Introduction
Gastric cancer is one of the most prevalent types of
human cancer. In Japan, ~50,000 people succumb due to gastric
cancer every year (1). Survival
prognoses for patients with gastric cancer remain poor, prompting
researchers to continue searching for novel treatment strategies.
To develop novel therapeutic options, it is important to understand
the molecular mechanisms of gastric cancer.
Previous studies have shown constitutive activation
of the Notch signaling pathway in various types of malignancies
(2). However, it remains unclear
whether this signaling pathway is activated in gastric cancer. The
Notch signaling pathway is an evolutionary conserved pathway that
is critical in various cellular processes, including cell fate
decisions, proliferation, development, adult homeostasis and stem
cell maintenance (3–7). NOTCH1 functions as a receptor in this
signaling pathway.
The NOTCH1 receptor binds with one of its ligands,
which include jagged 1 (JAG1), JAG2, delta-like 1
(Drosophila) (DLL1), DLL3 or DLL4 (2). The notch intracellular cytoplasmic domain
(NICD) of the NOTCH receptor is then subjected to processing by
proteases [a disintegrin and metalloproteinase (ADAM) protease or
γ-secretase] and subsequently translocates into the nucleus
(2). NICD then binds with target
proteins to activate downstream targets and promote Notch
signaling.
In the present study, immunostaining for NICD was
performed in 115 gastric cancer tissue samples collected during
gastric surgery to determine the correlation between localization
of NICD, clinicopathological characteristics and survival prognoses
in gastric cancer patients.
Materials and methods
Tissue samples
Tumor tissue samples were obtained from 115 gastric
cancer patients (comprising 74 males and 41 females; mean age, 63.1
years) who had undergone surgery at the Department of
Gastroenterological Surgery, Nagoya City University Graduate School
of Medical Science (Nagoya, Japan) between 1996 and 2007 without
pre-operative chemotherapy or radiation. All samples were
snap-frozen in liquid nitrogen and stored at −80°C. The tumors were
classified according to 6 th UICC guidelines for clinical and
pathological studies on gastric cancer (8). Written, informed consent was obtained
from each patient and approval was obtained from the ethical
committee on human research of Nagoya City University (code, no.
71).
Immunohistochemistry
Immunohistochemical staining was performed on
gastric cancer tissue samples that were fixed with 10% formalin for
1 day at room temperature, and then embedded in paraffin.
Paraffin-embedded 3-µm tumor sections were deparaffinized using
xylene (Wako Laboratory Chemicals, Osaka, Japan), rehydrated, and
heat-treated by microwaving in 10 mM citrate buffer (Cell Signaling
Technology, Tokyo, Japan) for 15 min for antigen retrieval. The
sections were cooled to room temperature and washed three times
with phosphate-buffered saline (PBS; Wako Laboratory Chemicals),
for 5 min each time. Sections were then treated with 0.3%
H2O2 in methanol for 30 min to neutralize
endogenous peroxidases, blocked with Block-Ace (Dainihon Sumitomo
Seiyaku, Osaka, Japan) for 10 min, and incubated with primary
monoclonal antibodies targeting human NOTCH1 (1:50; cat. no.
ST1028; Calbiochem; EMD Millipore, Billerica, MA, USA) overnight at
4°C. The sections were then washed with PBS three times. The
sections were incubated with a secondary antibody (1:10; EnVision+
kit; cat. no. K400211: Dako North America, Inc., Carpinteria, CA,
USA) for 30 min and washed with PBS three times. Detection of
immunoreactive proteins was facilitated with 3,3′-diaminobenzidine
buffer tablets (EMD Millipore) and the sections were counterstained
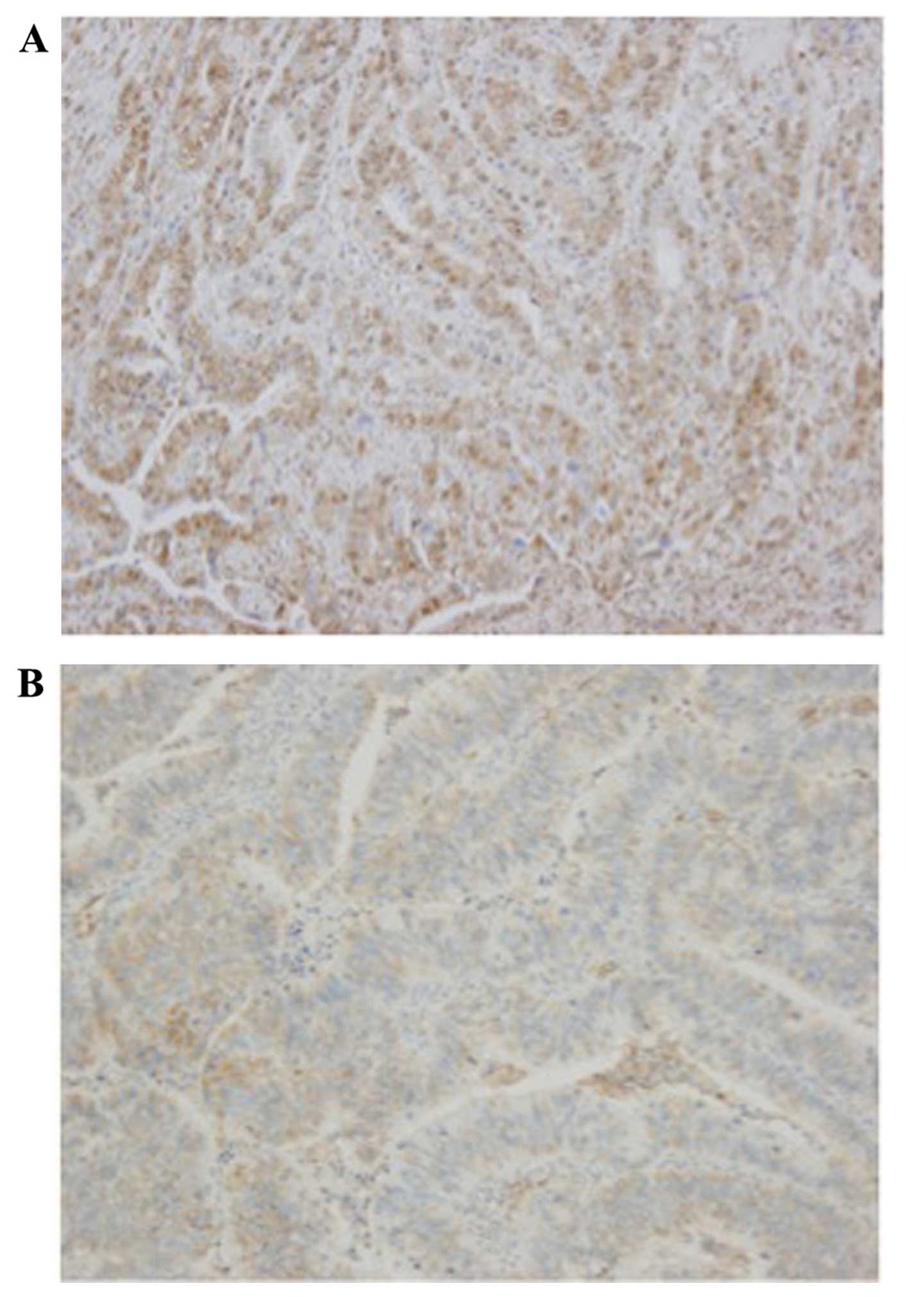
with hematoxylin. For the evaluation of NOTCH1 expression,
immunostaining was considered positive only when unequivocally
strong nuclear staining was present in >30% of the tumor cells,
as analyzed using a light microscope. Cases with faint staining
only were considered negative.
Statistical analysis
Statistical analysis was performed using the
StatView 5.0 software package (Abacus Concepts, Berkeley, CA, USA).
χ2 tests were used to analyse the associations between
NICD immunostaining and the clinical histopathological parameters
of the patients. The survival rate of gastric cancer patients after
surgery was examined using the Kaplan-Meier method, and survival
times were compared using the log-rank test. Cox regression
analysis of factors potentially associated with survival was
conducted to identify independent factors that may exert a
significant joint effect on survival. All tests were two-tailed and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression of NICD in gastric
cancer
First, NOTCH1 localization was examined by
immunostaining. Representative images are presented in Fig. 1. The 115 gastric cancer patients were
divided into two groups according to NICD expression: One group of
patients exhibited NICD-positive staining in >30% of all cancer
cell nuclei (n=61; positive group), and the other group had
NICD-positive staining in <30% of nuclei (n=54; negative
group).
Correlation between
clinicopathological factors and NICD in gastric cancer
Correlations between NICD nuclear localization and
the clinicopathological characteristics of the patients are
presented in Table I. No significant
correlation was observed between the NICD-positive group and age,
gender, location, lymphatic invasion or blood vessel invasion
(Table I). Significant correlations
were observed between NICD nuclear localization and
clinicopathological characteristics, such as tumor status (T
factor; P=0.0069), lymph node status (N factor; P=0.0118),
pathological stage (P=0.0160) and histological differentiation
(P=0.0232).
 | Table I.Correlation of nuclear NICD in gastric
cancer with clinicopathological factors, including patient and
tumor characteristics (n=115). |
Table I.
Correlation of nuclear NICD in gastric
cancer with clinicopathological factors, including patient and
tumor characteristics (n=115).
| Characteristics | NICD-positive
patients/total patients | P-value |
|---|
| Age at surgery
(years) |
| 0.1315 |
| ≤65 | 39/66 |
|
|
>65 | 22/49 |
|
| Gender |
| 0.4953 |
| Male | 41/74 |
|
|
Female | 20/41 |
|
| Location |
| 0.9236 |
|
Upper | 15/27 |
|
|
Middle | 21/39 |
|
|
Lower | 25/49 |
|
| Tumor status |
| 0.0069 |
| T1 | 23/57 |
|
| T2 | 9/15 |
|
| T3 | 9/17 |
|
| T4 | 20/26 |
|
| T1 vs.
T2-4 | 23/57 vs. 38/58 |
|
| Lymph node
status |
| 0.0118 |
| N0 | 30/69 |
|
|
N-positive | 31/46 |
|
| Pathological
stage |
| 0.0160 |
| I | 27/63 |
|
| II | 13/21 |
|
| III | 21/31 |
|
| I vs.
II–IV | 27/63 vs. 34/52 |
|
| Histological
differentiation |
| 0.0232 |
|
Differentiated | 38/82 |
|
|
Undifferentiated | 23/33 |
|
| Lymphatic
invasion |
| 0.1385 |
|
Negative | 20/45 |
|
|
Positive | 41/70 |
|
| Blood vessel
invasion |
| 0.0786 |
|
Negative | 25/56 |
|
|
Positive | 36/59 |
|
Survival curves and expression of
NICD
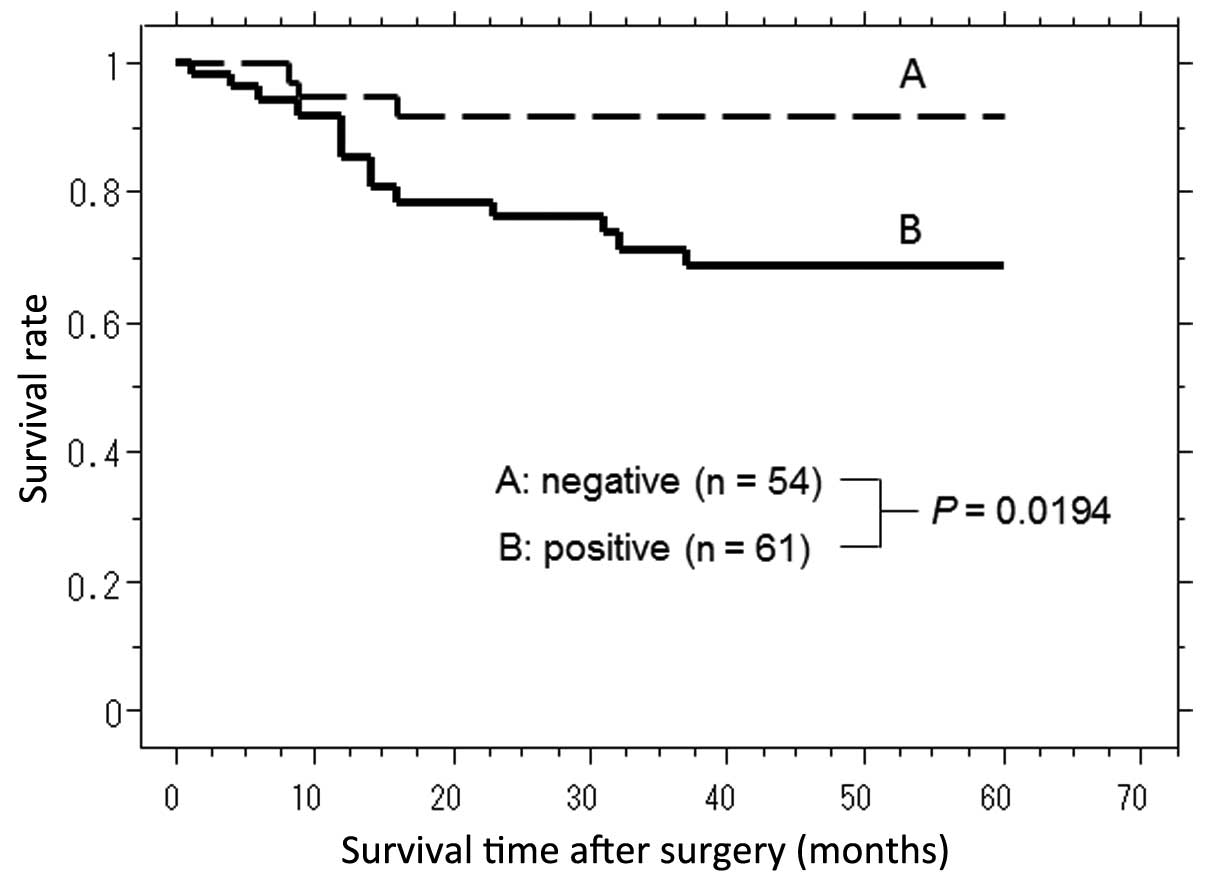
The correlation between nuclear localization of NICD
and survival time in gastric cancer patients after surgery was
investigated, and the mean follow-up was 32.53 months. The
NICD-positive group (n=61) had a significantly shorter survival
time following surgery when compared with the NICD-negative group
[n=54; Fig. 2 (P=0.0194, log-rank
test)].
Among the clinicopathological factors that were
evaluated, univariate analysis indicated that local invasiveness
[risk rate (RR)=10.870; P=0.0016], lymph node metastasis
(RR=41.667; P=0.0003), lymphatic invasion (RR=13.158; P=0.0125),
vein invasion (RR=25.00; P=0.0019) and NICD nuclear localization
(RR=3.937; P=0.0312) were statistically significant prognostic
factors (Table II). Multivariate
analysis revealed that only lymph node metastasis was an
independent prognostic factor (Table
III).
 | Table II.Univariate analysis. |
Table II.
Univariate analysis.
| Parameters | Risk ratio | 95% confidence
interval | P-value |
|---|
| Age at surgery
(years) |
|
|
|
| ≤65 | 1 |
|
|
|
>65 | 1.958 | 0.754–5.085 | 0.1677 |
| Gender |
|
|
|
| Male | 1 |
|
|
|
Female | 1.379 | 0.532–3.577 | 0.5085 |
| Primary tumor |
|
|
|
| T1 | 1 |
|
|
|
T2-4 | 10.870 | 2.481–47.619 | 0.0016 |
| Lymph node
metastasis |
|
|
|
| N0 | 1 |
|
|
|
N-positive | 41.667 | 5.435–41.667 | 0.0003 |
| Lymphatic
invasion |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 13.158 | 1.739–100.00 | 0.0125 |
| Vein invasion |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 25.000 | 3.289–200.00 | 0.0019 |
| Differentiation
status |
|
|
|
|
Differentiated | 1 |
|
|
|
Undifferentiated | 3.039 | 1.172–7.874 | 0.0223 |
| Immunostaining for
NICD |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 3.937 | 1.131–13.70 | 0.0312 |
 | Table III.Multivariate analysis including
NICD. |
Table III.
Multivariate analysis including
NICD.
| Parameters | Risk ratio | 95% confidence
interval | P-value |
|---|
| Primary tumor |
|
|
|
| T1 | 1 |
|
|
|
T2-4 | 0.371 | 0.0528–2.618 | 0.3204 |
| Lymph node
metastasis |
|
|
|
| N0 | 1 |
|
|
|
N-positive | 27.778 | 2.632–333.33 | 0.0056 |
| Lymphatic
invasion |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 0.924 | 0.104–8.197 | 0.9435 |
| Vein invasion |
|
|
|
|
Negative | 1 |
|
|
|
Positive | 8.403 | 0.827–83.333 | 0.0719 |
| Differentiation
status |
|
|
|
|
Differentiated | 1 |
|
|
|
Undifferentiated | 2.933 | 0.946–9.091 | 0.0623 |
| Immunostaining for
NICD |
|
|
|
|
Positive | 1 |
|
|
|
Negative | 2.0747 | 0.451–9.524 | 0.3486 |
Discussion
NOTCH proteins are single-pass transmembrane
receptors that regulate cell-fate decisions during development
(9). The NOTCH family includes four
receptors, NOTCH1, NOTCH2, NOTCH3 and NOTCH4, whose ligands include
JAG1, JAG2, DLL1, DLL3, and DLL4 (2).
The mature NOTCH1 receptor is a heterodimeric class I transmembrane
glycoprotein, generated by proteolytic processing of a precursor
polypeptide (proNOTCH1) in the trans-Golgi network (10). Receptors bind to their ligand NOTCH
receptor, which is then subjected to processing by proteases (ADAM
protease and γ-secretase) and translocate into the nucleus
(11). NICD activates its targets,
promoting protein-protein interactions (12). Data from the present study revealed
that the translocation of NICD into the nucleus occurred in
approximately half of the examined gastric cancer cases (53%;
Table I). Therefore, it was
hypothesized that the Notch signaling pathway is activated in
approximately half of all gastric cancer cases.
Oncogenic roles for Notch signaling have also been
discovered in Hodgkin's lymphoma (HL), anaplastic large-cell
non-HL, certain types of acute myeloid leukemia and B-cell chronic
lymphoid leukemia, gliomas, medulloblastomas, sarcomas, and various
epithelial malignancies of the breast, cervix, lung, colon,
prostate, head and neck, kidney, and pancreas (13–19).
However, while many of the mechanisms underlying the deregulation
in these malignancies remain unclear, the altered expression of
Notch receptors or other Notch signaling pathway components is
often associated with poor prognosis or tumor metastasis (20). Together, these facts indicate that
Notch signaling is oncogenic in a variety of types of human tumor.
Consistent with this, the present data indicates that NOTCH1 is
oncogenic in gastric cancer, as nuclear translocation of NOTCH1 was
correlated with the T and N factors, and a poor prognosis.
Conversely, Notch signaling is anti-oncogenic in
squamous cell carcinoma (SCC) of the skin and cervical uterus, and
for basal cell carcinoma (BCC) of the skin (21–23),
partially due to its interference with canonical WNT signaling.
Thus, it remains unclear whether NOTCH1 acts as a tumor suppressor
or oncogene. However, according to the present data, activation of
the Notch signaling pathway in gastric cancer is indicated to
promote cancer, since NICD expression correlated with tumor status
and lymph node metastasis (Table I).
Therefore, the present study hypothesizes that NOTCH1 acts as an
oncogene in gastric cancer.
The role of the Notch signaling pathway in gastric
cancer is particularly complicated, and it is not clear what
mechanisms regulate the NOTCH1 signaling pathway. However, a
previous study suggested that NOTCH1 signaling contributes to the
progression of human gastric cancer through induction of
prostaglandin-endoperoxide synthase 2 (PTGS2) expression (24).
The NOTCH1 gene is located on chromosome
9q34. A previous study reported that the most frequent chromosomal
changes in gastric adenoma, as analyzed by comparative genomic
hybridization, were gains on 9q (25).
Thus, amplification of NOTCH1 signaling via chromosomal alterations
may be involved in gastric cancer carcinogenesis.
In gastric cancer patients, prognostic markers,
including erb-b2 receptor tyrosine kinase 2 (ERBB2)
(26,27), CD44 (28,29) and
matrix metallopeptidase 12 (30) have
been reported. Additionally, a previous study by the present team
demonstrated that the expression of the microRNAs mir-20b
and mir-150 may be a prognostic marker for undifferentiated
gastric cancer (1). Thus, NOTCH1 may
be added to this list of prognostic markers. However, further
investigations into the role of NOTCH1 in gastric cancer
progression are required. Although the precise molecular mechanisms
involved in the activation of the NOTCH1 signaling pathway remain
to be clarified, the present data suggest that NOTCH1 may be a
molecular target for the development of an effective therapeutic
intervention for patients with gastric cancer.
Acknowledgements
The authors would like to thank Ms. Haruko Izuchi
for her excellent technical assistance.
References
|
1
|
Katada T, Ishiguro H, Kuwabara Y, Kimura
M, Mitui A, Mori Y, Ogawa R, Harata K and Fujii Y: MicroRNA
expression profile in undifferentiated gastric cancer. Int J Oncol.
34:537–542. 2009.PubMed/NCBI
|
|
2
|
Katoh M and Katoh M: Notch signaling in
gastrointestinal tract (Review). Int J Oncol. 30:247–251.
2007.PubMed/NCBI
|
|
3
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: Cell fate control and signal integration in
development. Science. 284:770–776. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lai EC: Notch signaling: control of cell
communication and cell fate. Development. 131:965–973. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Le Borgne R, Bardin A and Schweisguth F:
The roles of receptor and ligand endocytosis in regulating Notch
signaling. Development. 132:1751–1762. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bray SJ: Notch signalling: A simple
pathway becomes complex. Nat Rev Mol Cell Biol. 7:678–689. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Baron M: An overview of the Notch
signalling pathway. Semin Cell Dev Biol. 14:113–119. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang W, Sun XW, Li CF, Lv L, Li YF, Chen
YB, Xu DZ, Kesari R, Huang CY, Li W, et al: Comparison of the 6th
and 7th editions of the UICC TNM staging system for gastric cancer:
results of a Chinese single-institution study of 1,503 patients.
Ann Surg Oncol. 18:1060–1067. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Struhl G and Adachi A: Requirements for
presenilin-dependent cleavage of notch and other transmembrane
proteins. Mol Cell. 6:625–636. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Sulis ML, Williams O, Palomero T, Tosello
V, Pallikuppam S, Real PJ, Barnes K, Zuurbier L, Meijerink JP and
Ferrando AA: NOTCH1 extracellular juxtamembrane expansion mutations
in T-ALL. Blood. 112:733–740. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kopan R and Ilagan MX: The canonical Notch
signaling pathway: unfolding the activation mechanism. Cell.
137:216–233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brzozowa M, Mielańczyk L, Michalski M,
Malinowski L, Kowalczyk-Ziomek G, Helewski K, Harabin-Słowińska M
and Wojnicz R: Role of Notch signaling pathway in gastric cancer
pathogenesis. Contemp Oncol (Pozn). 17:1–5. 2013.PubMed/NCBI
|
|
13
|
Leong KG and Karsan A: Recent insights
into the role of Notch signaling in tumorigenesis. Blood.
107:2223–2233. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Koch U and Radtke F: Notch and cancer: a
double-edged sword. Cell Mol Life Sci. 64:2746–2762. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Miele L: Notch signaling. Clin Cancer Res.
12:1074–1079. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miele L, Miao H and Nickoloff BJ: NOTCH
signaling as a novel cancer therapeutic target. Curr Cancer Drug
Targets. 6:313–323. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Z, Banerjee S, Li Y, Rahman KM, Zhang
Y and Sarkar FH: Down-regulation of notch-1 inhibits invasion by
inactivation of nuclear factor-kappaB, vascular endothelial growth
factor, and matrix metalloproteinase-9 in pancreatic cancer cells.
Cancer Res. 66:2778–2784. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu H, Zhou X, Redfield S, Lewin J and
Miele L: Elevated Jagged-1 and Notch-1 expression in high grade and
metastatic prostate cancers. Am J Transl Res. 5:368–378.
2013.PubMed/NCBI
|
|
19
|
Yang Y, Yan X, Duan W, Yan J, Yi W, Liang
Z, Wang N, Li Y, Chen W, Yu S, et al: Pterostilbene exerts
antitumor activity via the Notch1 signaling pathway in human lung
adenocarcinoma cells. PLoS One. 8:e626522013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee SH, Jeong EG, Yoo NJ and Lee SH:
Mutational analysis of NOTCH1, 2, 3 and 4 genes in common solid
cancers and acute leukemias. APMIS. 115:1357–1363. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Radtke F and Raj K: The role of Notch in
tumorigenesis: oncogene or tumour suppressor? Nat Rev Cancer.
3:756–767. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Thélu J, Rossio P and Favier B: Notch
signalling is linked to epidermal cell differentiation level in
basal cell carcinoma, psoriasis and wound healing. BMC Dermatol.
2:72002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Proweller A, Tu L, Lepore JJ, Cheng L, Lu
MM, Seykora J, Millar SE, Pear WS and Parmacek MS: Impaired notch
signaling promotes de novo squamous cell carcinoma formation.
Cancer Res. 66:7438–7444. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yeh TS, Wu CW, Hsu KW, Liao WJ, Yang MC,
Li AF, Wang AM, Kuo ML and Chi CW: The activated Notch1 signal
pathway is associated with gastric cancer progression through
cyclooxygenase-2. Cancer Res. 69:5039–5048. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Buffart TE, Carvalho B, Mons T, Reis RM,
Moutinho C, Silva P, van Grieken NC, Vieth M, Stolte M, van de
Velde CJ, et al: DNA copy number profiles of gastric cancer
precursor lesions. BMC Genomics. 8:3452007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jørgensen JT and Hersom M: HER2 as a
prognostic marker in gastric cancer - a systematic analysis of data
from the literature. J Cancer. 3:137–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gravalos C and Jimeno A: HER2 in gastric
cancer: A new prognostic factor and a novel therapeutic target. Ann
Oncol. 19:1523–1529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Doventas A, Bilici A, Demirell F, Ersoy G,
Turna H and Doventas Y: Prognostic significance of CD44 and
c-erb-B2 protein overexpression in patients with gastric cancer.
Hepatogastroenterology. 59:2196–2201. 2012.PubMed/NCBI
|
|
29
|
Yamamichi K, Uehara Y, Kitamura N, Nakane
Y and Hioki K: Increased expression of CD44v6 mRNA significantly
correlates with distant metastasis and poor prognosis in gastric
cancer. Int J Cancer. 79:256–262. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng J, Chu D, Wang D, Zhu Y, Zhang X, Ji
G, Zhao H, Wu G, Du J and Zhao Q: Matrix metalloproteinase-12 is
associated with overall survival in Chinese patients with gastric
cancer. J Surg Oncol. 107:746–751. 2013. View Article : Google Scholar : PubMed/NCBI
|