Introduction
The process of enamel demineralization and the
development of white spot lesions around orthodontic brackets,
fixed during orthodontic treatment constitutes a serious health
issue, with a morbidity rate of ≤95% (1,2). The bonding
of orthodontic brackets on the enamel surface can promote dental
plaque accumulation, which results in the increased level of
cariogenic bacteria, particularly Streptococcus mutans.
Demineralization occurs when bacteria metabolize carbohydrates and
produce organic acids, dissolving the calcium phosphate mineral of
tooth structures (3). Cavitated
lesions then develop if no effective therapeutic option is used
(4). As the incidence of white spot
lesions is increased following orthodontic treatment (1,5), the
development of an effective preventive strategy against enamel
demineralization around orthodontic brackets is necessary. Novel
orthodontic bonding systems with antibacterial properties would be
a promising strategy for preventing enamel demineralization, as
cariogenic bacteria colonization and proliferation are the primary
pathogenesis of white spot lesions (6–8). Previous
research has focused on developing novel antibacterial agents for
improving orthodontic bonding systems, for example, fluoride,
nano-silver, titanium oxide, chlorhexidine and quaternary ammonium
(9–11).
The use of substances derived from natural products
has been extremely successful in the discovery of novel medicines
(12). Galla chinensis, a
traditional Chinese medicine, received much attention in the search
of new bioactive agents for anti-caries. The bioactive components
of Galla chinensis extract (GCE) can suppress the growth of
microcosm biofilms and inhibit lactic acid production (13). Furthermore, previous studies have
demonstrated that GCE not only has a significant inhibitory effect
on enamel demineralization (14), but
also effectively promotes the remineralization of initial enamel
carious lesion (15–17). Furthermore, GCE seems to be the only
natural product with the potential to regulate the balance of
enamel demineralization/remineralization (18).
We hypothesized that GCE could be a useful resource
for developing an orthodontic bonding system with antibacterial
properties. Therefore, the present study aimed to prepare a
GCE-containing adhesive cement for orthodontic use, and to evaluate
its antibacterial effects and bonding strength.
Materials and methods
Preparation of GCE-containing
cement
GCE was distilled as described previously (19). Briefly, Galla chinensis produced
in Sichuan province, China was dried at 60°C for 3 days, powdered,
double extracted with distilled water, and then dissolved in
ethanol for filtration and evaporation. Subsequently, the GCE was
further fractionated by adsorption chromatography and purified by
successive column chromatography. The obtained GCE was dissolved in
ethanol and adjusted to pH 5.5. The GCE was incorporated into the
liquid of resin-modified glass ionomer cement (GC Fuji ORTHO™ LC,
GC Corp., Tokyo, Japan) at certain mass fractions, which were
pre-set to ensure the mass fraction of GCE in cement mixture
(following mixing of the liquid with powder). These values were 0
(control group), 0.1, 0.2, 0.4, and 0.8% respectively. Once the
GCE-containing cement sample was required for use, the cement
liquid was mixed with the powder at a constant ratio, according to
the manufacturer's instructions. Following this, 20 sec of LED
light curing was performed (Ortholux, 3 M Unitek, Monrovia, CA,
USA) for complete polymerization of the cement.
Bacterial suspensions
As one of the most important cariogenic bacteria, S.
mutans (strain 25175; American Type Culture Collection, Manassas,
VA, USA) was selected for testing the antimicrobial properties of
the GCE-containing cement. The bacterial cells were cultured in
Brain Heart Infusion (BHI) broth (BD Diagnostics, Franklin Lakes,
NJ, USA) and incubated at 37°C in a jar with a microaerophilic
atmosphere enriched with 5% CO2 (Oxoid Campygen,
Basingstoke, UK) until reaching the initial stationary phase after
48 h (Optical density at 650 nm of 0.30), and then measured using a
Spectronic 20 (Milton Roy, Houston, TX, USA). The microbial
suspension was used to inoculate the agar diffusion test plates and
to perform the adhesion assay.
Agar diffusion test
An agar diffusion test was adapted to evaluate
antibacterial activity. S. mutans suspensions were inoculated on
three plates with 20 ml of BHI agar. Five blocks were loaded on
each plate and respective cement was introduced and
light-polymerized immediately. The plates were then incubated at
37°C for 48 h, followed by the measurement of the inhibition halo
diameters using a manual caliper. This was repeated three times,
and mean values used for analysis.
Bacteria colonization
susceptibility
Six tubes containing 0.5 ml of S. mutans suspension
were prepared. Cements with five mass fractions as described above,
were photopolymerized into blocks respectively and then cut in 1–2
mm size solid cubic particles with a scalpel blade. Three solid
particles of each cement were placed in respective tubes in order
to evaluate bacteria adhesion to cement specimens. A tube
containing a pasteurized inactivated bacterial suspension was used
as the negative control, in order to evaluate nonspecific adherence
of the microorganism. The tubes were then incubated at 37°C for 48
h in an atmosphere enriched with 5% CO2. Next, in order
to remove not firmly adhered bacteria, all particles were washed
three times in sterile culture medium and agitated for 10 sec at
1,400 rpm using an SA8 vortex mixer (Bibby Scientific Ltd., Stone,
UK). The specimens were then fixed with glutaraldehyde and osmium
tetroxide (both from Sigma-Aldrich, St. Louis, MO, USA),
sputter-coated with gold and examined using a Quanta 200 scanning
electron microscope (FEI, Hillsboro, OR, USA). Bacteria adhesion
was semi-quantitatively evaluated using a 6-level scoring system
defined as follows (20,21): 0, no bacterial growth; 1, rare, widely
dispersed single cells; 2, multiple bacterial cells forming a
monolayer film; 3, multiple cells showing active chain-like
proliferation with a multilayered film in some areas; 4, a
multilayered, homogenous film of bacteria, with the underlying
surface visible in some areas, and 5, multilayered, mature colonies
of bacterial cells with a spongiform structure and no visible
underlying surface.
Bond strength
A total of fifty freshly extracted human premolars
collected from orthodontic patients were stored in a 10% formalin
solution at room temperature. Prior to the experiment, the teeth
were removed from the preservation solution, washed completely in
distilled water and then etched with 37% phosphoric acid gel at
buccal side. All fifty premolars were randomly divided into 5
groups (10 teeth per group). Fifty metal brackets (Gemini, 3M
Unitek, Monrovia, USA) were bonded to etched enamel surfaces using
five groups of GCE-containing cement respectively. Following light
polymerization, the samples were immerged into in artificial saliva
(purchased from Canspec Scientific Instruments, Shanghai, China)
for three days to simulate intra-oral conditions. A universal
testing machine (MTS, Eden Prairie, USA) was used to detect shear
bond strength (SBS). The surface area of bracket base was measured,
and the breaking load (N) was recorded when bracket detached in the
shear mode at a crosshead speed of 0.5 mm/min. Bond strength was
calculated as follows: Bond strength (MPa) = Breaking load (N)/Area
of bracket base (mm2).
Statistical analysis
Data were presented as mean ± standard deviation.
One-way ANOVA with post hoc analysis using least significant
difference method was performed. All statistical analyses were
performed using the SPSS 17.0 software (SPSS, Inc., Chicago, IL,
USA) and P<0.05 was taken to indicate a statistically
significant difference.
Results
Agar diffusion test
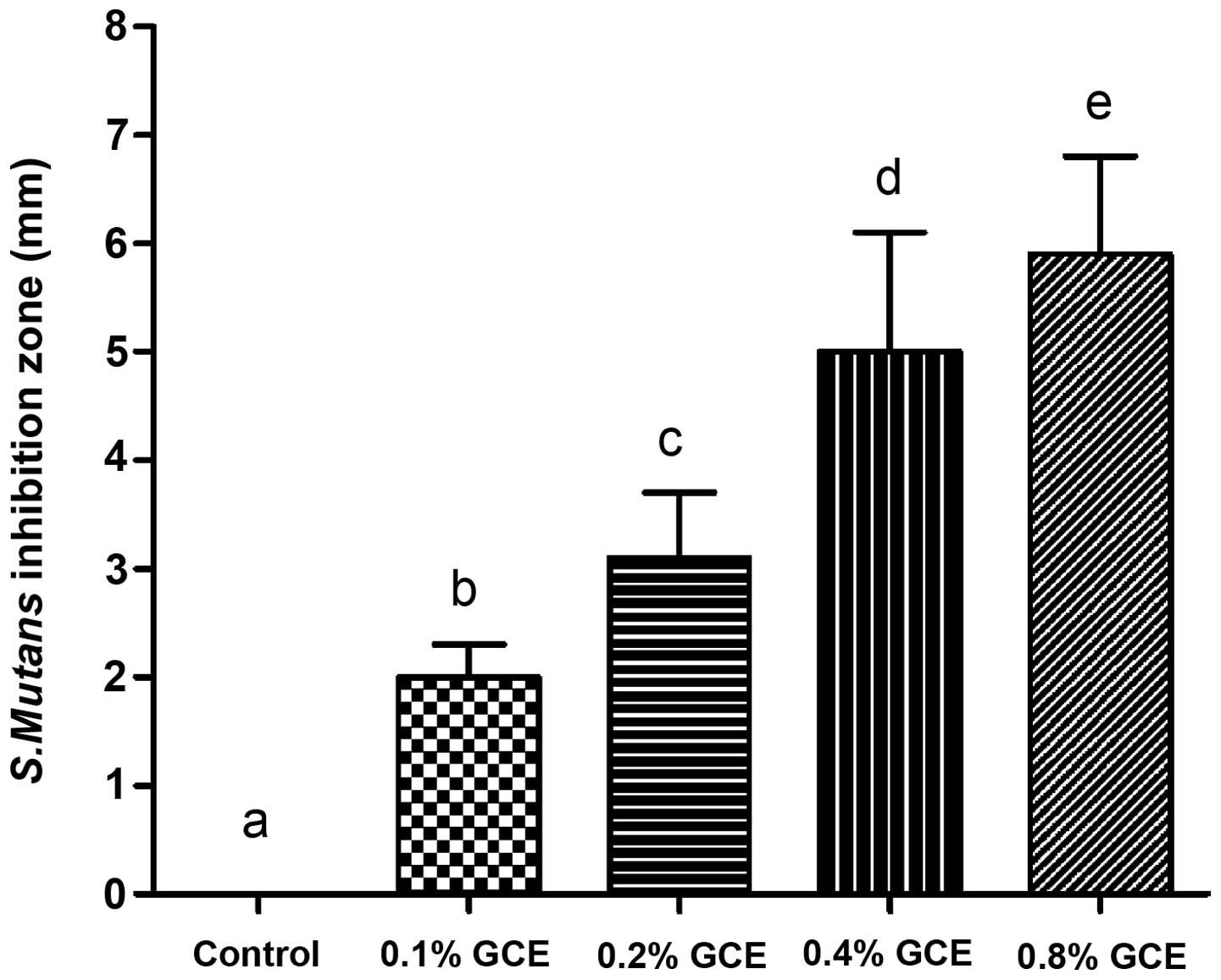
As Fig. 1 demonstrates,
all GCE-containing samples exhibited an inhibitory effect on S.
mutans and exhibited larger bacterial inhibition halos than the
control (0% GCE), with statistical significance (P<0.05). When
the GCE mass fraction increased, bacterial inhibition zone
presented a dose-dependent increase. In the present study, the 0.8%
GCE group had the largest diameter of inhibition halo (5.9±0.9
mm).
SEM evaluation for S mutans adherence
to cement specimens
The tube which contained a pasteurized inactivated
bacterial suspension had no specific adherence of S. mutans
(Fig. 2). Based on semi-quantitative
analysis, compared with control sample, S. mutans had a weaker
adherent capacity to all GCE-containing cements, however,
differences between each GCE-containing group were not
significant.
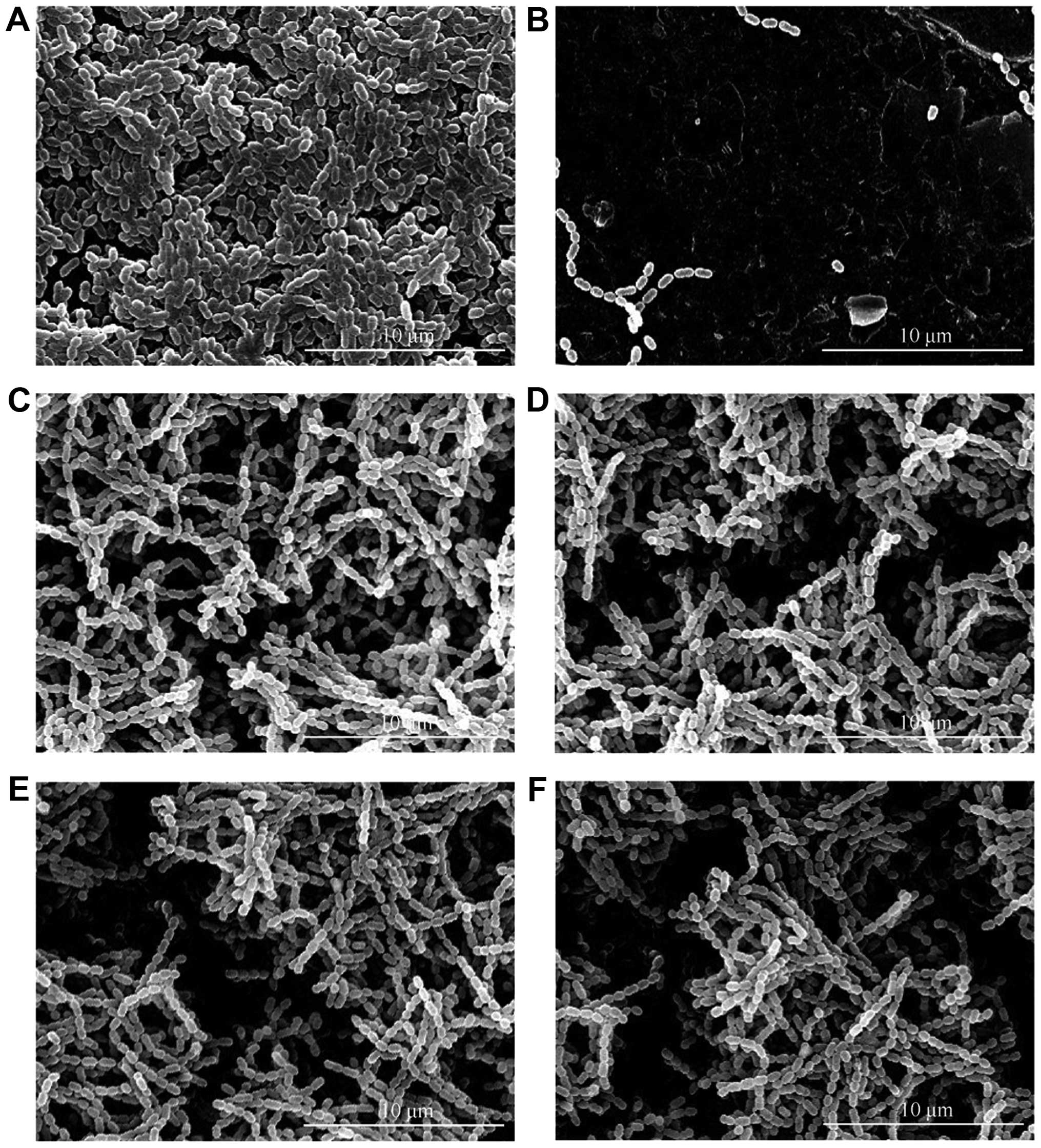 | Figure 2.SEM evaluation for S. mutans
adherence on cement specimens (20 kV, magnification x5,000). (A)
Control, (B) Inactive S. mutans adherence, (C) 0.1% GCE, (D)
0.2% GCE, (E) 0.4% GCE, (F) 0.8% GCE. S. mutans presented a
weaker adherent capacity to all GCE-containing cements compared
with control, but the difference between each GCE-containing group
was not significant. SEM, scanning electron microscopy; GCE,
Galla chinensis extract. |
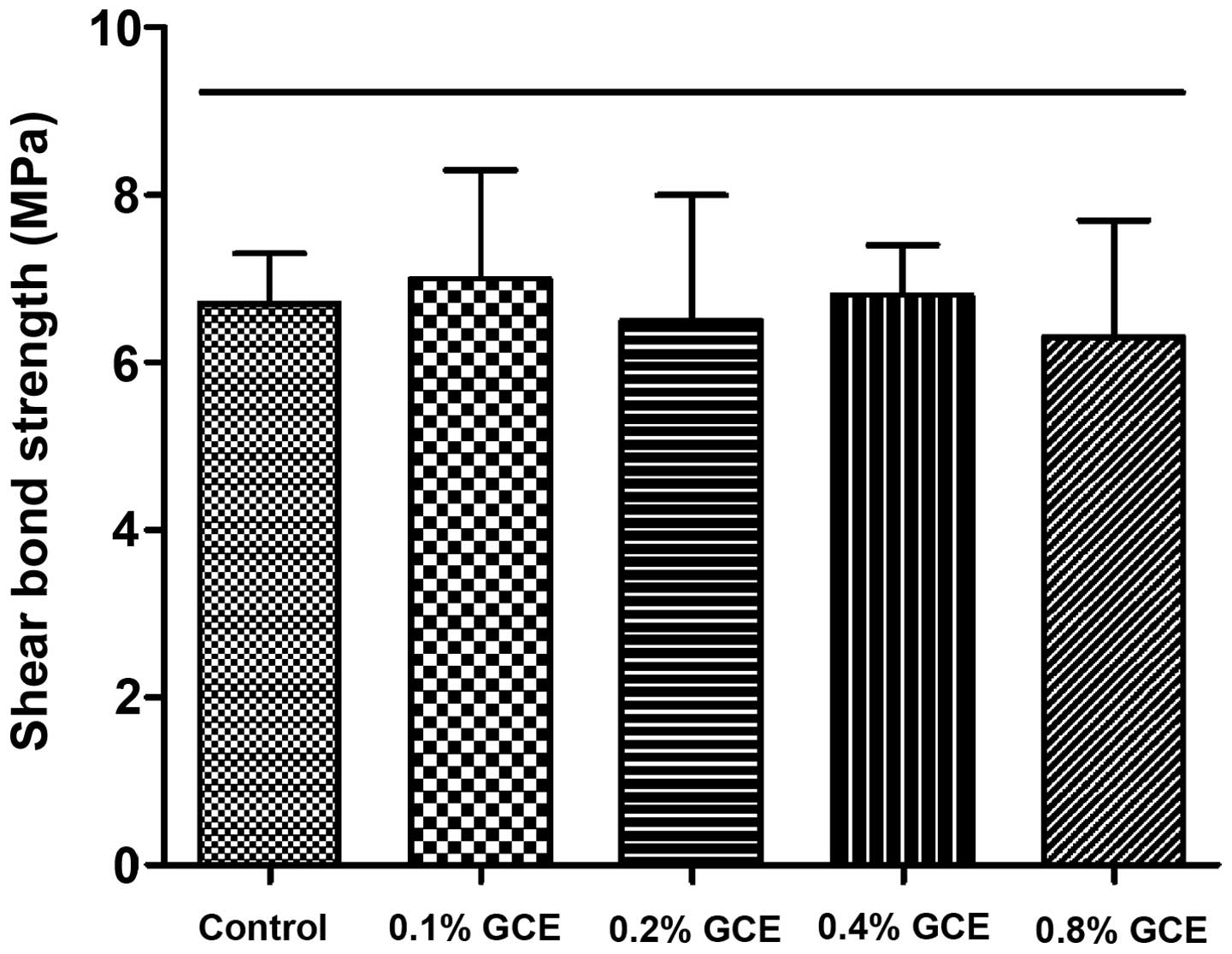
Evaluation of SBS
The SBS value of the control group (0% GCE) was
6.7±0.6 MPa. Despite the mass fraction of GCE rising from 0.1 to
0.8%, GCE-containing groups did not exhibit weaker SBS compared
with control group, indicating no bond strength impairment
(Fig. 3).
Discussion
It has been demonstrated previously that an
increased prevalence of cariogenic bacteria such as S.
mutans and Lactobacillus species in the dental biofilm
around brackets can promote enamel decalcification and the
formation of incipient caries in orthodontic patients (22,23).
Modified orthodontic bonding material containing antibacterial
components is effective at preventing white spot lesions around
brackets (24,25). In general, incorporated components
should have a strong ability of inhibiting cariogenic bacteria
growth and colonization, and the ability of promoting enamel
remineralization is preferred.
In the present study, GCE, a promising anti-caries
agent, was used for modifying orthodontic bonding material. GCE has
a wide range of biological properties, including
antiviral/antibacterial activity and accelerating blood coagulation
(26). GCE can not only inhibit
certain cariogenic bacteria growth/adherence/acid production
(27), but also has the ability of
regulating enamel demineralization/remineralization balance
(16,28). Chemical analyses have revealed that GCE
consists of several monomeric and polymeric polyphenols (e.g.,
gallotannin, gallic acid) and some other components (such as
carbohydrates and proteins). GCE can be isolated to four
subfractions (GCEs-A, B, C and D), however, these are less
effective than crude GCE regarding antimicrobial and mineralization
effects (16,27,28).
Therefore, crude GCE was used in the present study.
In the agar diffusion test, the 0.1, 0.2, 0.4 and
0.8% GCE-containing groups all exhibited a significant S.
mutans inhibition halo, and the antibacterial effect presented
a concentration-dependent enhancement. Similarly, 4 mg/ml GCE was
demonstrated to significantly inhibit the growth and metabolism of
oral biofilms in previous reports (13,27).
Although S. mutans had a weaker adhesion to all
GCE-containing cements compared with the control, it appeared that
S. mutans adherent capacity not be affected by the
concentration of GCE, which requires further verification.
SBS test results revealed that adding GCE did not
reduce the bond strength of adhesive cement. SBS values of all
GCE-containing groups were within adequate range for orthodontic
bonding (5.9–7.8 MPa), suggested previously (29,30),
indicating that modified adhesive cement containing 0.1–0.8% GCE
meets the required clinical bracket bonding.
Compared with other natural products, the
antibacterial activity of GCE appears unremarkable. However, it is
so far the only natural product able to regulate enamel
demineralization/remineralization balance, which makes it important
among various anti-caries natural products (18). The present study discussed the
antibacterial property of GCE-containing cement. Based on current
findings, it is likely that its effect in preventing enamel
demineralization or promoting remineralization would be even more
significant. The optimal concentration of incorporated GCE requires
further investigation.
In conclusion, GCE-containing adhesive cement
exhibits a promising inhibitory effect on S. mutans growth
and adhesion. Without impairing bond strength, adding GCE in
adhesive cement may be an attractive option for orthodontic
bonding.
References
|
1
|
Lovrov S, Hertrich K and Hirschfelder U:
Enamel demineralization during fixed orthodontic
treatment-incidence and correlation to various oral-hygiene
parameters. J Orofac Orthop. 68:353–363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richter AE, Arruda AO, Peters MC and Sohn
W: Incidence of caries lesions among patients treated with
comprehensive orthodontics. Am J Orthod Dentofacial Orthop.
139:657–664. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Brown WE, Gregory TM and Chow LC: Effects
of fluoride on enamel solubility and cariostasis. Caries Res.
11(Suppl 1): S118–S141. 1977. View Article : Google Scholar
|
|
4
|
Al Mulla AH, Al Kharsa S, Kjellberg H and
Birkhed D: Caries risk profiles in orthodontic patients at
follow-up using cariogram. Angle Orthod. 79:323–330. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gorelick L, Geiger AM and Gwinnett AJ:
Incidence of white spot formation after bonding and banding. Am J
Orthod. 81:93–98. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spencer CG, Campbell PM, Buschang PH, Cai
J and Honeyman AL: Antimicrobial effects of zinc oxide in an
orthodontic bonding agent. Angle Orthod. 79:317–322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Scherer W, Cooper H and Antonelli J:
Antimicrobial properties of dental dentin-enamel adhesives. J
Esthet Dent. 2:140–141. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chambers C, Stewart S, Su B, Sandy J and
Ireland A: Prevention and treatment of demineralisation during
fixed appliance therapy: A review of current methods and future
applications. Br Dent J. 215:505–511. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Passariello C, Sannino G, Petti S and
Gigola P: Intensity and duration of in-vitro antibacterial activity
of different adhesives used in orthodontics. Eur J Oral Sci.
122:154–160. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poosti M, Ramazanzadeh B, Zebarjad M,
Javadzadeh P, Naderinasab M and Shakeri MT: Shear bond strength and
antibacterial effects of orthodontic composite containing TiO2
nanoparticles. Eur J Orthod. 35:676–679. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Melo MA, Wu J, Weir MD and Xu HH: Novel
antibacterial orthodontic cement containing quaternary ammonium
monomer dimethylaminododecyl methacrylate. J Dent. 42:1193–1201.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harvey A: Strategies for discovering drugs
from previously unexplored natural products. Drug Discov Today.
5:294–300. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cheng L, Exterkate RA, Zhou X, Li J and
ten Cate JM: Effect of Galla chinensis on growth and
metabolism of microcosm biofilms. Caries Res. 45:87–92. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang L, Xue J, Li J, Zou L, Hao Y, Zhou X
and Li W: Effects of Galla chinensis on inhibition of
demineralization of regular bovine enamel or enamel disposed of
organic matrix. Arch Oral Biol. 54:817–822. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cheng L, Li J, Hao Y and Zhou X: Effect of
compounds of Galla chinensis and their combined effects with
fluoride on remineralization of initial enamel lesion in vitro. J
Dent. 36:369–373. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chu JP, Li JY, Hao YQ and Zhou XD: Effect
of compounds of Galla chinensis on remineralisation of
initial enamel carious lesions in vitro. J Dent. 35:383–387. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cheng L, Li J, Hao Y and Zhou X: Effect of
compounds of Galla chinensis on remineralization of enamel
surface in vitro. Arch Oral Biol. 55:435–440. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Djakpo O and Yao W: Rhus chinensis and
Galla chinensis-folklore to modern evidence: Review.
Phytother Res. 24:1739–1747. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cheng L, Li JY, Huang S and Zhou XD:
Effect of Galla chinensis on enhancing remineralization of enamel
crystals. Biomed Mater. 4:0341032009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martins Júnior W, De Rossi A, Samih
Georges, Abi Rached R and Rossi MA: A scanning electron microscopy
study of diseased root surfaces conditioned with EDTA gel plus
Cetavlon after scaling and root planing. J Electron Microsc
(Tokyo). 60:167–175. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brambilla E, Ionescu A, Fadini L, Mazzoni
A, Imazato S, Pashley D, Breschi L and Gagliani M: Influence of
MDPB-containing primer on Streptococcus mutans biofilm formation in
simulated Class I restorations. J Adhes Dent. 15:431–438.
2013.PubMed/NCBI
|
|
22
|
Forsberg CM, Brattström V, Malmberg E and
Nord CE: Ligature wires and elastomeric rings: Two methods of
ligation, and their association with microbial colonization of
Streptococcus mutans and lactobacilli. Eur J Orthod. 13:416–420.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosenbloom RG and Tinanoff N: Salivary
Streptococcus mutans levels in patients before, during and after
orthodontic treatment. Am J Orthod Dentofacial Orthop. 100:35–37.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Brown ML, Davis HB, Tufekci E, Crowe JJ,
Covell DA and Mitchell JC: Ion release from a novel orthodontic
resin bonding agent for the reduction and/or prevention of white
spot lesions. An in vitro study. Angle Orthod. 81:1014–1020. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Patil N, Jawale B, Redasani R, Chaudhari
L, Garde JB and Chauhan VS: In vitro caries-preventive effect of
fluoridated orthodontic resins against cariogenic challenge
stimulation. J Contemp Dent Pract. 13:452–455. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu-Yuan CD, Chen CY and Wu RT:
Gallotannins inhibit growth, water-insoluble glucan synthesis and
aggregation of mutans streptococci. J Dent Res. 67:51–55. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie Q, Li J and Zhou X: Anticaries effect
of compounds extracted from Galla chinensis in a multispecies
biofilm model. Oral Microbiol Immunol. 23:459–465. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zou L, Zhang L, Li J, Hao Y, Cheng L, Li W
and Zhou X: Effect of Galla chinensis extract and chemical
fractions on demineralization of bovine enamel in vitro. J Dent.
36:999–1004. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Reynolds IR and von Fraunhofer JA: Direct
bonding of orthodontic attachments to teeth: The relation of
adhesive bond strength to gauze mesh size. Br J Orthod. 3:91–95.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lopez JI: Retentive shear strengths of
various bonding attachment bases. Am J Orthod. 77:669–678. 1980.
View Article : Google Scholar : PubMed/NCBI
|