Introduction
With a rapidly aging population, osteoporosis is a
significant concern in Japan. While it is asymptomatic,
osteoporosis progresses gradually, leading to hip and lumbar
fractures, which may require extended nursing care and threaten
life expectancy (1). Therefore,
prevention of osteoporosis is important and the efficacy of
bisphosphonates in osteoporosis treatment has been supported by
previous studies (2). However, side
effects of bisphosphonate use are gastrointestinal (GI) disorders
(3,4). In
the ‘super-aging’ Japanese society, consumption of non-steroidal
anti-inflammatory drugs (NSAIDs) and aspirin increases with the
greater incidence of joint, ischemic heart and cerebrovascular
diseases, with NSAID-induced GI disorders becoming an increasing
concern (5,6). In addition, gastric acid secretion in
Japanese patients has gradually increased due to a trend towards
Westernized eating habits (7) and a
decrease in Helicobacter pylori infections, with the number
of gastroesophageal reflux disease (GERD) patients also increasing
rapidly (8). Proton pump inhibitors
(PPIs) are key first-line therapeutic strategies for the treatment
of NSAID-induced ulcers and GERD (9).
PPIs are often administered as a long-term treatment, and it is
common for PPIs to be used concomitantly with bisphosphonates. A
previous study suggested that PPI use was associated with a
dose-dependent loss of the anti-fracture efficacy of alendronate
(AD) (10). However, there are few
prospective studies that investigate the efficacy of AD on lumbar
bone mineral density (BMD) in osteoporotic patients using
concomitant PPIs. The aim of the present study was to investigate
the efficacy of AD on lumbar BMD in osteoporotic patients using
concomitant PPIs, comparing the effects versus alfacalcidol (AC) in
a prospective, randomized, open-label, comparative study.
Materials and methods
Study design
The present study was conducted as a prospective,
randomized, open-label, active control, comparative, single-center
study. From 2009 until 2013 at Juntendo University Hospital (Tokyo,
Japan), osteoporotic patients (age, ≥50 years) who were using PPIs
were enrolled in the study. After assignment to the AC (1 µg/day)
or AD (35 mg/week) groups, the patients were followed up for one
year of treatment. The AD group patients took the medication in the
early morning (after an overnight fast) with a glass of plain
water, and were instructed to remain upright for ≥30 min before
consuming the first food of the day. Patients from the two groups
were prohibited from taking any other medication affecting bone or
calcium metabolism during the treatment period. Patient profiles
[age, gender, body mass index (BMI), alcohol consumption, smoking,
comorbidities (type 2 diabetes mellitus and hypertension)] and
ongoing concomitant medications [calcium channel blockers (CCBs),
low-dose aspirin (LDAA), and 3-hydroxy-3-methylglutaryl-coenzyme A
(HMG-CoA) reductase inhibitors] were evaluated. BMI was calculated
as body weight divided by the square of body height in meters
(kg/m2). Patients that had used standard doses of CCBs,
LDAA, or HMG-CoA reductase inhibitors for >6 months were
identified as users of that specific therapy. We defined the cases
that used the usual dose of PPIs (10 mg rabeprazole or 20 mg
omeprazole or 30 mg lansoprazole) for >6 months as users of that
specific therapeutic strategy. The study was conducted in
accordance with the Declaration of Helsinki. The Juntendo
University Ethics Committee approved this study protocol (reference
no. 207-028) and patients signed an Ethics Committee-approved
informed consent document.
Exclusion criteria
Patients with osteoporosis were selected for the
present study, however, certain individuals were excluded according
to the following criteria: Patients who were currently or
previously being treated with glucocorticoids, hormone replacement
therapy, thyroid/parathyroid medication, psychotropic medication,
anticonvulsants, selective estrogen receptor modulators or calcium
were excluded. Patients with the following conditions were also
excluded: Gastrectomy, inflammatory bowel disease, malignant
disease (gastric, esophageal, colon, lung, pancreatic, liver, bile
duct, gallbladder, breast, uterine, ovarian, prostate, and bladder
cancer, malignant lymphoma, leukemia and multiple myeloma), chronic
kidney disease, type 1 diabetes mellitus, hypo/hyper-thyroidism,
hypo/hyper-parathyroid disorder, rheumatoid arthritis (including
other collagen diseases), and those female patients who were
premenopausal.
Measurement of lumbar BMD
BMD at lumbar vertebrae 2 through 4 (L2-4) was
measured by dual-energy X-ray absorptiometry using a Discovery
DXA® system (Hologic; Bedford, MA, USA) and the presence
of fragility fractures were investigated in the chest and lumbar
spine using lateral vertebral X-rays. The diagnosis of osteoporosis
was performed in accordance with the 2000 version of the Japanese
Diagnostic Criteria of the Japanese Society for Bone and Mineral
Research (11). Degenerative lesions
of the vertebrae were ruled out on diagnosing osteoporosis. An
osteoporotic patient was defined as one having lumbar BMD values
<70% of the young adult mean (YAM) in those without any
prevalent fragility fracture. Osteoporosis was also defined as the
presence of fragility fractures of any bone in a person with a BMD
of <80% of the YAM. Percentage change of lumbar BMD was
evaluated from baseline to the end of one year of treatment in the
AC and AD groups.
Measurement of bone turnover
markers
Serum bone-specific alkaline-phosphatase (BAP; a
biomarker of bone formation) and serum collagen type I cross-linked
N-telopeptide (NTX; a biomarker of bone resorption) were examined.
Percentage changes of NTX and BAP from baseline to the end of one
year of treatment were investigated in the AC and AD groups.
Upper GI findings and frequency scale
for the symptoms of GERD (FSSG)
The prevalence rate of upper GI endoscopy findings
were analyzed, including reflux esophagitis (RE), hiatal hernia
(HH) and peptic ulcer disease (PUD). RE was defined as grade A, B,
C, or D according to the Los Angeles Classification (12), and PUD as a gastric and/or duodenal
ulcer or ulcer scar. HH was defined as an apparent separation of
the esophagogastric junction and diaphragm impression by >2 cm
at endoscopy. The FSSG score was established via questionnaire
(13). Changes in the FSSG score from
the baseline to the end of one year of treatment, and findings of
upper GI endoscopy (RE, HH and PUD) at baseline and the end of one
year of treatment were evaluated.
Endpoint
In addition, the safety of use of the two
therapeutic agents over time was evaluated by assessing the side
effects experienced by patients in each group.
Statistical analysis
The baseline characteristics were compared between
the AC and AD groups by paired t-test or Fisher's exact test. The
percentage change from baseline to the end of one year of treatment
for lumbar BMD, NTX, and BAP was investigated by paired t-test. The
change of FSSG score from baseline to the end of one year of
treatment in each group was also investigated by paired t-test and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Baseline characteristics
Of the 22 patients enrolled in the present study,
six were excluded from evaluation [three patients in the AC group
(one was excluded due to medication side effects, one due to other
disease, and one dropped out of the study) and three patients in
the AD group (one due to medication side effects and two dropped
out of the study)].
The baseline characteristics are summarized in
Table I. Of the 16 eligible patients,
eight received AC treatment and eight received AD treatment. In the
AC and AD groups, respectively, 1 case vs. 3 cases received
rabeprazole; 5 cases vs. 1 case received omeprazole; and 2 cases
vs. 4 cases received lansoprazole. As shown in Table 1, there were no significant differences
in baseline characteristics between the two treatment groups. There
were no patients with fractures at the beginning of the study.
 | Table I.Patient baseline characteristics. |
Table I.
Patient baseline characteristics.
| Patient profile | Total (n=16) | Alfacalcidol
(n=8) | Alendronate
(n=8) | P-value |
|---|
| Age, years |
64.1±9.0 |
64.9±10.1 |
63.3±8.4 | 0.730 |
| Gender, n (%) |
|
|
| 1.000 |
| Male | 4
(25.0) | 2 (25.0) | 2
(25.0) |
|
|
Female | 12 (75.0) | 6 (75.0) | 6
(75.0) |
|
| Body mass index
(kg/m2) |
20.8±3.8 |
19.6±3.0 |
21.9±4.3 | 0.230 |
| Alcohol consumption,
n (%) |
|
|
| 1.000 |
|
Non-drinker | 15 (93.8) | 7 (87.5) |
8 (100.0) |
|
|
Drinker | 1 (6.2) | 1 (12.5) | 0 (0.0) |
|
| Smoking, n (%) |
|
|
| 0.569 |
|
Non-smoker | 12 (75.0) | 7 (87.5) | 5
(62.5) |
|
|
Smoker | 4 (25.0) | 1 (12.5) | 3
(37.5) |
|
| Comorbidities |
|
|
|
|
|
Diabetes mellitus, n (%) |
|
|
| 1.000 |
|
No | 15 (93.8) | 7 (87.5) |
8 (100.0) |
|
|
Yes | 1 (6.2) | 1 (12.5) | 0 (0.0) |
|
|
Hypertension, n (%) |
|
|
| 0.569 |
|
No | 12 (75.0) | 7 (87.5) | 5
(62.5) |
|
|
Yes | 4 (25.0) | 1 (12.5) | 3
(37.5) |
|
| Concomitant
medications |
|
|
|
|
| Calcium
channel blocker, n (%) |
|
|
| 1.000 |
|
Non-user | 13 (81.3) | 7 (87.5) | 6
(75.0) |
|
|
User | 3 (18.7) | 1 (12.5) | 2
(25.0) |
|
| Low
dose aspirin, n (%) |
|
|
| 1.000 |
|
Non-user | 10 (62.3) | 5 (62.5) | 5
(62.5) |
|
|
User | 6 (37.6) | 3 (37.5) | 3
(37.5) |
|
|
3-hydroxy-3-methylglutaryl-coenzyme
A reductase inhib-itors, n (%) |
|
|
| 1.000 |
|
Non-user | 11 (68.8) | 6 (75.0) | 5
(62.5) |
|
|
User | 5 (31.2) | 2 (25.0) | 3
(37.5) |
|
| Bone turnover
marker |
|
|
|
|
|
Bone-specific alkaline
phos-phatase, U/l |
25.4±7.8 |
27.4±8.4 |
23.5±7.1 | 0.338 |
|
Collagen type I
cross-linked |
14.0±6.0 |
12.4±1.9 |
15.6±8.3 | 0.302 |
| N
telopeptide, nnmol BCE/l |
|
|
|
|
| Lumber dual-energy
X-ray absorptiometry |
|
|
|
|
| Bone
mineral density, g/cm2 |
0.66±0.12 |
0.62±0.14 |
0.70±0.10 | 0.182 |
| Upper
gastrointestinal findings |
|
|
|
|
| Reflux
esophagitis, n (%) |
|
|
| 1.000 |
|
No |
16 (100.0) |
8 (100.0) |
8 (100.0) |
|
|
Yes | 0
(0.0) | 0 (0.0) | 0 (0.0) |
|
| Hiatal
hernia, n (%) |
|
|
| 0.608 |
|
No | 10 (62.3) | 4 (50.0) | 6
(75.0) |
|
|
Yes | 6
(37.6) | 4 (50.0) | 2
(25.0) |
|
| Peptic
ulcer disease, n (%) |
|
|
| 0.569 |
|
No | 12 (75.0) | 5 (62.5) | 7
(87.5) |
|
|
Yes | 4
(25.0) | 3 (37.5) | 1
(12.5) |
|
| Questionnaire |
|
|
|
|
|
Frequency scale for the
symptoms of gastroesopha-geal reflux disease score |
6.5±6.1 |
3.6±3.0 |
9.4±7.2 | 0.056 |
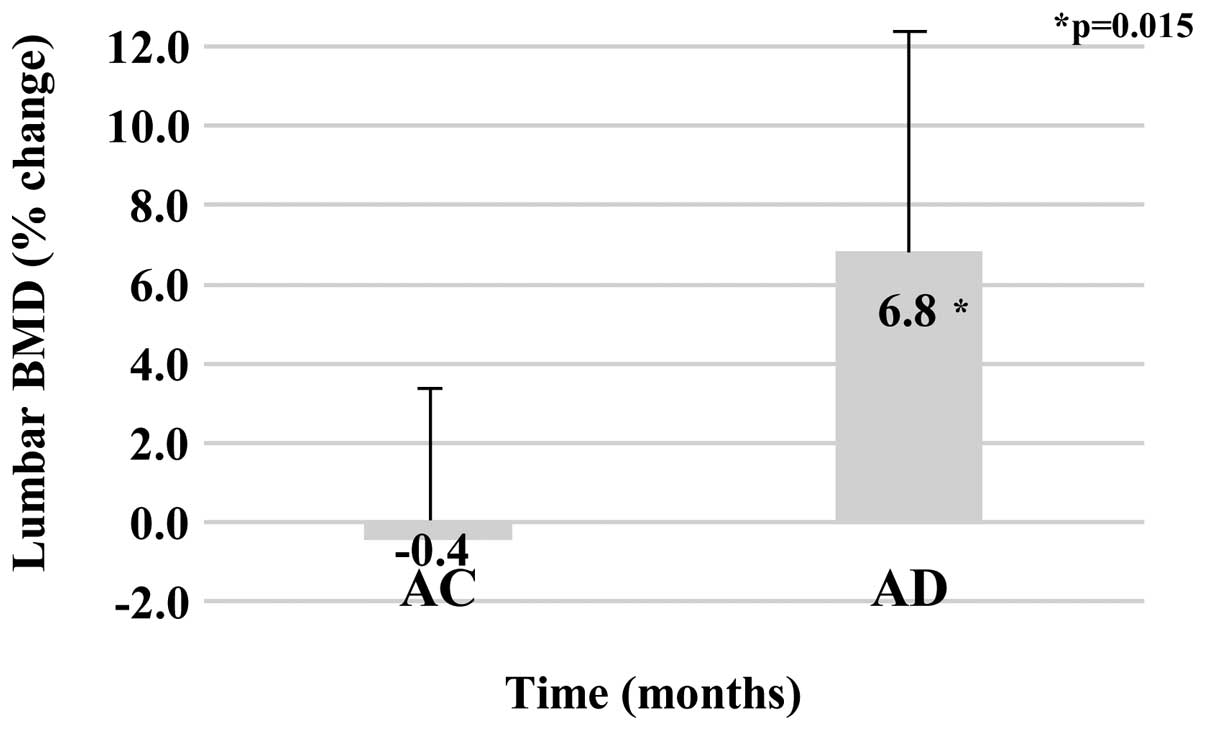
Lumbar BMD
Baseline values of lumbar BMD were 0.62±0.14
g/cm2 for the AC group and 0.70±010 g/cm2 for
the AD group (P=0.182). Following one year of treatment, the lumbar
BMD values were 0.61±0.12 g/cm2 for the AC group and
0.75±010 g/cm2 for the AD group. The percentage changes
in lumbar BMD from baseline to the end of one year of therapy were
−0.4±4.0% for the AC group and 6.8±6.3% for the AD group, with
significantly effective lumbar BMD findings identified in the AD
group (P=0.015; Fig. 1).
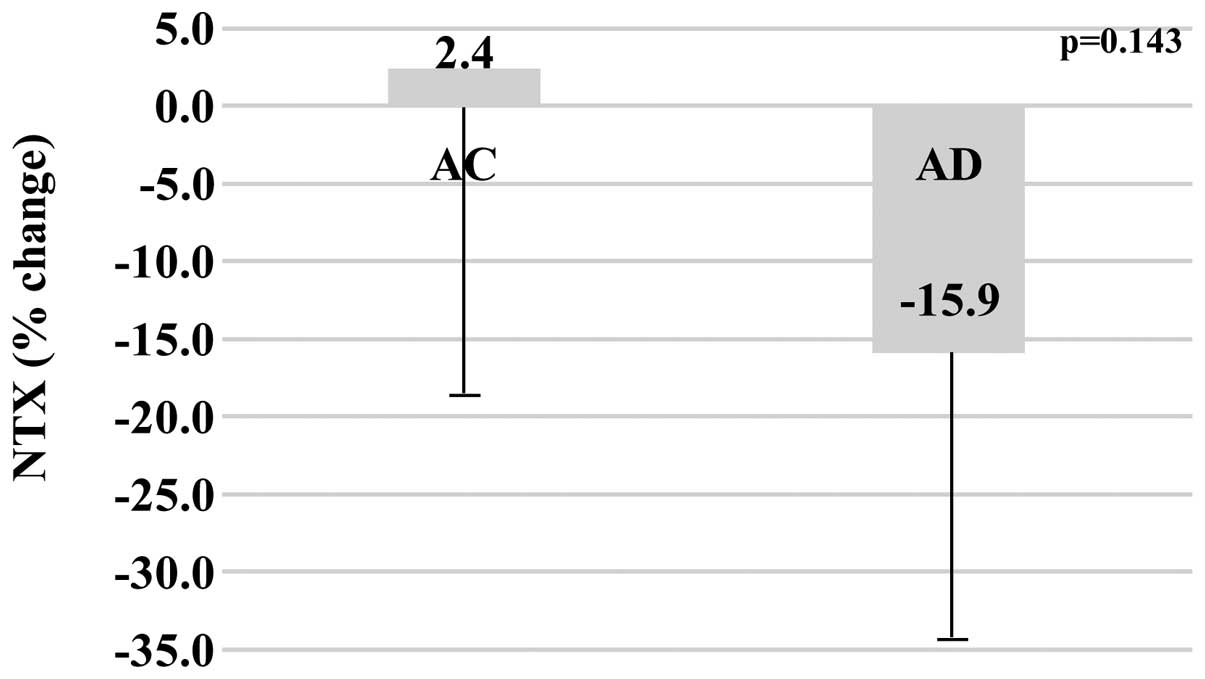
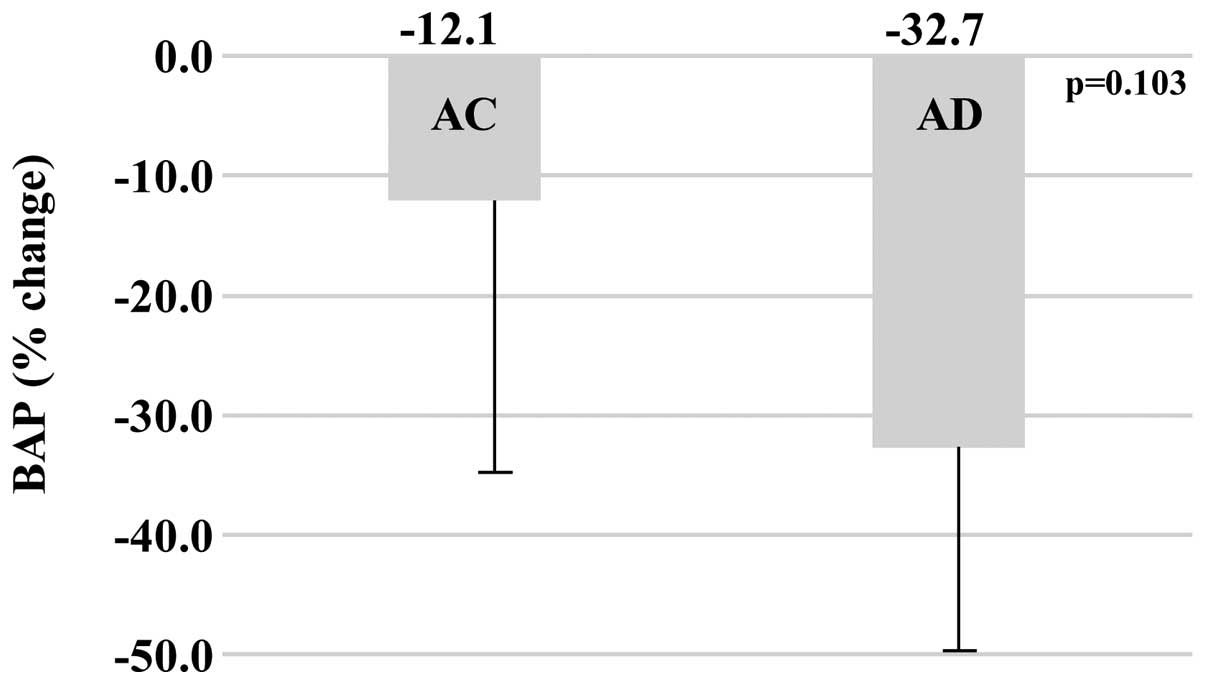
Bone turnover markers
The baseline values of bone turnover markers in the
AC and AD groups, respectively were as follows: BAP (U/l), 27.4±8.4
vs. 23.5±7.1 (P=0.338); NTX (nmol BCE/l), 12.4±1.9 vs. 15.6±8.3
(P=0.302). After one year of treatment, the values of bone turnover
markers in the AC and AD groups, respectively were as follows: BAP
(U/l), 23.6±7.3 vs. 15.1±4.1; NTX (nmol BCE/l), 12.8±4.5 vs.
12.6±8.7. When comparing the percentage change between baseline and
the end of one year of treatment, the AC group demonstrated less of
a change over time in BAP (−12.1±25.5%) compared with the AD group
(−32.7±21.7%) (P=0.103), and less of a change over time in NTX
(+2.4±25.7%) compared with the AD group (−15.9±21.3%) (P=0.143);
although the difference was not statistically significant (Figs. 2 and 3).
Upper GI findings
Baseline findings of the upper GI endoscopy
(Table I) indicated no RE in either
treatment group, HH in 50.0% of the AC group vs. 25.0% of the AD
group (P=0.608), PUD in 37.5% of the AC group vs. 12.5% of the AD
group (P=0.569). No novel findings of RE or PUD were observed from
baseline to the end of one year of treatment in either group.
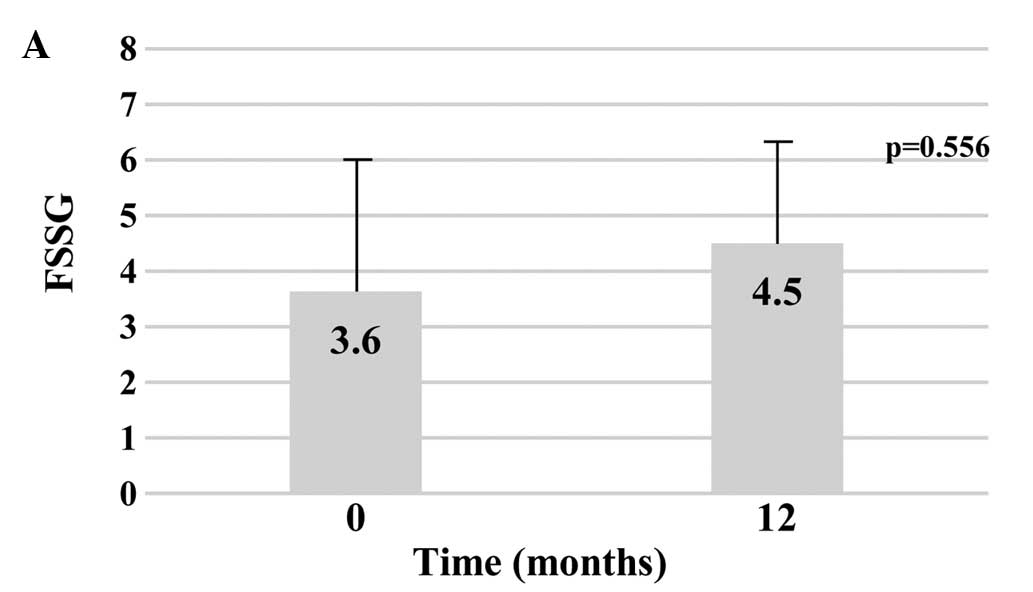
FSSG score
The mean baseline values for FSSG were 3.6±3.0 for
the AC group vs. 9.4±7.2 for the AD group (P=0.056). After one year
of treatment, the mean FSSG values were 4.5±2.8 for the AC group
and 8.3±5.1 for the AD group. The change from baseline FSSG scores
to the end of one year of treatment was not significantly different
in either group (AC group, P=0.556; AD group, P=0.723),
respectively (Fig. 4).
Safety analysis
Of the 22 patients that participated in this study,
six patients were excluded as mentioned above. In each group, the
side effect was mild nausea and the frequency of side effects was
the same between the two study groups (one case in each group). New
bone fractures were not observed from baseline to the end of one
year of treatment in either group.
Discussion
To the best of our knowledge, this is the first
prospective, randomized, open-label study to investigate the
therapeutic efficacy of AC and AD, as estimated by lumbar BMD
changes in osteoporotic patients using concomitant PPIs. Although a
recent prospective study comparing AD and AC indicated that AD was
more efficacious than AC in increasing lumber BMD (14), the current study of osteoporotic
patients using PPIs demonstrated that lumbar BMD change following a
one-year treatment with AD was greater than that observed with AC
treatment. Furthermore, a previous report indicated that PPI use
was associated with a dose-dependent loss of AD anti-fracture
efficacy (10). According to certain
Japanese prospective studies, the lumbar BMD increase at one year
after AD treatment was ~6% (14,15) and in
the current study, the lumbar BMD increase after one year of AD
treatment in osteoporotic patients using PPIs (6.8%) was similar to
a previous study. However, a limitation of the present study was
that the number of study subjects was small; thus, further large
prospective studies are required to determine the effect of AD in
osteoporotic patients using concomitant PPIs.
It is hypothesized that PPIs attenuate the
therapeutic effect of bisphosphonate as follows: PPIs may decrease
the absorption of calcium; the dissolution of calcium carbonate is
a pH-dependent process and gastric pH is particularly important for
calcium absorption (16). As PPIs
strongly suppress gastric acid secretion, the dissolution of
calcium carbonate is inhibited by gastric juice with a high pH and,
as a result, calcium absorption may decrease. Similarly, an
association between PPI use and bone fracture is suggested in
western countries (17); however,
certain studies did not report any association between PPI use and
bone fracture (18) and there are few
prospective studies regarding the association between PPI use and
bone fracture.
It was reported that the occurrence of GI adverse
events was an independent determinant of persistence with
bisphosphonate therapy (19) and
adherence to bisphosphonate treatment may decrease due to GI
adverse events. However adherence to the bisphosphonate treatment
schedule was good in the present study (data not shown).
It was reported that PPIs suppress bone resorption
adversely by inhibiting the vacuolar proton pump of the osteoclast
(20). However, it was reported that
the concentrations of PPIs required for the inhibition of the
osteoclast proton pump are markedly higher than the tolerable
physiological concentrations (21).
Gertz et al (22) reported that
increasing gastric pH by infusion of ranitidine was associated with
a doubling of AD bioavailability. Recently, Itoh et al
(23) reported that risedronate
administration in combination with a PPI may be more effective for
treating osteoporosis and for improving physical fitness than
treatment with risedronate alone, although they evaluated the BMD
of the trabecular bone using quantitative computer tomography
(23).
According to previous reports, the lumbar BMD
increase following one year of AC treatment was ~1% (24,25), while
the current study indicated a lumbar BMD change of −0.4% after one
year of AC treatment. It was previously reported that
bisphosphonates reduce ~50% of bone turnover markers (15); however, no significant difference in
bone turnover markers was identified in the present study. A
limitation of the current investigation was that the number of
study subjects was small; therefore, the effect of AD on bone
turnover markers may not have been apparent.
It was suggested that osteoporotic patients
exhibiting lumbar pressure fractures or kyphosis are at risk of
GERD (26,27). Miyakoshi et al (28) also reported that increases in the angle
of lumbar kyphosis and the number of lumbar vertebral fractures may
represent very important risk factors for GERD in osteoporotic
patients. However, there are few reports about the association
between GERD symptoms using FSSG and bisphosphonates. In the
current study, the FSSG score did not change after the one-year
treatment with AD; therefore, the increase in GERD symptoms
triggered by long-term use was not observed.
Since there were no significant changes in frequency
or severity of side effects in the two treatment groups in this
study, concomitant PPI use with AD may present as a safe
therapeutic strategy. Furthermore, no increased findings of RE or
PUD were observed over the one year of treatment with either AD or
AC. As the residence time in the esophagus of the medication was
considered a mechanism of the risk for esophagitis (4), the patients in the current study were
instructed comprehensively about the appropriate dosing regimen
(taking a tablet with enough water and remaining upright for ≥30
min before the first food of the day).
There were certain limitations of the current study.
The study was not a double-blind trial, but an open-labeled study.
The number of study subjects was particularly small; therefore, a
large prospective multicenter trial is required. Furthermore,
patients used different PPIs, which may have influenced certain
results, such as lumbar BMD and the FSSG score. Family histories of
osteoporosis or certain other important risk factors, such as
exercise, sunlight exposure, dietary calcium intake, or the use of
over-the-counter medication and other nutrients were not
investigated.
In conclusion, the present study demonstrated via
lumbar BMD change that a one-year treatment with AD was more
efficacious than with AC in osteoporotic patients using PPIs.
Furthermore, as with previous reports, the percentage change in
lumbar BMD following one year of AD treatment was similar (6.8%) in
osteoporotic patients using PPIs. However, additional, large
prospective multicenter trials are required to further clarify the
efficacy of bisphosphonate administration with PPIs in osteoporosis
treatment.
References
|
1
|
Iki M: Epidemiology of osteoporosis in
Japan. Clin Calcium. 22:797–803. 2012.(In Japanese). PubMed/NCBI
|
|
2
|
Liberman UA, Weiss SR, Bröll J, Minne HW,
Quan H, Bell NH, Rodriguez-Portales J, Downs RW Jr, Dequeker J,
Favus M, et al: The Alendronate Phase III Osteoporosis Treatment
Study Group: Effect of oral alendronate on bone mineral density and
the incidence of fractures in postmenopausal osteoporosis. N Engl J
Med. 333:1437–1443. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de Groen PC, Lubbe DF, Hirsch LJ, Daifotis
A, Stephenson W, Freedholm D, Pryor-Tillotson S, Seleznick MJ,
Pinkas H and Wang KK: Esophagitis associated with the use of
alendronate. N Engl J Med. 335:1016–1021. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Graham DY, Malaty HM and Goodgame R:
Primary amino-bisphosphonates: A new class of gastrotoxic drugs -
comparison of alendronate and aspirin. Am J Gastroenterol.
92:1322–1325. 1997.PubMed/NCBI
|
|
5
|
Yoshimura N, Muraki S, Oka H, Kawaguchi H,
Nakamura K and Akune T: Cohort profile: Research on
osteoarthritis/osteoporosis against disability study. Int J
Epidemiol. 39:988–995. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nagasue T, Nakamura S, Kochi S, Kurahara
K, Yaita H, Kawasaki K and Fuchigami T: Time trends of the impact
of Helicobacter pylori infection and nonsteroidal
anti-inflammatory drugs on peptic ulcer bleeding in Japanese
patients. Digestion. 91:37–41. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kinoshita Y, Kawanami C, Kishi K, Nakata
H, Seino Y and Chiba T: Helicobacter pylori independent
chronological change in gastric acid secretion in the Japanese.
Gut. 41:452–458. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujiwara Y and Arakawa T: Epidemiology and
clinical characteristics of GERD in the Japanese population. J
Gastroenterol. 44:518–534. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Malfertheiner P, Fass R, Quigley EM,
Modlin IM, Malagelada JR, Moss SF, Holtmann G, Goh KL, Katelaris P,
Stanghellini V, et al: Review article: From gastrin to
gastro-oesophageal reflux disease - a century of acid suppression.
Aliment Pharmacol Ther. 23:683–690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Abrahamsen B, Eiken P and Eastell R:
Proton pump inhibitor use and the antifracture efficacy of
alendronate. Arch Intern Med. 171:998–1004. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Orimo H, Hayashi Y, Fukunaga M, Sone T,
Fujiwara S, Shiraki M, Kushida K, Miyamoto S, Soen S, Nishimura J,
et al: Osteoporosis Diagnostic Criteria Review Committee: Japanese
Society for Bone and Mineral Research: Diagnostic criteria for
primary osteoporosis: Year 2000 revision. J Bone Miner Metab.
19:331–337. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lundell LR, Dent J, Bennett JR, Blum AL,
Armstrong D, Galmiche JP, Johnson F, Hongo M, Richter JE, Spechler
SJ, et al: Endoscopic assessment of oesophagitis: Clinical and
functional correlates and further validation of the Los Angeles
classification. Gut. 45:172–180. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kusano M, Shimoyama Y, Sugimoto S,
Kawamura O, Maeda M, Minashi K, Kuribayashi S, Higuchi T, Zai H,
Ino K, et al: Development and evaluation of FSSG: Frequency scale
for the symptoms of GERD. J Gastroenterol. 39:888–891. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shiraki M, Kushida K, Fukunaga M,
Kishimoto H, Taga M, Nakamura T, Kaneda K, Minaguchi H, Inoue T,
Morii H, et al: The Alendronate Phase III Osteoporosis Treatment
Research Group: A double-masked multicenter comparative study
between alendronate and alfacalcidol in Japanese patients with
osteoporosis. Osteoporos Int. 10:183–192. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Uchida S, Taniguchi T, Shimizu T, Kakikawa
T, Okuyama K, Okaniwa M, Arizono H, Nagata K, Santora AC, Shiraki
M, et al: Therapeutic effects of alendronate 35 mg once weekly and
5 mg once daily in Japanese patients with osteoporosis: A
double-blind, randomized study. J Bone Miner Metab. 23:382–388.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sipponen P and Härkönen M: Hypochlorhydric
stomach: A risk condition for calcium malabsorption and
osteoporosis? Scand J Gastroenterol. 45:133–138. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang YX, Lewis JD, Epstein S and Metz DC:
Long-term proton pump inhibitor therapy and risk of hip fracture.
JAMA. 296:2947–2953. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaye JA and Jick H: Proton pump inhibitor
use and risk of hip fractures in patients without major risk
factors. Pharmacotherapy. 28:951–959. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Penning-van Beest FJ, Goettsch WG, Erkens
JA and Herings RM: Determinants of persistence with
bisphosphonates: A study in women with postmenopausal osteoporosis.
Clin Ther. 28:236–242. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tuukkanen J and Väänänen HK: Omeprazole, a
specific inhibitor of H+-K+-ATPase, inhibits bone resorption in
vitro. Calcif Tissue Int. 38:123–125. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mattsson JP, Väänänen K, Wallmark B and
Lorentzon P: Omeprazole and bafilomycin, two proton pump
inhibitors: Differentiation of their effects on gastric, kidney and
bone H(+)-translocating ATPases. Biochim Biophys Acta.
1065:261–268. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gertz BJ, Holland SD, Kline WF,
Matuszewski BK, Freeman A, Quan H, Lasseter KC, Mucklow JC and
Porras AG: Studies of the oral bioavailability of alendronate. Clin
Pharmacol Ther. 58:288–298. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Itoh S, Sekino Y, Shinomiya K and Takeda
S: The effects of risedronate administered in combination with a
proton pump inhibitor for the treatment of osteoporosis. J Bone
Miner Metab. 31:206–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Orimo H, Shiraki M, Hayashi Y, Hoshino T,
Onaya T, Miyazaki S, Kurosawa H, Nakamura T and Ogawa N: Effects of
1 alpha-hydroxyvitamin D3 on lumbar bone mineral density and
vertebral fractures in patients with postmenopausal osteoporosis.
Calcif Tissue Int. 54:370–376. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shikari M, Kushida K, Yamazaki K, Nagai T,
Inoue T and Orimo H: Effects of 2 years' treatment of osteoporosis
with 1 alpha-hydroxy vitamin D3 on bone mineral density and
incidence of fracture: A placebo-controlled, double-blind
prospective study. Endocr J. 43:211–220. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yoshimura M, Nagahara A, Ohtaka K, Shimada
Y, Asaoka D, Kurosawa A, Osada T, Kawabe M, Hojo M, Yoshizawa T, et
al: Presence of vertebral fractures is highly associated with
hiatal hernia and reflux esophagitis in Japanese elderly people.
Intern Med. 47:1451–1455. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yamaguchi T, Sugimoto T, Yamada H, Kanzawa
M, Yano S, Yamauchi M and Chihara K: The presence and severity of
vertebral fractures is associated with the presence of esophageal
hiatal hernia in postmenopausal women. Osteoporos Int. 13:331–336.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyakoshi N, Kasukawa Y, Sasaki H, Kamo K
and Shimada Y: Impact of spinal kyphosis on gastroesophageal reflux
disease symptoms in patients with osteoporosis. Osteoporos Int.
20:1193–1198. 2009. View Article : Google Scholar : PubMed/NCBI
|