF inhibits tumor cell invasion and
mesenchymal transition through a MET/VEGFR2 complex. Cancer Cell.
22:21–35. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Eikesdal HP and Kalluri R: Drug resistance
associated with antiangiogenesis therapy. Semin Cancer Biol.
19:310–317. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wei L, Lin J, Xu W, Cai Q, Shen A, Hong Z
and Peng J: Scutellaria barbata D. Don inhibits tumor
angiogenesis via suppression of Hedgehog pathway in a mouse model
of colorectal cancer. Int J Mol Sci. 13:9419–9430. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yan B, Liu L, Zhao Y, Xiu LJ, Sun DZ, Liu
X, Lu Y, Shi J, Zhang YC, Li YJ, et al: Xiaotan Sanjie decoction
attenuates tumor angiogenesis by manipulating Notch-1-regulated
proliferation of gastric cancer stem-like cells. World J
Gastroenterol. 20:13105–13118. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Wang XF, Zhou QM, Du J, Zhang H, Lu YY and
Su SB: Baicalin suppresses migration, invasion and metastasis of
breast cancer via p38MAPK signaling pathway. Anticancer Agents Med
Chem. 13:923–931. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shen A, Lin J, Chen Y, Lin W, Liu L, Hong
Z, Sferra TJ and Peng J: Pien Tze Huang inhibits tumor angiogenesis
in a mouse model of colorectal cancer via suppression of multiple
cellular pathways. Oncol Rep. 30:1701–1706. 2013.PubMed/NCBI
|
|
49
|
Li F, Wang Y, Wang X, Li J, Cui H and Niu
M: Ganoderic acids suppress growth and angiogenesis by modulating
the NF-κB signaling pathway in breast cancer cells. Int J Clin
Pharmacol Ther. 50:712–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Wen-Sheng W: ERK signaling pathway is
involved in p15INK4b/p16INK4a expression and HepG2 growth
inhibition triggered by TPA and Saikosaponin a. Oncogene.
22:955–963. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Lee Y-C, Cheng TH, Lee JS, Chen JH, Liao
YC, Fong Y, Wu CH and Shih YW: Nobiletin, a citrus flavonoid,
suppresses invasion and migration involving FAK/PI3K/Akt and small
GTPase signals in human gastric adenocarcinoma AGS cells. Mol Cell
Biochem. 347:103–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Wang N and Feng Y, Cheung F, Wang X, Zhang
Z and Feng Y: A Chinese medicine formula Gegen Qinlian decoction
suppresses expansion of human renal carcinoma with inhibition of
matrix metalloproteinase-2. Integr Cancer Ther. 14:75–85. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Spurrell EL and Lockley M: Adaptive
immunity in cancer immunology and therapeutics.
Ecancermedicalscience. 8:4412014.PubMed/NCBI
|
|
54
|
Xiao W, Wu K, Yin M, Han S, Ding Y, Qiao
A, Lu G, Deng B, Bo P and Gong W: Wogonin inhibits tumor-derived
regulatory molecules by suppressing STAT3 signaling to promote
tumor immunity. J Immunother. 38:167–84. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Fridman WH, Pagès F, Sautès-Fridman C and
Galon J: The immune contexture in human tumours: Impact on clinical
outcome. Nat Rev Cancer. 12:298–306. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Huang CF, Lin SS, Liao PH, Young SC and
Yang C-C: The immunopharmaceutical effects and mechanisms of herb
medicine. Cell Mol Immunol. 5:23–31. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Peng Q, Cai H, Sun X, Li X, Mo Z and Shi
J: Alocasia cucullata exhibits strong antitumor effect in
vivo by activating antitumor immunity. PLoS One. 8:e753282013.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Ma YH, Cheng WZ, Gong F, Ma AL, Yu QW,
Zhang JY, Hu CY, Chen XH and Zhang DQ: Active Chinese mistletoe
lectin-55 enhances colon cancer surveillance through regulating
innate and adaptive immune responses. World J Gastroenterol.
14:5274–5281. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sun S, Zheng K, Zhao H, Lu C, Liu B, Yu C,
Zhang G, Bian Z, Lu A and He X: Regulatory effect of astragalus
polysaccharides on intestinal intraepithelial γδT cells of tumor
bearing mice. Molecules. 19:15224–15236. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Su ZY and Sheen LY: An evidence-based
perspective of Ganoderma Lucidum (Lucid Ganoderma) for
cancer patients. Evidence-based Anticancer Materia Medica. Cho
W.C.S.: Springer. 245–263. 2011. View Article : Google Scholar
|
|
61
|
de la Taille A, Hayek OR, Burchardt M,
Burchardt T and Katz AE: Role of herbal compounds (PC-SPES) in
hormone-refractoryprostate cancer: Two case reports. J Altern
Complement Med. 6:449–451. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Hsieh TC and Wu JM: Mechanism of action of
herbal supplement PC-SPES: Elucidation of effects of individual
herbs of PC-SPES on proliferation and prostate specific gene
expression in androgen-dependent LNCaP cells. Int J Oncol.
20:583–588. 2002.PubMed/NCBI
|
|
63
|
Gao Y, Zhou S, Jiang W, Huang M and Dai X:
Effects of ganopoly (a Ganoderma lucidum polysaccharide
extract) on the immune functions in advanced-stage cancer patients.
Immunol Invest. 32:201–215. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Donohoe CL, Ryan AM and Reynolds JV:
Cancer cachexia: Mechanisms and clinical implications.
Gastroenterol Res Pract. 2011:6014342011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Zhang L, Wu C, Zhang Y, Liu F, Wang X,
Zhao M and Hoffman RM: Comparison of efficacy and toxicity of
traditional Chinese medicine (TCM) herbal mixture LQ and
conventional chemotherapy on lung cancer metastasis and survival in
mouse models. PLoS One. 9:e1098142014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang L, Wu C, Zhang Y, Liu F, Zhao M,
Bouvet M and Hoffman RM: Efficacy comparison of traditional Chinese
medicine LQ versus gemcitabine in a mouse model of pancreatic
cancer. J Cell Biochem. 114:2131–2137. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Qi F, Li A, Inagaki Y, Gao J, Li J, Kokudo
N, Li XK and Tang W: Chinese herbal medicines as adjuvant treatment
during chemo- or radio-therapy for cancer. Biosci Trends.
4:297–307. 2010.PubMed/NCBI
|
|
68
|
Zhou QM, Wang XF, Liu XJ, Zhang H, Lu YY,
Huang S and Su SB: Curcumin improves MMC-based chemotherapy by
simultaneously sensitising cancer cells to MMC and reducing
MMC-associated side-effects. Eur J Cancer. 47:2240–2247. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shakibaei M, Kraehe P, Popper B, Shayan P,
Goel A and Buhrmann C: Curcumin potentiates antitumor activity of
5-fluorouracil in a 3D alginate tumor microenvironment of
colorectal cancer. BMC Cancer. 15:2502015. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Wang H, Chan YL, Li TL and Wu CJ:
Improving cachectic symptoms and immune strength of tumour-bearing
mice in chemotherapy by a combination of Scutellaria
baicalensis and Qing-Shu-Yi-Qi-Tang. Eur J Cancer.
48:1074–1084. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Li SG, Chen HY, Ou-Yang CS, Wang X-X, Yang
Z-J, Tong Y and Cho WC: The efficacy of Chinese herbal medicine as
an adjunctive therapy for advanced non-small cell lung cancer: A
systematic review and meta-analysis. PLoS One. 8:e576042013.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Singh SR, Tan M and Rameshwar P: Cancer
metabolism: Targeting metabolic pathways in cancer therapy. Cancer
Lett. 356(2 Pt A): 147–148. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Nowarski R, Gagliani N, Huber S and
Flavell RA: Innate immune cells in inflammation and cancer. Cancer
Immunol Res. 1:77–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Nobili S, Lippi D, Witort E, Donnini M,
Bausi L, Mini E and Capaccioli S: Natural compounds for cancer
treatment and prevention. Pharmacol Res. 59:365–378. 2009.
View Article : Google Scholar : PubMed/NCBI
|















