Introduction
Ovarian cancer is one of the three most common
malignant tumors in the female reproductive system. It has an
insidious onset with a difficult early diagnosis (1). In approximately, 70% of all cases of
ovarian cancer, the disease is not diagnosed before reaching an
advanced stage (2). The 5-year
survival rate associated with ovarian cancer is <30% (3). Over 90% of all cases of ovarian masses
detected in premenopausal and ≤60% in postmenopausal women, are
benign (4). The early diagnosis of
ovarian malignant tumor becomes a key factor in improving the
survival rate of patients. Tools currently in use for
differentiating between low- and high-risk patients with ovarian
cancer are the tumor markers carbohydrate antigen-125 (CA-125) and
the human epididymis protein 4 (HE4), as well as the index value of
risk of ovarian malignancy algorithm (ROMA) (5).
The tumor marker CA-l25 has been used for 30 years
for the monitoring of ovarian cancer, diagnosis, effective
evaluation, and recurrence (6).
Although clinical application of CA-125 has been extensive, its
specificity as a marker of malignant tumor or early diagnosis of
ovarian cancer requires reassesment (7). In premenopausal women, the detection of
CA-125 in ovarian cancer sensitivity and specificity is not ideal
because of the menstrual cycle, pregnancy and other effects
(8).
The introduction of HE4, a type of gynecological
tumor marker, has attracted much attention. HE4 has shown a
sensitivity and specificity of 72.9 and 95%, respectively, for
differentiating between types of ovarian masses, which is better
than that of CA-125 detection (9). HE4
is highly expressed in ovarian cancer, endometrial cancer tissues
and in the adjacent tissues, normal tissues and benign tumors
(10). Consequently, as an ideal tumor
marker, HE4 has received increased attention. It has been confirmed
(10–14)
that HE4 has an obvious difference in the expression level between
benign gynecological diseases such as ovarian cyst, uterine
fibroids, endometriosis, endometrial polyps and other ovarian
cancers, including endometrial and cervical cancer, which can be
used for the differential diagnosis of the disease. However, in
order to utilize the value of existing detection and to further
improve the accuracy of early diagnosis of ovarian cancer while
simultaneously assessing the risk of ovarian cancer and combining
the research results and the relevant statistical analysis, the
ROMA index value (11–16) has been introduced (17). The ROMA index value is an algorithm
that takes into account the levels of CA-125 and HE4 together with
menopausal status using quantitative and objective parameters
(18). The sensitivity and specificity
of ROMA are 88.7 and 74.7%, respectively, when applied in cohorts
of pre- and postmenopausal women (17). Previous investigations on the
application of HE4 and ROMA in ovarian cancer with results
indicated improvement in the diagnostic accuracy of ovarian cancer.
In the present study, we evaluated the values of these tools in the
global and differential diagnosis of ovarian cancer. We thus
analyzed the sera levels of HE4, CA-125 and determined the values
of the ROMA index combined with menopausal status in patients
suffering ovarian carcinoma, as confirmed by surgical treatment in
Xuzhou Central Hospital (Jiangsu, China). The period of the study
was from October 2013 to May 2015.
Subjects and methods
Clinical data
In total, the present study included 158 cases,
which were divided into the ovarian cancer, benign ovarian disease
and healthy control groups. Selected patients did not receive
chemotherapy or hormonal therapy, or a combination thereof for
other tumors or serious heart, liver and kidney disease, or
diabetes. A total of 64 patients in the ovarian cancer group were
selected between October 2013 and May 2015 in Xuzhou Central
Hospital with pelvic mass, which was examined and confirmed by
postoperative pathological findings. There were 14 cases of
papillary serous cystadenocarcinoma, 1 case of clear cell
carcinoma, 7 cases of mucinous cystadenocarcinoma and 42 cases of
serous cystadenocarcinoma.
According to the staging method of the International
Federation of Gynaecology and Obstetrics, 10 cases were stage I, 18
cases of stage II, 23 cases of stage III and 13 cases of stage IV.
The patients were aged 30–51 years with an average age of 55±11.9
years. Twenty-seven patients were in premenopausal status (aged
30–51 years, average age 43.8±6.08 years) while 37 patients were in
postmenopausal status (aged 47–81 years, average age 63±7.9 years).
The 64 patients were in the benign ovarian disease group (6 cases
of ovarian serous cystadenoma, 14 cases of ovarian mucinous
cystadenoma, 30 cases of mature ovarian teratoma, 5 cases of theca
cell tumor and 9 cases of ovarian endometriosis cyst). The patients
were aged 24–82 years, with an average age of 47.81±13.9 years. Of
the 64 patients, 40 patients were in premenopausal status (aged
24–47 years, average age 38.9±6.8 years), while 24 patients were in
postmenopausal status (aged 50–82 years, average age 62.7±9.2
years). Thirty normal females in the healthy control group
identified during the same period with no liver and kidney disease
and no tumor history, were included. The patients were aged 30–63
years, with an average age of 45.2±8.25 years. Of the 30 cases, 21
cases were at a premenopausal status, aged 30–49 years with an
average age of 40.8±5 years. Nine cases were of postmenopausal
status with an age of 51–63 years and an average age of 55.7±3.4
years.
All the subjects provided written inform consent.
The study was approved by the Ethics Committee of the Xuzhou
Central Hospital.
Sample collection
Samples were collected from all the patients prior
to surgery and 3 ml blood was collected. Serum was centrifuged at
2000 × g and stored at −20 and −80°C until use.
Sample detection
Serum CA-125 and HE4 were detected using the full
automatic chemiluminescence analyzer Cobs601 and the corresponding
kit according to manufacturer's protocol (Roche Diagnostics,
Indianapolis, IN, USA). Briefly, serum HE4 and CA-125 levels were
calculated for ROMA index value using the Roche ROMA index of
ovarian cancer risk assessment software. Serum HE4 and CA-125
reference range was <140 pmol/l and <35 U/ml,
respectively.
ROMA index calculation
The ROMA index was calculated according to the
levels of HE4 and CA-125. HE4 and CA-125 values were input to the
ovarian cancer risk assessment software, followed by automatic
calculation of the corresponding ROMA index. The premenopausal
calculation formula of the ROMA index was: 12+2.38 × LN(HE4)+0.062
6 × LN(CA-125). The postmenopausal calculation formula of the ROMA
index was: 8.09+1.04 × LN(HE4)+0.732 × LN(CA-125). When Roche
Elecsys specificity was 75%, premenopausal women with a ROMA value
≥11.4, had a higher risk of ovarian cancer. Postmenopausal women
with ROMA value ≥29.9 had a higher risk of ovarian cancer.
Statistical analysis
SPSS 16.0 statistical software (SPSS, Inc., Chicago,
IL, USA) was used for statistical analysis. HE4, CA-125, ROMA index
and other non-normal measurement data were shown as the quartile
interval. The count data were shown using rate. The use of the rank
sum test (Man-whitney U test) and Chi-square test data were
statistically analyzed. The area under curve (AUC) of receiver
operating characteristic (ROC) were calculated for a comparison of
the three test methods. P<0.05 was considered to indicate a
statistically significant difference.
Results
Comparison of the difference of serum
HE4 and CA-125 levels and the ROMA index between groups
The serum levels of HE4, CA-125 and ROMA index in
the ovarian cancer group were significantly higher than those in
the benign tumor and healthy control groups, and there was
significant difference (P<0.05). The expression level of HE4 and
ROMA index in the benign tumor group was not significantly
different. The expression level of CA-125 in serum was
significantly higher than that in the healthy control group
(P<0.05, Table I).
 | Table I.Sera levels of HE4, CA-125 and ROMA
index of three groups. |
Table I.
Sera levels of HE4, CA-125 and ROMA
index of three groups.
| Parameters | Healthy control
group | Benign tumor
group | Ovarian cancer
group |
|---|
| Cases | 30 | 64 | 64 |
| HE4 | 39.04±8.38 | 54.76±42.35 |
739.03±860.04a,b |
| CA-125 | 15.08±5.28 |
49.07±175.61a |
868.85±1204.08a,b |
| ROMA index | 6.18±2.21 | 10.15±11.98 |
76.30±28.57a,b |
Evaluation of serum HE4, CA-125 and
ROMA in the diagnosis of ovarian cancer
The patients in the ovarian benign disease and
healthy control groups were further divided into the pre- and
postmenopausal groups. The patients with ovarian cancer were
divided into the pre- and postmenopausal groups. The serum levels
of HE4, CA-125 and ROMA index were detected to evaluate the
sensitivity, specificity, positive predictive value and negative
predictive value of HE4, CA-125 and ROMA standardized with
pathological diagnosis (Table II).
The ROMA index, and a comparison of the sera levels of CA-125 and
HE4 in the diagnosis of ovarian cancer in each group indicated
significant differences between the three groups (P<0.001,
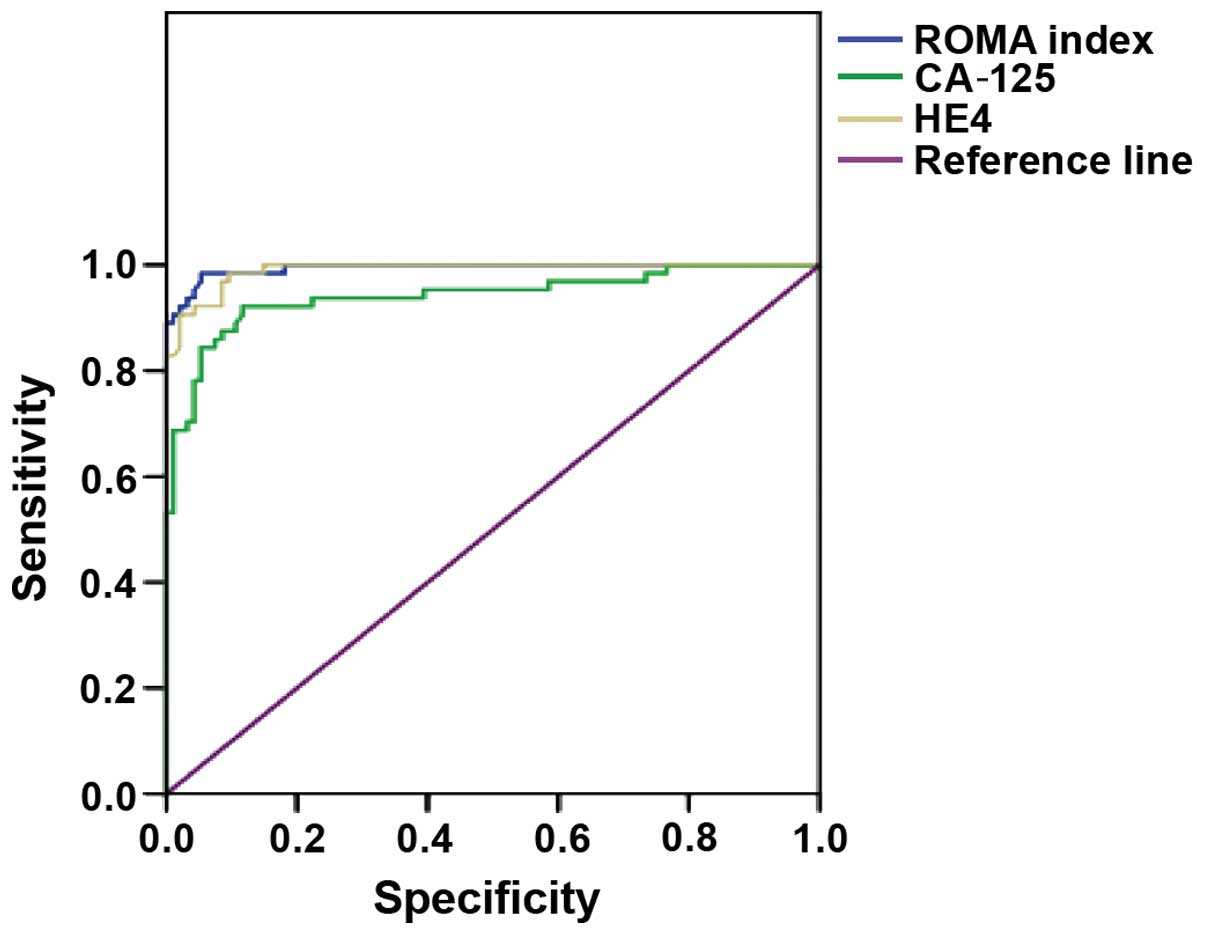
Table III). The AUC of ROC of the
ROMA index, HE4 and CA-125 in the diagnosis of ovarian cancer
gradually decreased to 0.994, 0.990 and 0.941, respectively
(Fig. 1).
 | Table II.The diagnostic values of CA-125, HE4
and ROMA in ovarian cancer compared with the golden standard. |
Table II.
The diagnostic values of CA-125, HE4
and ROMA in ovarian cancer compared with the golden standard.
| Characteristics | Sensitivity (%) | Specificity (%) | Positive predictive
value (%) | Negative predictive
value (%) |
|---|
| Total |
|
|
CA-125 | 85.07 (57/64) | 92.31 (84/94) | 90.6 (57/67) | 89.36 (84/91) |
| HE4 | 75 (48/64) | 97.87 (92/94) | 96 (48/50) | 85.19
(92/108) |
| ROMA
index | 93.75 (60/64) | 92.55 (87/94) | 89.55 (60/67) | 86.14
(87/101) |
| Premenopausal |
|
|
CA-125 | 92.59 (25/27) | 88.52 (54/61) | 78.13 (25/32) | 96.43
(54/56) |
| HE4 | 70.37 (19/27) | 98.36 (60/61) | 95.00 (19/20) | 88.24
(60/68) |
| ROMA
index | 96.3 (26/27) | 88.52 (54/61) | 78.79 (26/33) | 98.18
(54/55) |
| Postmenopausal |
|
|
CA-125 | 86.49 (32/37) | 90.9 (30/33) | 91.43 (32/35) | 90.90
(30/35) |
| HE4 | 78.38 (29/37) | 96.97 (32/33) | 96.67 (29/30) | 80.00
(32/40) |
| ROMA
index | 91.89 (34/37) | 96.97 (32/33) | 97.14 (34/35) | 91.43
(32/35) |
 | Table III.The diagnostic values of CA-125, HE4
and ROMA in ovarian cancer. |
Table III.
The diagnostic values of CA-125, HE4
and ROMA in ovarian cancer.
|
| CA-125 | HE4 |
|---|
|
|
|
|
|---|
| Characteristics | Positive | Negative | Positive | Negative |
|---|
| Total |
|
| ROMA
index |
|
|
Positive | 57 | 10 | 48 | 18 |
|
Negative | 10 | 81 | 2 | 90 |
|
χ2 | 86.721 |
| 89.755 |
|
|
P-value | <0.001 |
| <0.001 |
|
| Premenopausal |
|
| ROMA
index |
|
|
Positive | 25 | 8 | 19 | 13 |
|
Negative | 7 | 48 | 1 | 55 |
|
χ2 | 35.41 |
| 39.27 |
|
|
P-value | <0.001 |
| <0.001 |
|
| Postmenopausal |
|
| ROMA
index |
|
|
Positive | 32 | 2 | 29 | 5 |
|
Negative | 3 | 33 | 1 | 35 |
|
χ2 | 51.47 |
| 48.62 |
|
|
P-value | <0.001 |
| <0.001 |
|
Discussion
The early diagnosis of ovarian malignancies is one
of the key factors for improving the survival rate of patients
(19). CA-125 has been used as a tumor
marker for the diagnosis and monitoring of ovarian cancer for 30
years, and is also used for efficacy evaluation and monitoring of
recurrence (8). Data have shown that
the serum levels of CA-125, HE4 and ROMA in ovarian cancer patients
were significantly higher than those of the patients with ovarian
benign disease and healthy women (20). The specificity and positive predictive
value of HE4 for ovarian cancer was the highest, and the
sensitivity of ROMA index was the highest. In the present study,
the 158 cases were divided into the premenopausal and
postmenopausal group to evaluate the three indicators in the
diagnostic value of ovarian cancer. The ROMA index demonstrated the
highest sensitivity and negative predictive value for ovarian
cancer. HE4 had the highest specificity and positive predictive
value. The specificity of HE4 for ovarian cancer was higher in the
postmenopausal women, as reported elsewhere (21). The sensitivity, specificity, positive
predictive value and negative predictive value of the ROMA index in
ovarian cancer were the highest (91.89, 96.97, 97.14 and 91.45%),
respectively. CA-125 and HE4 were significantly different from the
ROMA index, and the ROMA index was significantly better than CA-125
and HE4 in the diagnosis of ovarian cancer. In addition, the ROC
curve drawn in this study for the benign tumor of ovary and healthy
control groups, identified that the area under the ROC curve of
CA-125, HE4 and ROMA index was increased by 0.941, 0.990 and 0.994,
respectively. This result confirmed the clinical diagnostic value
of the ROMA index (5). It also showed
that detection of ROMA index in the diagnosis of ovarian cancer was
higher than CA125 and HE4.
In conclusion, application of the ROMA index and HE4
for the diagnosis of ovarian cancer was found to be effective and
it has good clinical application value, which may be useful for
clinicians.
References
|
1
|
Smith LH and Oi RH: Detection of malignant
ovarian neoplasms: A review of the literature. I. Detection of the
patient at risk; clinical, radiological and cytological detection.
Obstet Gynecol Surv. 39:313–328. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang Z, Bast RC Jr, Yu Y, Li J, Sokoll
LJ, Rai AJ, Rosenzweig JM, Cameron B, Wang YY, Meng XY, et al:
Three biomarkers identified from serum proteomic analysis for the
detection of early stage ovarian cancer. Cancer Res. 64:5882–5890.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heintz AP, Odicino F, Maisonneuve P, Quinn
MA, Benedet JL, Creasman WT, Ngan HY, Pecorelli S and Beller U:
Carcinoma of the fallopian tube. FIGO 26th Annual Report on the
Results of Treatment in Gynecological Cancer. Int J Gynaecol
Obstet. 95(Suppl 1): S145–S160. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Enakpene CA, Omigbodun AO, Goecke TW,
Odukogbe AT and Beckmann MW: Preoperative evaluation and triage of
women with suspicious adnexal masses using risk of malignancy
index. J Obstet Gynaecol Res. 35:131–138. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Karlsen MA, Sandhu N, Høgdall C,
Christensen IJ, Nedergaard L, Lundvall L, Engelholm SA, Pedersen
AT, Hartwell D, Lydolph M, et al: Evaluation of HE4, CA125, risk of
ovarian malignancy algorithm (ROMA) and risk of malignancy index
(RMI) as diagnostic tools of epithelial ovarian cancer in patients
with a pelvic mass. Gynecol Oncol. 127:379–383. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Folk JJ, Botsford M and Musa AG:
Monitoring cancer antigen 125 levels in induction chemotherapy for
epithelial ovarian carcinoma and predicting outcome of second-look
procedure. Gynecol Oncol. 57:178–182. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Urban N, McIntosh MW, Andersen M and
Karlan BY: Ovarian cancer screening. Hematol Oncol Clin North Am.
17:989–1005, ix. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jacobs I and Bast RC: The CA 125
tumour-associated antigen: A review of the literature. Hum Reprod.
4:1–12. 1989.PubMed/NCBI
|
|
9
|
Moore RG, Brown AK, Miller MC, Badgwell D,
Lu Z, Allard WJ, Granai CO, Bast RC and Lu K: Utility of a novel
serum tumor biomarker HE4 in patients with endometrioid
adenocarcinoma of the uterus. Gynecol Oncol. 110:196–201. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levanon K, Crum C and Drapkin R: New
insights into the pathogenesis of serous ovarian cancer and its
clinical impact. J Clin Oncol. 26:5284–5293. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Holcomb K, Vucetic Z, Miller MC and Knapp
RC: Human epididymis protein 4 offers superior specificity in the
differentiation of benign and malignant adnexal masses in
premenopausal women. Am J Obstet Gynecol. 205:358.e1–6. 2011.
View Article : Google Scholar
|
|
12
|
Hamed EO, Ahmed H, Sedeek OB, Mohammed AM,
Abd-Alla AA and Abdel Ghaffar HM: Significance of HE4 estimation in
comparison with CA125 in diagnosis of ovarian cancer and assessment
of treatment response. Diagn Pathol. 8:112013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kadija S, Stefanovic A, Jeremic K,
Radojevic MM, Nikolic L, Markovic I and Atanackovic J: The utility
of human epididymal protein 4, cancer antigen 125, and risk for
malignancy algorithm in ovarian cancer and endometriosis. Int J
Gynecol Cancer. 22:238–244. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sandri MT, Bottari F, Franchi D, Boveri S,
Candiani M, Ronzoni S, Peiretti M, Radice D, Passerini R and Sideri
M: Comparison of HE4, CA125 and ROMA algorithm in women with a
pelvic mass: Correlation with pathological outcome. Gynecol Oncol.
128:233–238. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Moore RG, Jabre-Raughley M, Brown AK,
Robison KM, Miller MC, Allard WJ, Kurman RJ, Bast RC and Skates SJ:
Comparison of a novel multiple marker assay vs the Risk of
Malignancy Index for the prediction of epithelial ovarian cancer in
patients with a pelvic mass. Am J Obstet Gynecol. 203:228.e1–6.
2010. View Article : Google Scholar
|
|
16
|
Park Y, Kim Y, Lee EY, Lee JH and Kim HS:
Reference ranges for HE4 and CA125 in a large Asian population by
automated assays and diagnostic performances for ovarian cancer.
Int J Cancer. 130:1136–1144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Moore RG, McMeekin DS, Brown AK,
DiSilvestro P, Miller MC, Allard WJ, Gajewski W, Kurman R, Bast RC
and Skates SJ: A novel multiple marker bioassay utilizing HE4 and
CA125 for the prediction of ovarian cancer in patients with a
pelvic mass. Gynecol Oncol. 112:40–46. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Toss A, De Matteis E, Rossi E, Casa LD,
Iannone A, Federico M and Cortesi L: Ovarian cancer: Can proteomics
give new insights for therapy and diagnosis? Int J Mol Sci.
14:8271–8290. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yancik R: Ovarian cancer. Age contrasts in
incidence, histology, disease stage at diagnosis, and mortality.
Cancer. 71(Suppl 2): 517–523. 1993.PubMed/NCBI
|
|
20
|
Molina R, Escudero JM, Augé JM, Filella X,
Foj L, Torné A, Lejarcegui J and Pahisa J: HE4 a novel tumour
marker for ovarian cancer: Comparison with CA 125 and ROMA
algorithm in patients with gynaecological diseases. Tumour Biol.
32:1087–1095. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lowe KA, Shah C, Wallace E, Anderson G,
Paley P, McIntosh M, Andersen MR, Scholler N, Bergan L, Thorpe J,
et al: Effects of personal characteristics on serum CA125,
mesothelin, and HE4 levels in healthy postmenopausal women at
high-risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev.
17:2480–2487. 2008. View Article : Google Scholar : PubMed/NCBI
|