Introduction
Gout is characterized by the recurrent attacks of
acute inflammatory arthritis with marked suffering. Serum uric acid
reduction is crucial as elevation of uric acid concentrations, the
cause of gout, may result in metabolic syndrome (1). However, the incidence of gout has rapidly
increased over the last 30 years in Japan, corresponding with
increases in meat, fish and alcohol consumption, which is a result
of Westernization. Uric acid is the final oxidation product of
purine metabolism in humans in the absence of the hepatic enzyme
uricase. Increased production and/or decreased uric acid excretion
elevated serum uric acid levels. The former is caused by an
excessively purine-rich diet and purine metabolism overactivation,
whereas the latter is caused by renal impairment and certain drugs.
Hyperuricemia is diagnosed when serum uric acid levels exceed the
limit of solubility (7.0 mg/dl), and increases the risk of
monosodium urate or uric acid crystal deposition, which could
result in acute gouty arthritis, gouty arthropathy, chronic
tophaceous gout, uric acid urolithiasis or gouty nephropathy
(2). In addition, hyperuricemia is
considered as a risk factor for the incidence of cardiovascular
diseases (3). Thus, long-term
reduction of serum urate concentrations to subsaturation levels is
vital in the treatment of gout (4).
Clinical management of serum uric acid levels often
includes using a xanthine oxidase inhibitor (allopurinol) and
uricosurics (probenecid and benzbromarone), which facilitate
urinary excretion. However, their use can induce several adverse
reactions, such as fever, skin rash, worsened renal function and
Stevens-Johnson syndrome (5,6). The risk-benefit balance for using such
drugs among gout-free patients with hyperuricemia is not favorable
according to the guidelines for the management of hyperuricemia and
gout (7). Therefore, non-medication
treatments, including a low-purine diet, exercise therapy and
natural products, are recommended for gout-free individuals with
insignificantly high serum uric acid. In particular, it is commonly
assumed that regular ingestion of dietary supplements is easier in
comparison to dietetics or exercise therapy for the individuals
with insignificantly high serum uric acid and no gout pain.
Although the majority of physicians have not regarded dietary
supplements to be efficacious on hyperuricemia, the ingredients
have been investigated for hypouricemic activity.
Imidazole compounds (L-histidine, anserine and
carnosine) are found at high concentrations in the muscles and
brains of vertebrates (8). Various
studies have indicated that the imidazole compounds have
pH-buffering (9) and antioxidant
properties (10), which are
attributable to their imidazole moiety. The imidazole compounds
have been reported to affect the activities of enzymes involved in
glycogenolysis (11) and
gluconeogenesis (12). These
properties suggest that the imidazole compounds may affect the pH
of body fluids, renal functions and organic acid levels that affect
uric acid reabsorption in the glycolytic pathway, resulting in
decreased serum uric acid levels. In addition, an early
investigation proposed a tuna extract containing the imidazole
compounds as a hypouricemic agent in a preliminary clinical study
(13). Therefore, the present study
evaluated the efficacy and safety of the tuna extract containing a
high concentration of the imidazole compounds on serum uric acid
levels in subjects with insignificantly high serum uric acid.
Materials and methods
Subjects and eligibility
All the volunteers were recruited from the Mareesia
Garden Clinic (Tokyo, Japan), and were Japanese male volunteers
(aged 20–64 years) whose serum uric acid concentrations were
6.5–8.0 mg/dl, the subject of lifestyle guidance, unless they had
any complications. Women were excluded from the present study due
to the small size of the sample population. The exclusion criteria
were gout, serious nephropathy, hepatopathy, cardiac disease,
anaphylaxis reaction to the test compound and other abnormalities
(as deemed by the investigator). Furthermore, volunteers already
receiving urate-lowering therapies, including drugs and dietary
supplements, were excluded. The final exclusion criterion was
participation in another clinical study at the beginning of the
present study. In total, 48 subjects who fulfilled the eligibility
criteria for the study agreed to participate. All 48 subjects were
enrolled in the study prior to random allocation into the three
groups.
Approval of the study was obtained from the
institutional review board of the Mareesia Garden Clinic, according
to an ethical principle and experimental plan based on the Helsinki
Declaration and the Ethical Guidelines for Epidemiological Research
by the Japanese Government. Prior to enrollment in the study, a
complete explanation regarding the study objective and methodology
was provided to the subjects by the doctor; and participants in the
study provided written informed consent.
Study design
The study was performed according to a randomized,
double-blind, placebo-controlled design. Following screening and
enrollment, 48 subjects were assigned using a random number table
to one of three groups: The placebo and the test supplement at low
and high doses of tuna extract. The subjects in the test supplement
groups were provided with hydroxypropyl methylcellulose capsules
filled with a commercial tuna extract, Marine Active 10 (Yaizu
Suisankagaku Industry Co., Ltd., Shizuoka, Japan). Marine Active 10
is produced by ion-exchange purification and spray drying from a
hot water extract of skipjack and yellowfin tuna, without enzymatic
hydrolysis. Dextrin was added as an excipient, and the purine base
was reduced to the level of 0.01 g/100 g. Quantification of amino
acids and histidine-containing dipeptides in this product was
performed using an amino acid analyzer (L-8500A; Hitachi, Tokyo,
Japan) and high-performance liquid chromatography (14). The subjects in the placebo group were
provided with hydroxypropyl methylcellulose capsules filled with
dextrin. The contents of the placebo and the test supplement (6
capsules/day) are shown in Table
I.
 | Table I.Daily intake of the placebo and the
test supplement (6 capsules/day). |
Table I.
Daily intake of the placebo and the
test supplement (6 capsules/day).
|
|
| Test supplement dose,
mg |
|---|
|
|
|
|
|---|
| Extract | Placebo, mg | Low | High |
|---|
| Tuna extract | 0.0 | 238.6 | 477.1 |
|
Anserine | 0.0 | 25.0 | 50.0 |
|
Carnosine | 0.0 | 2.2 | 4.4 |
|
Histidine | 0.0 | 16.9 | 33.9 |
| Other
amino acids | 0.0 | 4.5 | 9.1 |
| Other
proteins and peptides | 0.0 | 11.9 | 23.9 |
|
Dextrin | 0.0 | 167.0 | 334.0 |
| Purine
base | 0.0 | 0.0 | 0.0 |
| Dextrin | 1,250.9 | 1,012.3 | 773.7 |
| Calcium stearate | 65.8 | 65.8 | 65.8 |
| Total | 1,316.7 | 1,078.1 | 839.5 |
The subjects were treated with 2 capsules containing
the placebo or the test supplement after each meal for 4 weeks.
They visited the clinic at 7 days before the intervention, at weeks
2 and 4 during the intervention, and at week 2 after the
intervention for follow-up assessment. The subjects were prohibited
from drinking alcohol for 3 days and had to fast for 8 h before
blood collection. During the visiting days, 24 ml of blood was
collected from each subject by venipuncture, which was then
subjected to serum uric acid analysis (primary endpoint) and safety
monitoring; this involved complete blood counts, qualitative
urinalysis and measurement of body weight, body mass index, blood
pressure, cardiac rate, total protein, albumin, albumin/globulin
ratio, lactate dehydrogenase, alkaline phosphatase, aspartate
aminotransferase, alanine aminotransferase, γ-glutamyl
transpeptidase, creatinine, blood urea nitrogen, triglycerides,
total cholesterol, high-density lipoprotein cholesterol,
low-density lipoprotein cholesterol, glucose, sodium, potassium,
chloride, calcium and inorganic phosphorus. Blood components were
determined using conventional methods by Japan Clinical
Laboratories, Inc. (Tokyo, Japan). Adverse events were assessed by
the investigator at each study visit. Furthermore, subjects were
requested to record the number of capsules ingested, subjective
symptoms, any medications received and their meals for 3 days
before the visit. The daily intakes of nutrients and purine were
calculated by nutritionists from the food diary and pictures of
meals ingested by the subjects for the 3 days before each
visit.
Statistical analysis
Values are expressed as the mean ± standard error.
Baseline data of subjects, daily intakes of nutrients and safety
data were compared by Dunnett's test among the placebo and the test
supplement (at low and high doses of tuna extract) groups. In
addition, the changes of serum uric acid were determined by
Williams' test (15) among the placebo
and the test supplement groups. Data were analyzed using R 3.2.2
scripting and statistical software (R Foundation for Statistical
Computing, Vienna, Austria; https://www.r-project.org/). All the hypotheses were
tested at the 5% level of significance. P<0.05 was considered to
indicate a statistically significant difference.
Results
Characteristics of the subjects
During the intervention, 1 subject in the placebo
group withdrew from the study due to his own request. The remaining
47 subjects completed the study and were included in the
statistical analysis for efficacy and safety. At the baseline, no
significant differences were observed for age, physiological
characteristics (body weight, body mass index, systolic blood
pressure, diastolic blood pressure and pulse rate) and serum uric
acid among the groups of placebo and the test supplement (at low
and high doses of tuna extract) (Table
II).
 | Table II.Baseline clinical characteristics of
the study subjects. |
Table II.
Baseline clinical characteristics of
the study subjects.
|
|
| Test supplement
dose |
|---|
|
|
|
|
|---|
| Variables | Placebo (n=15) | Low (n=16) | High (n=16) |
|---|
| Age, years |
44.2±1.8 |
45.0±1.9 |
43.8±2.1 |
| Body weight, kg |
74.1±2.7 |
74.8±2.0 |
74.9±3.1 |
| Body mass index,
kg/m2 |
24.7±0.7 |
25.5±0.7 |
25.7±0.7 |
| Systolic blood
pressure, mmHg |
125.6±4.0 |
124.3±3.8 |
125.7±2.5 |
| Diastolic blood
pressure, mmHg |
79.5±2.6 |
83.0±3.0 |
77.9±2.0 |
| Pulse rate, beats
min−1 |
71.1±1.6 |
70.1±1.4 |
72.7±2.0 |
| Serum uric acid,
mg/dl |
7.1±0.1 |
7.2±0.1 |
7.2±0.1 |
Effect of tuna extract ingestion on
serum uric acid levels
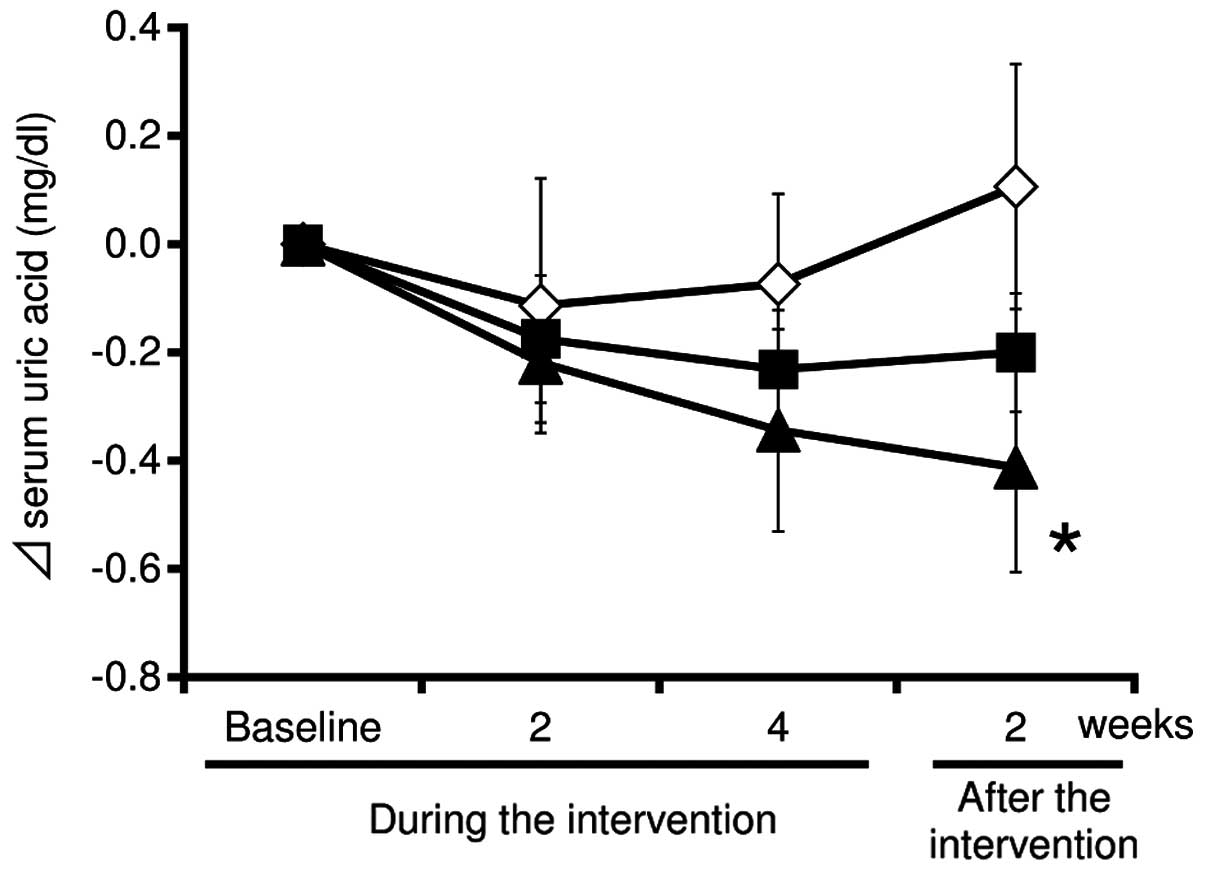
Fig. 1 shows the
changes of serum uric acid during the 4-week intervention and the
subsequent 2-week post-intervention follow-up. During the
intervention, the uric acid levels in the test supplement (at low
and high doses of tuna extract) groups were substantially decreased
compared with the placebo group at weeks 2 and 4. In addition, the
uric acid levels in the test supplement group at a high-dose of
tuna extract was significantly decreased compared with the placebo
group at week 2 after the intervention (mean change from baseline:
High-dose, −0.49±0.19 mg/dl; placebo, 0.14±0.22 mg/dl;
P<0.05).
The mean adherence rates were not different among
the placebo and the test supplement groups (95.7±0.5, 95.9±0.8 and
96.0±0.2% for the placebo, low and high dose, respectively). During
and subsequent to the intervention, no significant differences were
observed in the daily intakes of nutrients and purine between the
placebo and the test supplement (at low and high doses of tuna
extract) groups (Table III).
Therefore, the oral administration of tuna extract was likely to
decrease the serum uric acid in subjects with insignificantly high
serum uric acid.
 | Table III.Mean daily intakes of nutrients and
purine for 3 days before the bi-weekly blood collection. |
Table III.
Mean daily intakes of nutrients and
purine for 3 days before the bi-weekly blood collection.
|
| During the
intervention | After the
intervention |
|---|
|
|
|
|
|---|
| Nutrients | Baseline | Week 2 | Week 4 | Week 2 |
|---|
| Energy, kj/day |
|
|
|
|
|
Placebo |
7,976±346 |
8,541±550 |
8,831±446 |
8,393±552 |
| Low
dose |
8,855±353 |
9,256±469 |
9,529±486 |
9,072±456 |
| High
dose |
7,568±333 |
7,978±412 |
8,705±510 |
8,070±487 |
| Protein, g/day |
|
|
|
|
|
Placebo |
65.4±3.7 |
71.0±5.3 |
73.9±3.7 |
71.1±4.1 |
| Low
dose |
70.5±3.3 |
76.1±5.3 |
76.8±4.6 |
76.1±4.4 |
| High
dose |
68.5±4.7 |
65.9±4.1 |
72.2±4.2 |
70.8±4.9 |
| Fat, g/day |
|
|
|
|
|
Placebo |
63.6±5.1 |
62.3±5.8 |
71.7±5.8 |
67.3±5.7 |
| Low
dose |
72.6±3.0 |
80.4±4.3 |
85.7±5.9 |
78.1±3.8 |
| High
dose |
57.7±4.6 |
68.2±7.4 |
77.2±6.4 |
71.2±8.4 |
| Carbohydrate,
g/day |
|
|
|
|
|
Placebo |
256.0±12.0 |
286.8±17.7 |
279.4±15.1 |
266.3±17.6 |
| Low
dose |
284.0±15.4 |
284.8±15.9 |
287.3±16.8 |
278.0±16.5 |
| High
dose |
243.3±11.2 |
247.1±9.9 |
262.8±17.0 |
240.8±13.4 |
| Purine, g/day |
|
|
|
|
|
Placebo |
276.1±20.6 |
296.8±28.1 |
307.8±20.3 |
302.0±23.1 |
| Low
dose |
259.6±13.2 |
306.9±32.0 |
301.8±24.7 |
320.2±34.7 |
| High
dose |
296.1±22.9 |
265.3±20.9 |
292.0±19.1 |
276.6±19.8 |
Adverse effects during regular tuna
extract ingestion
During and subsequent to the intervention, 44
adverse events were reported in 5, 7 and 10 subjects receiving
placebo, and test supplements at low and high doses, respectively.
Relatively frequent adverse events reported were cold symptoms,
gastric distress and headache. These events were generally mild and
none were judged by the investigator to be associated with the
study treatment. Furthermore, routine physical and cardiovascular
characteristics, hematology and blood chemistry did not show any
significant abnormalities during the intervention and follow-up
periods in all the three groups (data not shown).
Discussion
The present study performed a randomized controlled
study and investigated the effect of oral administration of the
tuna extract (at low and high doses) on serum uric acid levels in
gout-free subjects with insignificantly high serum uric acid. The
results indicated that administration of the tuna extract
containing the imidazole compounds resulted in a significant
reduction in serum uric acid levels compared with administration of
placebo. Moreover, there was a dose-dependent trend of uric acid
reduction at week 4 during the intervention and week 2 after the
intervention. Therefore, these results suggest that the oral
administration of tuna extract containing the imidazole compounds
exhibits a hypouricemic action on the individuals with
insignificantly high serum uric acid.
Only a limited number of studies have investigated
the hypouricemic effect of the imidazole compounds. The study by
Noguchi et al (16) fed a diet
containing 1% inosine and 1% anserine to Wistar rats (male, 7 weeks
old) ad libitum. After 1 week of administration, the serum
uric acid levels in the rats in the inosine with anserine group
were lower compared to those in the inosine group (as a control).
Microarray analysis showed a marked increase in the expression of
hypoxanthine phosphoribosyltransferase and lactate dehydrogenase in
the livers of the rats in the inosine with anserine group compared
with those in the inosine group. Hypoxanthine
phosphoribosyltransferase salvages hypoxanthine and guanine to
renew purine synthesis (17),
resulting in the inhibition of uric acid synthesis by the tuna
extract containing anserine. Lactate dehydrogenase catalyzes the
interconversion of pyruvate and lactate. Lactate directly activates
urate transporter 1 (URAT1), contrary to several uricosurics. URAT1
is the most important element in the mechanism involved in the
reabsorption and urinary excretion of uric acid (18). In addition, ingested anserine is
absorbed intact in human blood, and is hydrolyzed to
π-methylhistidine and β-alanine by serum and tissue carnosinases
for 4 h after ingestion (19). Intact
anserine and π-methylhistidine (8% anserine and 82%
π-methylhistidine of the ingested amount of anserine) are excreted
into the urine (20). Therefore,
anserine and π-methylhistidine, with their proton-buffering
capacity, may stabilize the pH of urine to improve the solubility
of uric acid and accelerate excretion, similar to the mechanism of
sodium potassium citrate (21). These
hypotheses require further investigation. Moreover, although almost
all the anserine is excreted into the urine over the 24 h after
ingestion, the serum uric acid levels were reduced at week 2 after
the intervention. This phenomenon cannot be accounted for by the
aforementioned hypotheses alone. Baguet et al (22) reported changes in the carnosine content
in human skeletal muscles subsequent to β-alanine ingestion. In the
present study, muscle carnosine increased and returned to baseline
values within 9 weeks. Therefore, carnosine was synthesized by
β-alanine-derived anserine, and may be correlated with the
hypouricemic effect.
Previous studies have supported the safety of
anserine and carnosine ingestion. Chicken breast extract (CBEX)
containing ≥35% anserine and carnosine did not result in
mutagenicity, assessed using the Ames assay, and no adverse effects
were observed in a 90-day chronic toxicity test (2,000 mg/kg/day)
in either male or female rats (23).
Similarly, in humans, no adverse events or abnormal changes were
observed in the blood examination, urinalysis or physical
examination after regular ingestion of CBEX (1,200 mg/day of
anserine and carnosine) for 4 weeks (24). In the present study, no severe adverse
events or marked changes were observed in the general condition of
the patients, hematology or blood biochemistry during regular
ingestion of the tuna extract. Therefore, continuous administration
of the tuna extract to subjects with insignificantly high serum
uric acid is not likely to cause adverse health effects.
In conclusion, these results suggest that the tuna
extract may be a beneficial and safe food ingredient for
individuals with insignificantly high serum uric acid. Since a
number of studies have shown antihypertensive (25) and hypoglycemic effects (26) of anserine, its ingestion results in
overall health benefits to individuals. However, although no
significant differences were observed between the tuna extract and
placebo groups during the intervention, a significant difference
was identified following the intervention; this could be due to the
small sample size, insufficient study duration or dose tested.
Therefore, additional studies are required for more accurate
assessment of the efficacy of the tuna extract. Moreover, further
studies on the evaluation of uric acid excretion into the urine are
required to evaluate the mechanism of serum uric acid level
reduction instigated by tuna extract ingestion, including the
imidazole compounds contribution.
Acknowledgements
The authors would like to thank Dr Shin Fujimori and
Dr Kiyoko Kaneko (Teikyo University) for their assistance in
discussions regarding the results. Furthermore, the authors also
thank Ms. Fumiyo Ohya-Nakano for her assistance in the validation
of analysis and study preparation. The authors would like to thank
Enago (www.enago.jp) for the English language
review.
References
|
1
|
Choi HK and Ford ES: Prevalence of the
metabolic syndrome in individuals with hyperuricemia. Am J Med.
120:442–447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wortmann RL: Gout and hyperuricemia. Curr
Opin Rheumatol. 14:281–286. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ishizaka N, Ishizaka Y, Toda E, Nagai R
and Yamakado M: Association between serum uric acid, metabolic
syndrome, and carotid atherosclerosis in Japanese individuals.
Arterioscler Thromb Vasc Biol. 25:1038–1044. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Shoji A, Yamanaka H and Kamatani N: A
retrospective study of the relationship between serum urate level
and recurrent attacks of gouty arthritis: Evidence for reduction of
recurrent gouty arthritis with antihyperuricemic therapy. Arthritis
Rheum. 51:321–325. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roujeau JC, Kelly JP, Naldi L, Rzany B,
Stern RS, Anderson T, Auquier A, Bastuji-Garin S, Correia O, Locati
F, et al: Medication use and the risk of Stevens-Johnson syndrome
or toxic epidermal necrolysis. N Engl J Med. 333:1600–1607. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hande KR, Noone RM and Stone WJ: Severe
allopurinol toxicity. Description and guidelines for prevention in
patients with renal insufficiency. Am J Med. 76:47–56. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yamanaka H, Ueda T and Ohno I: Guideline
for the management of hyperuricemia and gout (2nd). Medical Review
Co., Ltd. Tokyo: 2010.
|
|
8
|
Kohen R, Yamamoto Y, Cundy KC and Ames BN:
Antioxidant activity of carnosine, homocarnosine, and anserine
present in muscle and brain. Proc Natl Acad Sci USA. 85:3175–3179.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abe H: Role of histidine-related compounds
as intracellular proton buffering constituents in vertebrate
muscle. Biochemistry (Mosc). 65:757–765. 2000.PubMed/NCBI
|
|
10
|
Boldyrev A, Bulygina E, Leinsoo T,
Petrushanko I, Tsubone S and Abe H: Protection of neuronal cells
against reactive oxygen species by carnosine and related compounds.
Comp Biochem Physiol B Biochem Mol Biol. 137:81–88. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Severin SE, Skolysheva LK, Shur SA and
Vulfson PL: The pH-dependent conformational transition in glycogen
phosphorylase b. The effect of carnosine and anserine on its
activity. Biochem Int. 20:227–238. 1990.PubMed/NCBI
|
|
12
|
Ikeda T, Kimura K, Hama T and Tamaki N:
Activation of rabbit muscle fructose 1,6-bisphosphatase by
histidine and carnosine. J Biochem. 87:179–185. 1980.PubMed/NCBI
|
|
13
|
Chang WTH: Composition containing
dipeptide of histidine and alanine for reducing uric acid and
method for reducing uric acid using the dipeptide. US Patent
7498301. Filed December 22, 2006; issued March 3. 2009.
|
|
14
|
Abe H and Ohmama S: Effect of starvation
and sea-water acclimation on the concentration of free l-histidine
and related dipeptides in the muscle of eel, rainbow trout and
Japanese dace. Comp Biochem Physiol Part B Comp Biochem.
88:507–511. 1987. View Article : Google Scholar
|
|
15
|
Williams DA: A test for differences
between treatment means when several dose levels are compared with
a zero dose control. Biometrics. 27:103–117. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Noguchi Y, Shimizu J, Mano H, Matahira Y
and Wada M: Effect of anserine administration on purine metabolism.
Proceedings of 60th Annual Meeting of Japan Society of Nutrition
and Food Science (Tokyo). 2682006.
|
|
17
|
Curto R, Voit EO and Cascante M: Analysis
of abnormalities in purine metabolism leading to gout and to
neurological dysfunctions in man. Biochem J. 329(Pt 3): 477–487.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Enomoto A, Kimura H, Chairoungdua A,
Shigeta Y, Jutabha P, Cha SH, Hosoyamada M, Takeda M, Sekine T,
Igarashi T, et al: Molecular identification of a renal urate anion
exchanger that regulates blood urate levels. Nature. 417:447–452.
2002.PubMed/NCBI
|
|
19
|
Kubomura D, Matahira Y, Masui A and
Matsuda H: Intestinal absorption and blood clearance of
L-histidine-related compounds after ingestion of anserine in humans
and comparison to anserine-containing diets. J Agric Food Chem.
57:1781–1785. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Abe H, Okuma E, Sekine H, Maeda A and
Yoshiue S: Human urinary excretion of L-histidine-related compounds
after ingestion of several meats and fish muscle. Int J Biochem.
25:1245–1249. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kessler T and Hesse A: Cross-over study of
the influence of bicarbonate-rich mineral water on urinary
composition in comparison with sodium potassium citrate in healthy
male subjects. Br J Nutr. 84:865–871. 2000.PubMed/NCBI
|
|
22
|
Baguet A, Reyngoudt H, Pottier A, Everaert
I, Callens S, Achten E and Derave W: Carnosine loading and washout
in human skeletal muscles. J Appl Physiol 1985. 106:837–842. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sato M, Karasawa N, Shimizu M, Morimatsu F
and Yamada R: Safety evaluation of chicken breast extract
containing carnosine and anserine. Food Chem Toxicol. 46:480–489.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Aoyagi S, Sugino T, Kajimoto Y and
Nishitani M: Safety of excess administration of CBEX-Dr-containing
drink on healthy people. Jpn Pharmacol Ther. 36:199–212. 2008.
|
|
25
|
Tanida M, Shen J, Kubomura D and Nagai K:
Effects of anserine on the renal sympathetic nerve activity and
blood pressure in urethane-anesthetized rats. Physiol Res.
59:177–185. 2010.PubMed/NCBI
|
|
26
|
Kubomura D, Matahira Y, Nagai K and
Niijima A: Effect of anserine ingestion on hyperglycemia and the
autonomic nerves in rats and humans. Nutr Neurosci. 13:183–188.
2010. View Article : Google Scholar : PubMed/NCBI
|