Introduction
Femoral head necrosis, also known as avascular
necrosis, is caused by partial or complete ischemia of the femoral
head, followed by the pathological necrosis of bone cells, bone
marrow cells and fat cells (1).
Current treatments focus on surgery. However, due to its
significant cost, high risk, short life of joint replacement and
frequent surgeries, the majority of patients cannot afford the
expense and the pain associated with the treatment (2,3). Therefore,
it is more practical to identify a medicine that is low cost and
has fewer side effects (4).
Chlorogenic acid is anti-inflammatory and antibacterial, and may
effectively protect osteoblasts and cure femoral head necrosis. The
present study established a rat hormone model of femoral head
necrosis at the cellular level, and discussed the treatment
mechanism of chlorogenic acid on activating osteoblasts and
decreasing apoptosis, which may be of a specific clinical
significance.
Materials and methods
Materials
Neonatal Wistar rats (SPF class) were purchased from
the Animal Center of Sun Yat-sen University (Guangzhou, China).
Dulbecco's modified Eagle's medium (DMEM)/F12 medium and fetal
bovine serum were purchased from Gibco (Thermo Fisher Scientific,
Inc., Waltham, MA, USA). B-cell lymphoma 2 (Bcl-2) monoclonal mouse
anti-rabbit primary antibody (cat. no. 1790-1; 1:500) and Bax
monoclonal mouse anti-rabbit primary antibody (cat. no. 1119-3;
1:500) were from Sigma-Aldrich (St. Louis, MO, USA), trypsin was
from Hyclone, Co. (Thermo Fisher Scientific, Inc.) and the
prednisolone acetate injection was purchased from Xianju
Pharmaceutical Co., Ltd. (Zhejiang, China).
The instruments used were a CO2 incubator
(Forma series; Thermo Fisher Scientific, Inc.); clean benches
(Suzhou Purification Plant, Suzhou, China); microplate reader
(BioTek, Swindon, UK); an inverted microscope (Nikon, Tokyo,
Japan); flow cytometer (FACSCalibur; BD Biosciences, San Diego, CA,
USA); electrophoresis tank, protein transfer tank, electrophoresis
apparatus and power (all Bio-Rad, Berkeley, CA, USA); basic
electronic balance (Mettler-Toledo Ltd., Leicester, UK); and DDL-5
centrifuge (Shanghai Anting Scientific Instrument Factory,
Shanghai, China).
Establishing the rat model
A total of 60 rats were divided into 5 groups of 12
rats, as follows: A, the normal group, which was treated with
saline; B, the model group, which was treated with saline; C,
chlorogenic acid low-dose group, which was treated with a 1 mg/kg
dose; D, chlorogenic acid middle-dose group, which was treated with
a 10 mg/kg dose; and E, chlorogenic acid high-dose group, which was
treated with a 20 mg/kg dose. Following the modeling, group B was
treated daily with saline for 4 weeks, and groups C-E were treated
daily at the respective doses for 4 weeks.
Hemorheological measurement
Blood samples were obtained from all the rats, and
the whole blood high shear value (200/sec), whole blood middle
shear value (30/sec), whole blood low shear value (3/sec),
erythrocyte sedimentation rate (mm/h), hematocrit (l/l) and the
plasma viscosity (mPa.sec) were measured according to the
manufacturer's instructions.
Detection of the bone mineral
density
The left lower limb femur of the rats was obtained,
the muscles and fascia were removed, and the sample was maintained
under a 4°C environment. Subsequently, the sample was placed in a
dual-energy X-ray absorptiometry for the bone mineral density
measurement.
Hematoxylin and eosin (H&E)
pathological observation
Samples of femoral head muscle of rats were removed,
fixed by 10% neutral formalin and underwent decalcification with 5%
nitric acid for 5 days. The bone tissue was pricked with a pin to
test zero resistance, and following this was rinsed with clean
water for 24 h. The sample was placed in a dryer for dehydration,
embed with paraffin, sliced and H&E stained to observe the
cartilage bone cells under light microscopy.
Detecting the hydroxyproline (HOP) and
hexosamine (HOM) contents
The rats were anesthetized with ether, and the
orbital blood was removed. A modified chloramine-T method was used
to detect the contents of HOP and HOM.
Preparation of drug-containing serums
and culture of osteoblasts
Following modeling and treatment, the orbital blood
and blood serum were removed from the rats, mixed within the same
group, and frozen to study the impact of drug-containing serum on
the culture of osteoblasts in vitro. A total of 5 newborn
1-day-old rats were used, their skulls were removed under sterile
conditions and placed into a petri dish containing D-Hank's
solution. The inner and outer membrane were carefully removed, the
sutural bone tissue was cut away, and subsequently rinsed with
D-Hank's solution 3 times. The tissue was placed into 5 ml of 0.25%
trypsin and agitated at 37°C for a 20-min digestion. The
supernatant was removed with a sterile syringe by suction, and the
sample was cut into 1×1-mm sections and transferred into flasks.
Following this, 5 ml of 0.1% type II collagenase was added and the
samples were agitated under 37°C for digestion, and the digestive
liquid was collected.
The aforementioned process was repeated once more,
and the two digestive liquids were mixed, passed through a 200-mesh
sieve and centrifuged at 279.5 × g for 10 min. The lower layer was
removed and washed twice with culture solution. Following this, 2
ml DMEM culture solution containing 15% fetal bovine serum was
added, and the precipitated cell clumps were percussed to disperse
uniformly. Following counting, the cells were seeded in culture
flasks, with each flask containing 2×105 cells/ml, and
the flasks were placed in a 37°C humidified incubator with 5%
CO2 for 12 h. Subsequently, the solution was changed to
remove the non-adherent cells, once every 2 days. After the cells
were seeded in a monolayer, the sample was washed three times with
D-Hank's solution, and digested with 0.25% trypsin for 5 min,
passaged, and the passaged osteoblast suspension was collected.
Detecting osteoblast proliferation by
the MTT assay
The passaged osteoblast suspension was centrifuged
at 279.5 × g for 10 min, the supernatant was discarded and the
sample was washed twice with balanced salt solution. Following
counting, the cell density was adjusted to 1×105/ml. The
cells were seeded in a 96-well plate, diluted 1:40 in the
drug-containing serum with culture medium, and 0.1 ml was added
into each well, with 3 compound wells for each group. The samples
were placed in the 5% CO2 cell incubator at 37°C for 48
h, and the MTT solution was added prior to reading the colorimetric
absorbance of each well, at wavelengths of 570 and 630 nm.
Measuring the cycle changes of the
osteoblasts with flow cytometry
After 48 h, the 4 groups were digested with trypsin,
and the cell concentration was adjusted to 5×106/ml,
fixed with 70% ethanol for 1 h at 4°C, centrifuged at 279.5 × g and
washed with deionized water. Following this, 1 ml PI-containing DNA
fluorescence staining solution was placed into each well, and the
samples were dyed for 30 min at 4°C. Subsequent to filtering, the
apoptotic cells in the G1 phase, S phase and the
G2/M cell numbers were measured with flow cytometry.
Measuring Bax and Bcl-2 expression
levels
After 2 h of spinal cord injury treatment, the
spinal tissue was removed with 1 cm around the injury center, and
the sample was immediately placed at −80°C for extracting the
protein. The spinal tissue was homogenized, centrifuged and the
supernatant was decanted. The protein content was measured using
the bicinchoninic acid assay method. A 15% gel was formulated,
loaded and electrophoresis occurred prior to transferring to a
membrane, which was closed with skimmed milk powder, and the
samples were reacted with the primary Bcl-2 antibody, secondary
antibody (goat anti-mouse) and chemiluminescence was performed.
Statistical analysis
All the experiments were repeated three times, and
SPSS 13.0 (SPSS, Inc., Chicago, IL, USA) was used for the
Bonferroni test and one-way analysis of variance, and pairwise
comparisons were made between multiple samples. P<0.05 was
considered to indicate a statistically significant difference.
Results
Bone mineral density test
Following injection with prednisolone acetate, in
comparison with group A, the femoral head bone mineral density of
the group B exhibited significant differences, as well as the
femoral neck sites, indicating the success of modeling. Following
treatment, the bone mineral density of the femoral head and neck
increased at different degrees corresponding to the doses of
chlorogenic acid (Table I).
 | Table I.Impact of drugs on bone mineral
density. |
Table I.
Impact of drugs on bone mineral
density.
| Group | Bone density of
femoral head position | Femur bone mineral
density neck site |
|---|
| A |
0.24±0.02a–c |
0.16±0.01a–c |
| B |
0.09±0.00 |
0.07±0.00 |
| C |
0.13±0.02d |
0.10±0.01 |
| D |
0.17±0.01d,e |
0.13±0.01d |
| E |
0.20±0.02a,b,f |
0.14±0.02a,c,f |
H&E staining
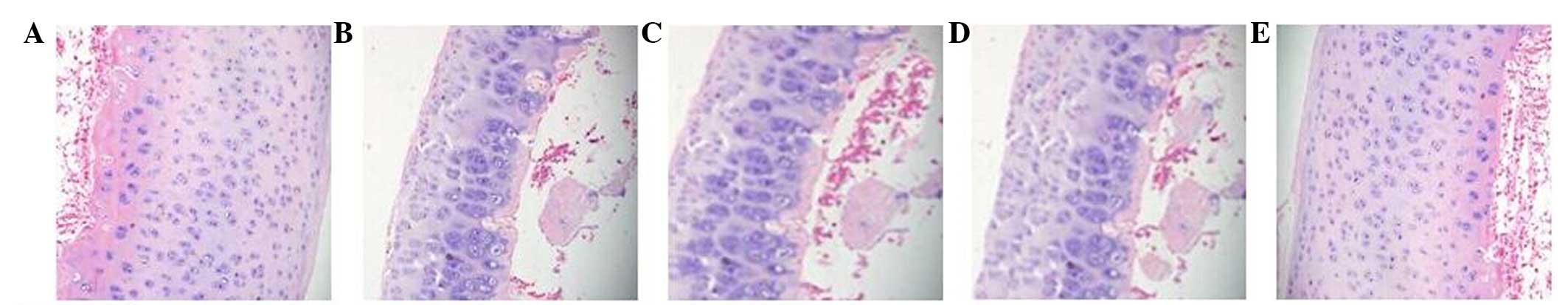
The H&E staining observations were as follows:
Group A exhibited a smooth cartilage surface, no inflammatory
articular cavum and no chondrocyte necrosis, while group B
demonstrated necrosis and disappearance of cartilage cell, as well
as cartilage depression. Instead, there were proliferations of
fibrous connective tissues, a small hemorrhage around, and more red
blood cells in the marrow cavity. Group E (high-dose) exhibited
mild depression of the cartilage surface, certain cell degeneration
and a clearly visible joint cavity. Group D (middle-dose) revealed
a smooth cartilage surface, few punctate depressions, some cell
necrosis and a clearly visible joint cavity. Group C (low-dose)
demonstrated focal cartilage surface defect, surrounding cell
necrosis, some proliferation of fibrous connective tissue and
chronic inflammatory cell infiltration in the marrow cavity
(Fig. 1).
HOM/HOP analysis
Following injection with the hormones, the bone
structure was destroyed and absorbed, collagen catabolism
increased, and the amount of HOP metabolites was also increased.
The main component of bone tissue, such as mucopolysaccharides,
decomposed, leading to the decrease of HOM, the main component of
mucopolysaccharides. Thus, the HOM/HOP value decreased relatively
(when comparing groups A and B) (Table
II).
 | Table II.Impact of drugs on HOM and HOP under
femoral head avascular necrosis. |
Table II.
Impact of drugs on HOM and HOP under
femoral head avascular necrosis.
| Group | HOM, µg/mg | HOP, µg/mg | HOM/HOP |
|---|
| A |
9.04±0.35a,b |
51.42±2.39a,c |
0.19±0.01a,b |
| B |
5.34±0.61 |
72.77±8.81 |
0.07±0.00 |
| C |
7.43±0.52d |
62.11±5.01 |
0.10±0.01 |
| D |
7.89±0.11d |
60.02±6.23d |
0.11±0.02 |
| E |
8.43±0.55a,b |
53.21±2.43a,b |
0.16±0.01b,d |
Hemorheological assessment
The hemorheological results showed that under the
condition of femoral head necrosis, whole blood viscosity
significantly reduced and blood viscosity improved when comparing
groups D and E with group B. An increase of the erythrocyte
sedimentation rate and hematocrit indicated ‘strong’ (plasma
viscosity), and chlorogenic acid can make them weak. While the
increase of blood viscosity means ‘condensate’ (high shear
viscosity), and chlorogenic acid can dilute it (Table III).
 | Table III.Hemorheological results of drugs on
rat blood. |
Table III.
Hemorheological results of drugs on
rat blood.
| Group | High shear viscosity,
200/sec | Middle shear
viscosity, 30/sec | Low shear viscosity,
3/sec | Hematocrit, mm/h | Erythrocyte
sedimentation, l/l | Plasma viscosity,
mPa.sec |
|---|
| A |
14.21±1.23a,b |
6.47±1.08a,c |
4.56±1.07a,b |
1.32±0.10a,b |
0.34±0.14a,b |
1.38±0.08a,b |
| B |
23.55±2.06 |
9.27±1.41 |
6.98±0.32 |
2.78±0.55 |
0.72±0.24 |
2.18±1.01 |
| C |
17.99±2.93 |
7.64±1.72 |
5.87±0.09 |
2.09±0.17 |
0.59±0.35 |
1.81±0.56 |
| D |
16.41±1.57d |
7.21±1.71d |
5.22±0.56d |
1.66±0.24c,d |
0.48±0.18 |
1.65±0.28c,d |
| E |
15.42±1.10a,c |
6.90±1.54a |
5.00±0.43a,c |
1.49±0.21a |
0.39±0.08c,d |
1.44±0.32a,b |
Impact of the drug-containing serum on
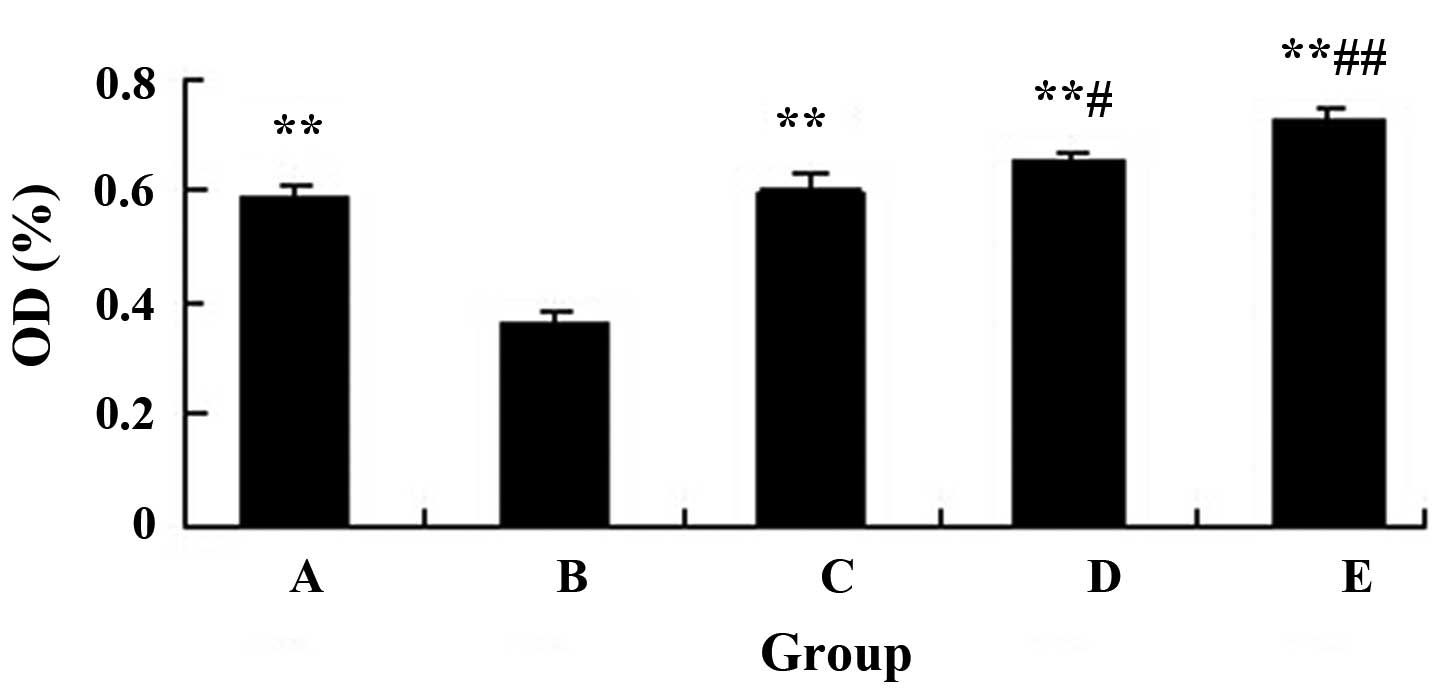
osteoblast proliferation
The drug-containing serum enhanced the proliferation
of osteoblasts, and there were significant differences between
groups B and C-E (Fig. 2).
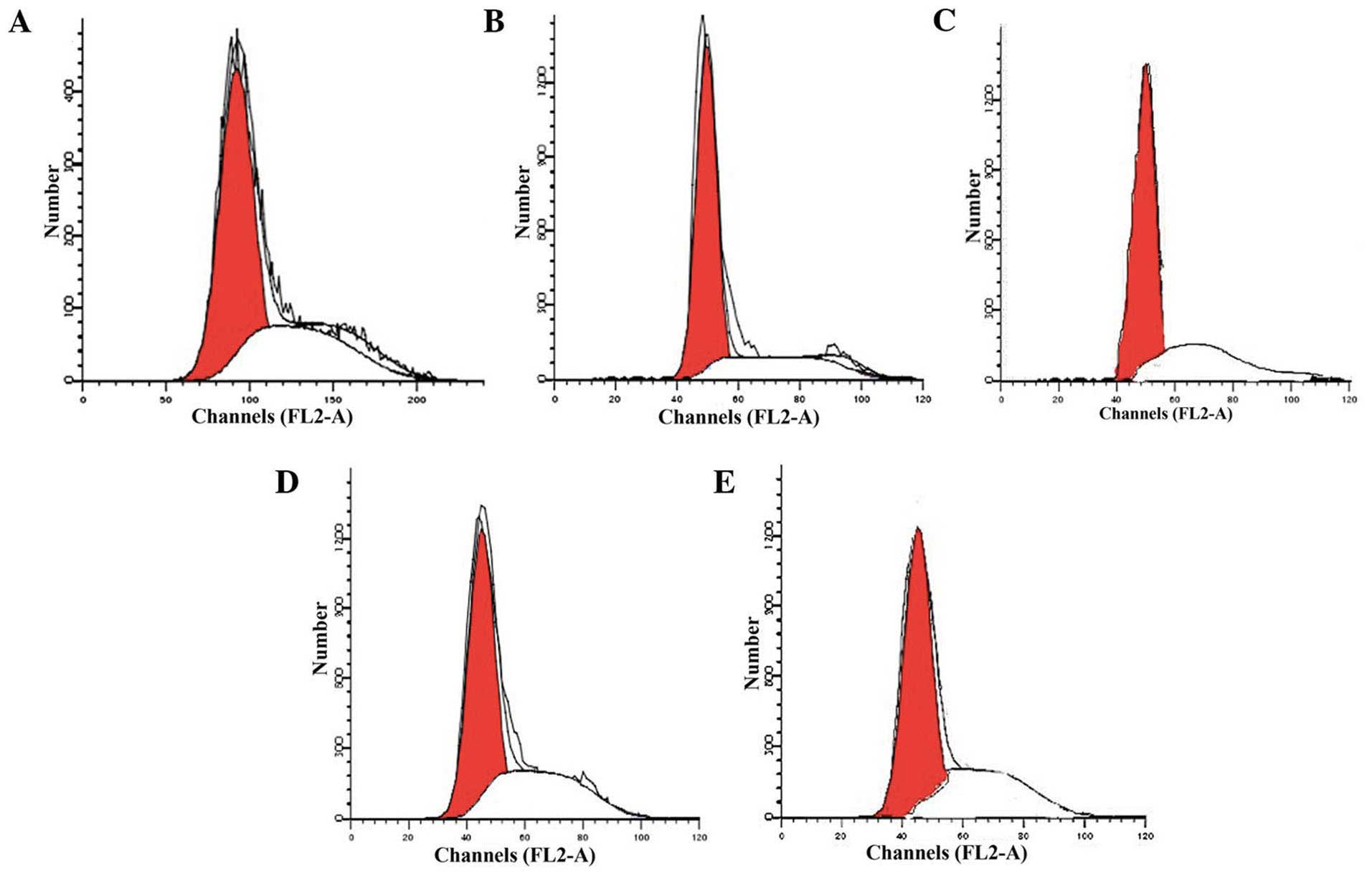
Measurement of apoptosis by flow
cytometry
The G1 phase cell cycle is the
preparation of DNA and protein synthesis, indicating the start of
the proliferation stage. The flow cytometry analysis indicated that
a large number of osteoblasts of the group B rats remained in the
G1 phase, and only a small number remained in the S
phase, indicating that prednisolone acetate can cause numerous
osteoblasts to remain in the G1 phase, thereby blocking
cells into the S phase, and thus, blocking DNA synthesis,
inhibiting cell mitosis and inducing apoptosis. However, following
the addition of the drug-containing serum, osteoblasts in the
G1 phase significantly reduced, and cells in the S phase
increased, suggesting that the serum can facilitate osteoblast
proliferation and reduce apoptosis (Fig.
3).
Measuring the expression levels of Bax
and Bcl-2 using western blotting
The western blotting results showed that when
compared with the normal group, the Bax expression level increased
and the Bcl-2 expression level decreased. Following treatment, the
Bax expression in the middle- and high-dose groups reduced
significantly; however, the reduction in the low-dose group was
smaller. By contrast, the Bcl-2 expression level increased
significantly in the high- and middle-dose groups, and the increase
was small in the low-dose group. Therefore, the treatment regulated
the expression levels of these proteins, and improved the
capabilities of osteoblast differentiation and maturity (Fig. 4).
Discussion
Femoral head necrosis is a common orthopedic disease
with one of the highest incidences of bone disease, and has high
mortality and morbidity. It is known to cause significant suffering
to patients (5,6). Chlorogenic acid can improve
cardiovascular and cerebrovascular blood flow, enhance
hematopoietic and immune functions, as well as bone metabolism. The
present study is based on this type of metabolism.
Osteoblasts, common in the growth of bone tissue,
gather in the newly formed bone surface, and have an important role
in the process of bone tissue development, bone metabolism and bone
mass balance maintenance and damage repair (7–9). Femoral
head necrosis is a common intractable clinical orthopedic disease,
and previous studies found that it may be closely associated with
the dysfunction of osteoblasts and bone marrow stromal cells
(10–12). The mechanism is that bone marrow
stromal cells differentiate into adipocytes, and hormones regulate
bone marrow stem cell differentiation into fat cells. Following
this, the stem cell pool cannot produce enough bone cells, and the
adipose tissue accumulates and fills in the bone marrow cavity,
thus resulting in increased internal pressure within the bone so
that microvascular arterial compression occurs. Following this,
venous obstruction decreases, blood stasis is blocked, metabolites
accumulate, capillary permeability increases, plasma extravasation
occurs and bone marrow stromal edema is observed. A vicious cycle
is formulated, leading to a large incidence of osteoblast ischemic
and anoxia necrosis, and avascular necrosis eventually occurs
(13).
In patients with osteonecrosis, reduced viability of
bone marrow stromal cells was observed in hematopoietic and
interstitial tissues; proliferation was inhibited and no adequate
osteoblasts were present to repair lesions in the early stage of
osteonecrosis remodeling. Furthermore, in the femoral head
ultrastructure necrosis, osteoblast-like cell death and lysis were
also identified (14). This indicated
that femoral head necrosis is closely associated with the change in
the number of bone cells.
The cell MTT results suggest that chlorogenic acid
of different densities can facilitate the proliferation of
osteoblasts. The flow cytometry results also demonstrated that the
G1 phase is the preparation stage of DNA and protein
synthesis, and the marker into the proliferation phase. Numerous
osteoblasts of the model group rats remained in phase
G1, and only a few remained in the S phase, indicating
that prednisolone acetate can cause them to remain in the
G1 phase, thereby blocking their entrance into the S
phase, which inhibits DNA synthesis and cell mitosis, and thus
induces apoptosis. However, following the addition of
drug-containing serums, the majority of osteoblasts did not remain
in the G1 phase, but moved to the S phase, indicating
that the serum can enhance osteoblast proliferation and reduce
apoptosis. In summary, the drug can facilitate osteoblast growth
and reduce apoptosis.
The use of corticosteroid is a major factor causing
avascular necrosis, and additionally, it is the most common cause
of femoral head necrosis (15–17). In recent years, it can be argued that
the application of hormones induced the increase of bone marrow
stromal cell differentiation into fat cells and the decrease of
those into the bone system, which have important roles in the
pathogenesis of femoral head necrosis.
Hormone-induced cell injury is one of the important
factors leading to aseptic necrosis of the femoral head, while
glucocorticoids can inhibit osteoblasts growth, accelerate
osteoblast differentiation and apoptosis of bone cells, accelerate
the osteoclast differentiation, and induce bone marrow stromal
cells transition into fat cells.
Bax and Bcl-2 are two important regulatory genes of
cell apoptosis. An excess of Bax expression can accelerate
apoptosis, while overexpression of Bcl-2 can inhibit apoptosis.
When the Bax/Bcl-2 ratio is greater than normal, the cells tend to
undergo apoptosis, and when the ratio is smaller than normal,
apoptosis is inhibited (18,19). In the present study, chlorogenic acid
treatment with different doses, particularly the high-dose group,
can suppress the reduction of Bcl-2 and the increase of Bax in the
process of osteoblast apoptosis, thereby inhibiting apoptosis and
necrosis.
A previous study has shown the presence of the blood
hypercoagulable state in femoral head necrosis (20), and in blood rheology, this equates to
‘sticky, thick and poly tendencies’, for example, an increased
blood viscosity. When inflammation occurs, plasma fibrinogen
components increase, and stickiness, whole blood viscosity, and
erythrocyte aggregation and hematocrit all increase, leading to the
hypercoagulable state of blood, and slower blood circulation. When
femoral head blood perfusion is reduced, cell ischemia, anoxia and
fat embolism form, intraosseous pressure increases, venous stasis
and microcirculation dysfunction occurs, thus resulting in femoral
head blood supply disorders, and ultimately the formation of
avascular necrosis (21).
The present study also reported that chlorogenic
acid of different concentrations can improve all hemorheological
indicators in a varying degree, suggesting that the drug can
inhibit platelet aggregation, is anti-thrombotic, lowers blood
viscosity, directly affects perfusion in bone microcirculation,
slows or prevents high pressure inside the bone, femoral head blood
flow is smoothed, removes metabolic waste in a timely manner,
relieves the state of ischemia and hypoxia, and enhances the
vitality of bone cells, thereby preventing and delaying the
occurrence and development of femoral head necrosis.
In conclusion, different doses of chlorogenic acid
have proved therapeutically effective in the treatment of hormonal
femoral head necrosis, in vivo and in vitro,
protecting osteoblasts, curing necrosis and reducing apoptosis,
which may be applicable for future treatment.
References
|
1
|
Wang XS, Zhuang QY, Weng XS, Lin J, Jin J
and Qian WW: Etiological and clinical analysis of osteonecrosis of
the femoral head in Chinese patients. Chin Med J (Engl).
126:290–295. 2013.PubMed/NCBI
|
|
2
|
Zhao DW and Hu YC: Chinese experts'
consensus on the diagnosis and treatment of osteonecrosis of the
femoral head in adults. Orthop Surg. 4:125–130. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Martel C, Ferlay J, Franceschi S,
Vignat J, Bray F, Forman D and Plummer M: Global burden of cancers
attributable to infections in 2008: A review and synthetic
analysis. Lancet Oncol. 13:607–615. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kim HA and Blanco FJ: Cell death and
apoptosis in osteoarthritic cartilage. Curr Drug Targets.
8:333–345. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rahman WA, Garbuz DS and Masri BA: Total
hip arthroplasty in steroid-induced osteonecrosis: Early functional
and radiological outcomes. Can J Surg. 56:41–46. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Helbig L, Simank HG, Kroeber M,
Schmidmaier G, Grützner PA and Guehring T: Core decompression
combined with implantation of a demineralised bone matrix for
non-traumatic osteonecrosis of the femoral head. Arch Orthop Trauma
Surg. 132:1095–1103. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu HY, Wu AT, Tsai CY, Chou KR, Zeng R,
Wang MF, Chang WC, Hwang SM, Su CH and Deng WP: The balance between
adipogenesis and osteogenesis in bone regeneration by platelet-rich
plasma for age-related osteoporosis. Biomaterials. 32:6773–6780.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
James AW, Leucht P, Levi B, Carre AL, Xu
Y, Helms JA and Longaker MT: Sonic Hedgehog influences the balance
of osteogenesis and adipogenesis in mouse adipose-derived stromal
cells. Tissue Eng Part A. 16:2605–2616. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Erken HY, Ofluoglu O, Aktas M, Topal C and
Yildiz M: Effect of pentoxifylline on histopathological changes in
steroid-induced osteonecrosis of femoral head: Experimental study
in chicken. Int Orthop. 36:1523–1528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vélez R, Hernández-Fernández A, Caminal M,
Vives J, Soldado F, Fernández A, Pla A and Aguirre M: Treatment of
femoral head osteonecrosis with advanced cell therapy in sheep.
Arch Orthop Trauma Surg. 132:1611–1618. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stuss M, Rieske P, Cegłowska A,
Stêpień-Kłos W, Liberski PP, Brzeziańska E and Sewerynek E:
Assessment of OPG/RANK/RANKL gene expression levels in peripheral
blood mononuclear cells (PBMC) after treatment with strontium
ranelate and ibandronate in patients with postmenopausal
osteoporosis. J Clin Endocrinol Metab. 98:E1007–E1011. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kim BS, Jung JS, Jang JH, Kang KS and Kang
SK: Nuclear Argonaute 2 regulates adipose tissue-derived stem cell
survival through direct control of miR10b and selenoprotein N1
expression. Aging Cell. 10:277–291. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Youm YS, Lee SY and Lee SH: Apoptosis in
the osteonecrosis of the femoral head. Clin Orthop Surg. 2:250–255.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hwang Y, Park J, Choi SH and Kim G:
Traumatic and Non-traumatic osteonecrosis in the femoral head of a
rabbit model. Lab Anim Res. 27:127–131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Takano-Murakami R, Tokunaga K, Kondo N,
Ito T, Kitahara H, Ito M and Endo N: Glucocorticoid inhibits bone
regeneration after osteonecrosis of the femoral head in aged female
rats. Tohoku J Exp Med. 217:51–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gangji V and Hauzeur JP: Treating
osteonecrosis with autologous bone marrow cells. Skeletal Radiol.
39:209–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Seamon J, Keller T, Saleh J and Cui Q: The
pathogenesis of nontraumatic osteonecrosis. Arthritis (Egypt).
2012:6017632012.
|
|
18
|
Xie XH, Wang XL, He YX, Liu Z, Sheng H,
Zhang G and Qin L: Promotion of bone repair by implantation of
cryopreserved bone marrow-derived mononuclear cells in a rabbit
model of steroid-associated osteonecrosis. Arthritis Rheum.
64:1562–1571. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vucic D: Apoptotic pathways as targets for
therapeutic intervention. Current Cancer Drug Targets. 8:862008.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jung HS, Kim YH and Lee JW: Duration and
magnitude of extracellular signal-regulated protein kinase
phosphorylation determine adipogenesis or osteogenesis in human
bone marrow-derived stem cells. Yonsei Med J. 52:165–172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Imhoff BR and Hansen JM: Differential
redox potential profiles during adipogenesis and osteogenesis. Cell
Mol Biol Lett. 16:149–161. 2011. View Article : Google Scholar : PubMed/NCBI
|