Introduction
Garlic (Allium sativum) is a plant closely
associated with onion, shallot and leek. For thousands of years, it
has been used as a medicinal agent for prevention and treatment of
diseases due to its potential effects, which include antimicrobial,
antithrombotic, hypolipidemic, antiarthritic, hypoglycemic,
immunomodulatory and antitumor activities (1–5). Kurozu is a
type of traditional Japanese black vinegar made from unpolished
rice. During its manufacturing processes, saccharification, ethanol
fermentation and acetic fermentation occur in one pot. Due to its
appearance, the vinegar is known as black vinegar. Previous studies
have suggested the benefits of black vinegar, including
antioxidant, hypotensive and antitumor effects (6–9).
Black-vinegar-mash-garlic is a food produced by pickling garlic in
black vinegar mash and allowing further fermentation thereafter.
The extracts of the whole fermentation product are rich in amino
acids, organic acids, vitamins and minerals, and are commercially
distributed as a health supplement.
Diet can be one of the factors that modify immune
responses (10). Our preliminary
investigation suggested that the intake of
black-vinegar-mash-garlic-containing food (the commercial brand
‘Jukusei Kurozuninniku Premium’) enhanced the immune function as
represented by stimulation of secretory immunoglobulin A (sIgA)
release in the saliva (data unpublished). Salivary sIgA is
synthesized as dimeric IgA by plasma cells in salivary glands and
constitutes a section of the first line of defense against pathogen
invasion (11). It can serve as a
marker reflecting the immune function in the intraoral and upper
respiratory tract (12). Increasing
evidence suggests the potential use of salivary sIgA to predict
mucosal and systemic immunity (13).
In the present study, the effect of
black-vinegar-mash-garlic-containing food on salivary sIgA release
was investigated in a randomized, double-blind, placebo-controlled,
parallel-group trial.
Materials and methods
Test articles
The test articles, placebo food and
black-vinegar-mash-garlic-containing food (active food) were
produced by Kenkoukazoku Co., Ltd. (Kagoshima, Japan). The
ingredients of the test articles are shown in Table I. Subjects were administered 6 capsules
(a total of 2.49 g) of either the placebo or active food every day
for 8 weeks during the test period. The dose of
black-vinegar-mash-garlic-containing food was defined based on its
recommended daily intake.
 | Table I.Composition of the test foods. |
Table I.
Composition of the test foods.
| Composition | Placebo | Active |
|---|
| Ingredient |
|
|
| Linseed
oil, mg | 1,098.0 | 1,035.0 |
| Starch,
mg | 430.8 | 430.8 |
| Acetic
acid flavor, mg | 90.0 | 0.0 |
| Caramel
pigment, mg | 30.0 | 0.0 |
|
Black-vinegar-mash-garlic
powder, mg | 0.0 | 300.0 |
| Glycerol,
mg | 363.6 | 363.6 |
| Beeswax,
mg | 252.0 | 195.0 |
| Gelling
agent, mg | 165.6 | 165.6 |
| Nutritional
content |
|
|
| Energy,
kcal | 15.67 | 14.69 |
| Protein,
g | 0.001 | 0.06 |
| Fat,
g | 1.35 | 1.07 |
|
Carbohydrate, g | 0.88 | 1.20 |
| Sodium,
mg | 8.19 | 6.72 |
Study design
A randomized, double-blind, placebo-controlled,
parallel-group study was conducted at two centers, Yaesu
Sakura-dori Clinic (presently Nihonbashi Sakura Clinic, Tokyo,
Japan), and Umeda Oak Clinic (Osaka, Japan), under the supervision
by the principle investigators Dr Takayuki Azuma (Yaesu Sakura-dori
Clinic) and Dr Norimasa Sato (Umeda Oak Clinic). The study was
conducted between May and October 2014. The study conformed to the
principles of the Declaration of Helsinki and was approved on April
24, 2014 by the institutional review board of the incorporated
medical institution of Aiseikai Aisei Hospital Ueno Clinic (Tokyo,
Japan) on the basis of the protocol and information on the test
study. The study was registered as the ID no. UMIN000013933
(Effects of food containing Kurozu Moromi and garlic powder on
immune function: A randomized, double blind, placebo-controlled
study) at the UMIN Clinical Trials Registry, Japan.
The details of this study were disclosed to subjects
prior to the start of the study, and the investigators obtained
informed consent from each subject. The candidates underwent
pretrial testing (life style questionnaire, health check,
somatometry, physical examination, fasting blood tests, urinalysis,
salivary sIgA determination and T-cell subset analysis) and began
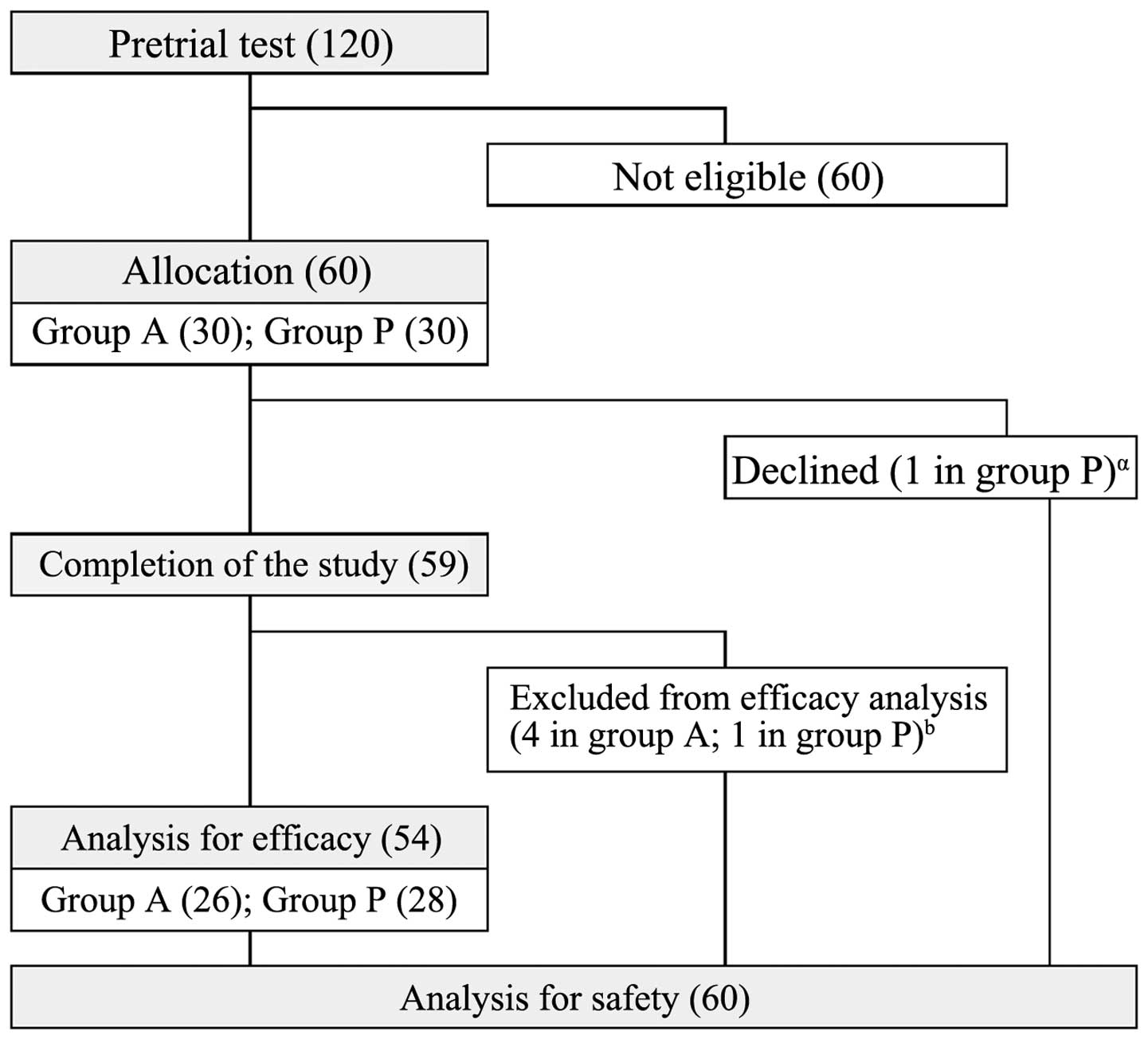
documenting the pretrial lifestyle. As outlined in Fig. 1, 60 subjects (34 males and 26 females)
were included out of 120 subjects who underwent pretrial testing.
The major criteria for inclusion of subjects were those with a
moderately slow rate of salivary sIgA release and with their age
between 30 and 60 years. Inclusion criteria for the study were: i)
Males or females whose ages were between 30 and 60 years; ii) those
with a moderate rate of salivary sIgA release. Exclusion criteria
for the study were: i) Those who habitually consume foods rich in
black vinegar or garlic; ii) those who habitually take health food
or a supplement that may enhance immune function; iii) those with a
case history of an allergic disease such as pollinosis, allergic
rhinitis, asthma, atopic dermatitis and allergic conjunctivitis;
iv) those who are receiving a treatment that will affect this study
such as desensitization therapy; v) those who have been receiving
within 1 month before the entry to this study or are planning to
receive dental or intraoral treatment; vi) those who have dental or
intraoral trouble; vii) those who have been engaged in shift work
or heavy physical work; viii) those who habitually undergo hard
exercise such as long distance running; ix) those who have
difficulties undergoing testing in this study, such as sampling of
saliva; x) those who have suffered from any disease or who require
any medication; xi) those who are suffering from or have a case
history of disease such as diabetes mellitus, liver disease, kidney
disease, heart disease, or disease that affects corticoid
secretion; xii) those who may be liable to allergies in response to
the test article; xiii) those who are judged inappropriate as
participants from the pretrial test; xiv) those who are planning to
get pregnant or nurse a baby during this study; xv) those who have
been participating in any other clinical trial within 1 month or
who are planning to do such after agreeing informed consent of
joining this study; xvi) those who are judged inappropriate as
participants from the answers to the lifestyle questionnaire; xvii)
those who are judged inappropriate as participants from the view of
the principle investigators. The selected candidates were assigned
to the placebo food group (n=30) or active food group (n=30) based
on random number tables. The allocation was performed by Mr.
Yoshihisa Kibune (TTC Co., Ltd., Tokyo, Japan) and was concealed
from the subjects, the investigators and the researchers who
recruited and assessed the participating subjects. The participants
ingested the placebo or active food every day for 8 weeks and noted
the time and amount of their test article intake, as well as their
use of pharmaceuticals. At weeks 4 and 8 of the study, participants
underwent a health check, somatometry, physical examination,
salivary sIgA determination and T-cell subset analysis. At week 8,
participants also underwent fasting blood tests and urinalysis.
During this trial, the principal investigators and
their assistants directed the participants with respect to the
following points: i) No change to lifestyle, including diet,
alcohol consumption, exercise and sleeping; ii) avoid
over-exercise, abstemious eating or overeating; iii) no change to
exercise habits; iv) no consumption of health food, dietary
supplement, lactic acid bacteria beverage, yogurt, kimchi or
lactobacillus preparation; v) log the name and dose when a
participant uses pharmaceuticals; vi) consume the specified amount
of the test article and to log the time and amount of the intake
every day; vii) avoid alcohol consumption and ingestion of
pharmaceuticals on the day before testing, and avoid hard exercise
on the day and the day before testing; ix) to finish eating a meal
by ~10:00 p.m. and thereafter not to take any meal other than warm
water until the end of testing; x) to go to bed by 12:00 p.m. on
the day before testing; xi) to brush teeth without using toothpaste
on the day and the day before testing; and xii) to avoid smoking
following toothbrushing until the end of the testing on that
day.
The primary outcome measure was the rate of salivary
sIgA release, and the secondary outcome measure was distribution of
T-cell subset population [percentages of cluster of differentiation
4+ (CD4+) and CD8+ T cells in
peripheral blood lymphocytes, as well as the ratio of
CD4+ T cells to CD8+ T cells].
Measurements of salivary sIgA
Subjects rinsed their mouth twice and gargled twice
with 150 ml of water for each time before testing. After sitting
for 15 min, subjects underwent sampling of their saliva using a
Salisoft (Sarstedt, Numbrecht, Germany) for 120 sec. The amount of
saliva recovered was recorded, and the salivary concentration of
sIgA was determined by immunonephelometry according to the standard
laboratory protocol of the BML Inc. (Tokyo, Japan).
T-cell subset analysis
The cell surface CD4 and CD8 antigens on peripheral
blood lymphocytes were analyzed by flow cytometry using
fluorescent-labeled monoclonal antibodies specific for the
respective cell surface antigens (cat. nos. 340133 and 347313,
respectively; BD Biosciences, Tokyo, Japan) according to the
standard laboratory protocol of the LSI Medience Corporation
(Tokyo, Japan).
Statistical analysis
All the measured values are expressed as the mean ±
standard error, or as indicated, the error bars may represent the
standard error of the mean. For comparison of the values in the
placebo and active food groups, χ2 test (for gender) or
unpaired Student's t-test (for others) were employed, and P<0.05
was considered to indicate a statistically significant
difference.
Results
Subjects
In total, 60 subjects were enrolled and randomly
allocated to each of the 2 groups: Group P in which participants
received placebo food, or group A in which participants received
black-vinegar-mash-garlic-containing food (Fig. 1). The subjects ingested 6 capsules of
the respective test article every day. One participant in group P
excluded themselves from the study at day 4. The remaining 59
participants completed the study. However, 5 subjects were judged
to fall into exclusion criteria and were eliminated from the
efficacy analysis for the following reasons: Diagnosed as diabetes
mellitus (n=1 in group A), lifestyle change due to hospitalization
for lumbar disc herniation (n=1 in group A), and long-term cold
that may affect sIgA release (n=2 in group A and n=1 in group P).
Thus, the data obtained with 54 participants (n=26 in group A and
n=28 in group P) were used for the efficacy analysis (Fig. 1).
Background data
The background data of the participants who were
subjected to efficacy analysis are shown in Table II. No significant difference was
observed in the values between groups A and P for each item,
including the rate of salivary sIgA release and T-cell subset
population.
 | Table II.Background characteristics of the
subjects. |
Table II.
Background characteristics of the
subjects.
| Characteristics | Active food
group | Placebo food
group | P-value |
|---|
| Subject number | 26 (male 11; female
15) | 28 (male 19; female
9) | 0.057 |
| Age, year | 44.5±7.9 | 44.2±8.3 | 0.871 |
| Height, cm | 163.52±9.25 | 167.25±7.44 | 0.108 |
| Body weight, kg | 59.67±12.31 | 63.89±10.48 | 0.180 |
| Body mass | 22.13±2.93 | 22.77±2.96 | 0.427 |
| index,
kg/m2 |
| Rate of salivary sIgA
release, µg/min | 173.46±58.22 | 179.67±54.35 | 0.686 |
| CD4+
T-cell, % | 42.5±6.6 | 45.0±7.7 | 0.195 |
| CD8+
T-cell, % | 28.1±6.3 | 27.1±5.2 | 0.537 |
|
CD4+/CD8+ ratio | 1.58±0.54 | 1.71±0.57 | 0.367 |
Effects of
black-vinegar-mash-garlic-containing food intake
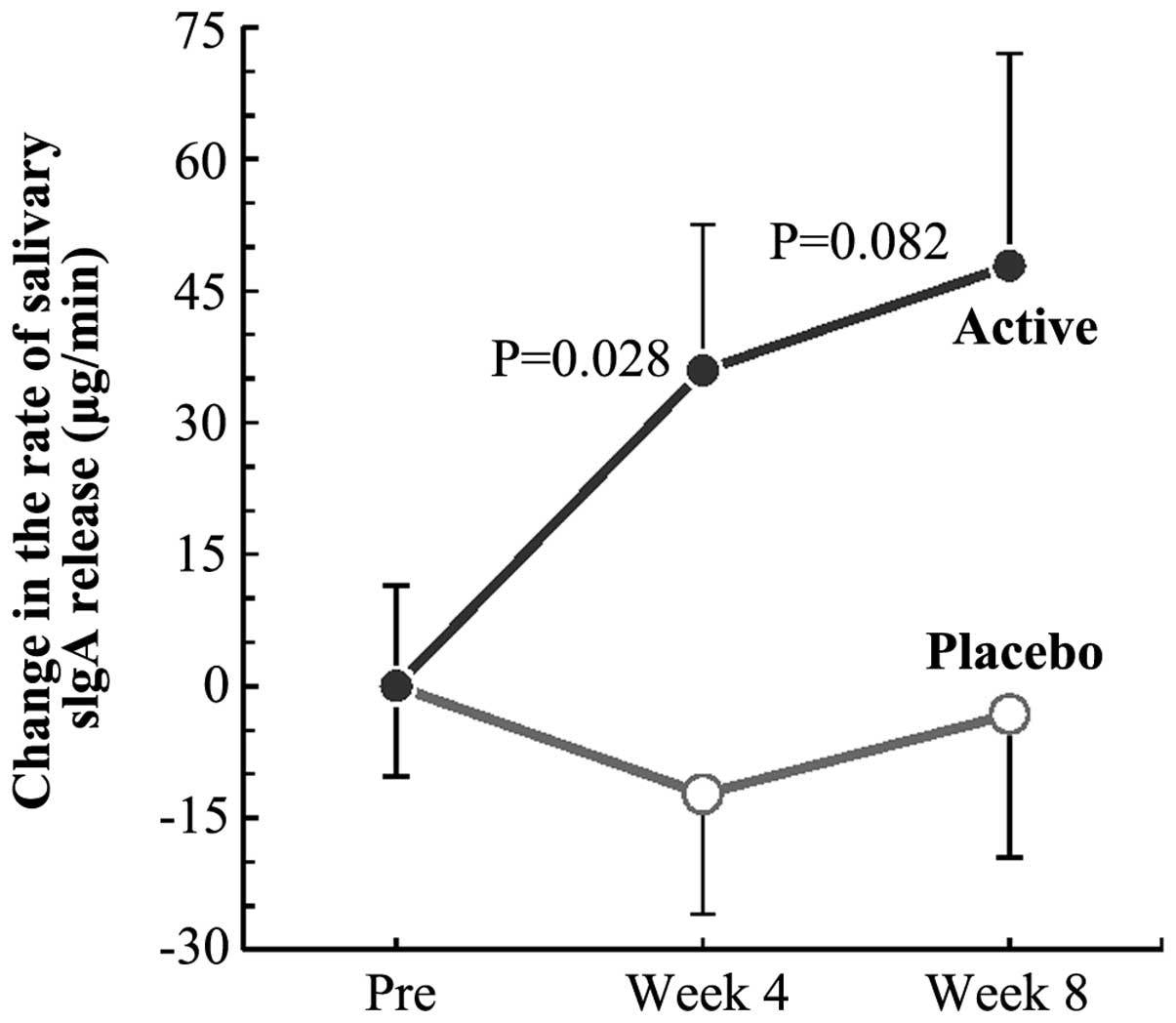
In group A, the rate of salivary sIgA release was
significantly high (P=0.028) compared to that in group P at week 4
of the study (Fig. 2). The rate in
group A at week 8 was also higher than that in group P. The time
course of the changes from the pretrial values suggested a trend of
elevation of the rate of sIgA secretion following the intake of
active test article (black-vinegar-mash-garlic-containing food)
(Fig. 2). No significant difference
was observed between groups A and P in the population distribution
of CD4+ and CD8+ peripheral lymphocytes
following the test article consumption (Table III).
 | Table III.Effect of the intake of
black-vinegar-mash-garlic-containing food on the distribution of
T-cell subset population. |
Table III.
Effect of the intake of
black-vinegar-mash-garlic-containing food on the distribution of
T-cell subset population.
|
|
|
| Change from pretrial
value |
|---|
|
|
|
|
|
|---|
| T-cell subset
distribution | Group | Total, n | Week 4 | Week 8 |
|---|
| CD4+
T-cell, % | A | 26 | −2.2±3.2 | −0.1±3.1 |
|
| P | 28 | −3.1±4.0 | −0.2±3.9 |
| CD8+
T-cell, % | A | 26 | 0.3±3.5 | 0.5±3.3 |
|
| P | 28 | −0.9±4.0 | −0.5±3.8 |
|
CD4+/CD8+ ratio | A | 26 | −0.09±0.28 | 0.01±0.35 |
|
| P | 28 | −0.04±0.28 | 0.06±0.25 |
Adverse event
There was one serious adverse event, the herniation
of the lumbar disc, with a subject in group A. Since its emergence
was accidental associated with exercise, the principle investigator
judged this case unrelated to the test article consumption. There
were certain other non-serious adverse events during this study.
Except for one observation in one subject in group P, which was a
stomach ache that relieved following cessation of test article
intake and was judged associated with the test article intake, the
other events were judged by the principle investigators to be
unrelated to test article consumption.
Discussion
The present study aimed to investigate the
immunomodulatory effect of the intake of
black-vinegar-mash-garlic-containing food, which has been
commercially distributed as a health food since February 2012.
Following this, <93 million capsules have been consumed. The
results in the present study demonstrated that the subjects who
ingested the black-vinegar-mash-garlic-containing food for 4 weeks
had a significantly higher rate of salivary release of sIgA
compared to those who consumed the placebo food. Thus, it is
suggested that the intake of black-vinegar-mash-garlic-containing
food is beneficial in enhancing intraoral mucosal immunity.
sIgA constitutes a major mucosal immune effector
molecule and provides an important first line of defense against
pathogens, such as Streptococcus mutans and Porphyromonas
gingivalis (14,15). Luminal sIgA is postulated to interfere
with pathogen adherence to mucosal epithelial cells and promote
intracellular neutralization of pathogens. IgA-secreting cells
differentiate in lymphoid tissues associated with mucosa (15). The majority of the IgA-secreting cells
reside in the gut mucosa. Increasing evidence suggests that the
migration of antibody-secreting cells to the gut is important in
conferring protective immune responses (16). The test food used in the present study
was provided as a capsule, and the gastrointestinal tract was the
first tissue to be exposed to the ingredients it contained. Thus,
it is likely that the immune system in the gastrointestinal tract
is first stimulated by the intake of
black-vinegar-mash-garlic-containing food, and this may in turn
affect salivary sIgA release through migration of the
antibody-secreting cells to the intraoral mucosal tissues.
In conclusion, the present findings indicate that
the intake of black-vinegar-mash-garlic-containing food stimulates
intraoral immune function. There was no adverse event associated
with the consumption of the food. Further investigations, including
in vitro experiments and animal studies, may reveal the
essential ingredient molecule and mechanism of its action involved
in this immunomodulatory action.
Acknowledgements
YN is an employee of Kenkoukazoku Co., Ltd., which
provided the research expenses to TTC Co., Ltd. YN was not involved
in the interpretation of the results, and did not influence the
outcomes at any stage of the clinical trial. A randomized,
double-blind, placebo-controlled, parallel-group study was
conducted with the aid of a fund from the Kenkoukazoku Co.,
Ltd.
References
|
1
|
Thomson M and Ali M: Garlic [Allium
sativum]: A review of its potential use as an anti-cancer
agent. Curr Cancer Drug Targets. 3:67–81. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bayan L, Koulivand PH and Gorji A: Garlic:
A review of potential therapeutic effects. Avicenna J Phytomed.
4:1–14. 2014.PubMed/NCBI
|
|
3
|
Schäfer G and Kaschula CH: The
immunomodulation and anti-inflammatory effects of garlic
organosulfur compounds in cancer chemoprevention. Anticancer Agents
Med Chem. 14:233–240. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kyo E, Uda N, Kasuga S and Itakura Y:
Immunomodulatory effects of aged garlic extract. J Nutr.
131:1075S–1079S. 2001.PubMed/NCBI
|
|
5
|
Lamm DL and Riggs DR: Enhanced
immunocompetence by garlic: Role in bladder cancer and other
malignancies. J Nutr. 131:1067S–1070S. 2001.PubMed/NCBI
|
|
6
|
Shimoji Y, Tamura Y, Nakamura Y, Nanda K,
Nishidai S, Nishikawa Y, Ishihara N, Uenakai K and Ohigashi H:
Isolation and identification of DPPH radical scavenging compounds
in Kurosu (Japanese unpolished rice vinegar). J Agric Food Chem.
50:6501–6503. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nanda K, Miyoshi N, Nakamura Y, Shimoji Y,
Tamura Y, Nishikawa Y, Uenakai K, Kohno H and Tanaka T: Extract of
vinegar ‘Kurosu’ from unpolished rice inhibits the proliferation of
human cancer cells. J Exp Clin Cancer Res. 23:69–75.
2004.PubMed/NCBI
|
|
8
|
Fukuyama N, Jujo S, Ito I, Shizuma T,
Myojin K, Ishiwata K, Nagano M, Nakazawa H and Mori H: Kurozu
moromimatsu inhibits tumor growth of Lovo cells in a mouse model in
vivo. Nutrition. 23:81–86. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sugiyama S, Kishi M, Fushimi S, Oshima Y
and Kajimoto O: Hypotensive effect and safety of brown rice vinegar
with high concentration of GABA on mild hypertensive subjects. Jpn
Pharmacol Ther. 36:429–444. 2008.
|
|
10
|
Lin WW and Karin M: A cytokine-mediated
link between innate immunity, inflammation, and cancer. J Clin
Invest. 117:1175–1183. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Woof JM and Mestecky J: Mucosal
immunoglobulins. Immunol Rev. 206:64–82. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dodds MW, Johnson DA and Yeh CK: Health
benefits of saliva: A review. J Dent. 33:223–233. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brandtzaeg P: Do salivary antibodies
reliably reflect both mucosal and systemic immunity? Ann N Y Acad
Sci. 1098:288–311. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Russell MW, Hajishengallis G, Childers NK
and Michalek SM: Secretory immunity in defense against cariogenic
mutans streptococci. Caries Res. 33:4–15. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Woof JM and Kerr MA: The function of
immunoglobulin A in immunity. J Pathol. 208:270–282. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mora JR and von Andrian UH:
Differentiation and homing of IgA-secreting cells. Mucosal Immunol.
1:96–109. 2008. View Article : Google Scholar : PubMed/NCBI
|