Introduction
It is widely known that improving the embryo
implantation rate is one of the main objectives of assisted
reproductive technologies (ARTs). The most common method to achieve
better results is to obtain and transfer multiple embryos. The
surplus embryos produced using ARTs may be cryopreserved for
subsequent use (1). The success of
cryopreservation may undoubtedly increase the cumulative pregnancy
rates of ARTs. However, these results appear to indicate a
reduction in the clinical pregnancy and implantation rates compared
with fresh embryos (2).
A glycoprotein layer, known as the zona pellucida
(ZP), surrounds human embryos and permits only acrosome-intact
sperm to fertilize the oocyte by blocking the entry of multiple
sperm. After fertilization, the ZP compresses and shapes the
embryo, protecting it from microorganisms and immune cells. At the
blastocyst stage, the embryo breaks out of the ZP to begin the
developmental process; failure at this stage can prevent
implantation. Hatching of the embryo is a critical step in the
sequence of physiological events that culminate in implantation.
Failure to hatch, due to intrinsic abnormalities in the blastocyst
or ZP, may be the main factors limiting human-assisted reproductive
efficiency, and the effects of zona hardening on embryo hatching
are probably one of the consequences of the process of freezing and
thawing embryos (3). Therefore,
artificial thinning of the zona or drilling of the zona may improve
the embryo's potential and thus improve the clinical outcome of the
thawing cycle. Since the 1980s, assisted hatching (AH) has been
used to improve the chances of implantation during ARTs (1).
AH using a 1.48-µm diode laser yields a better
outcome than AH using mechanical or chemical methods (4,5). However, to
the best of our knowledge, no single study has been able to
demonstrate sufficient evidence favourable to AH, and the current
research conclusions are not unanimous (6–13). In a
large meta-analysis, Martins et al showed that laser-AH
(LAH) is currently one of the best, safest and most effective AH
methods (14). LAH can improve the
clinical pregnancy rate of the thawing cycle. Since LAH can be
divided into artificial thinning of the zona and drilling of the
zona, previous findings have shown that the former is better than
that the latter in vitro (15,16). Hiraoka
et al have shown that vitrification can increase the
hardness of zona, and the embryo implantation and clinical
pregnancy rates in the half ZP thinning FET cycle was superior to
that of a quarter ZP, although these studies lacked a control group
(1). However, to the best of our
knowledge, no study has included a sufficient sample to properly
evaluate the effect of LAH on assisted reproduction outcomes
(1,17).
In the present study, we used vitrified frozen-thawed sister
embryos and performed laser-assisted ZP thinning to reduce a
quarter, a half and two-thirds area of the zona in the experimental
groups.
The aim of the current study was to observe the rate
of blastocyst formation and the complete hatching rate to determine
which size of ZP thinning by LAH is optimal for embryonic
development and to determine the best LAH method.
Materials and methods
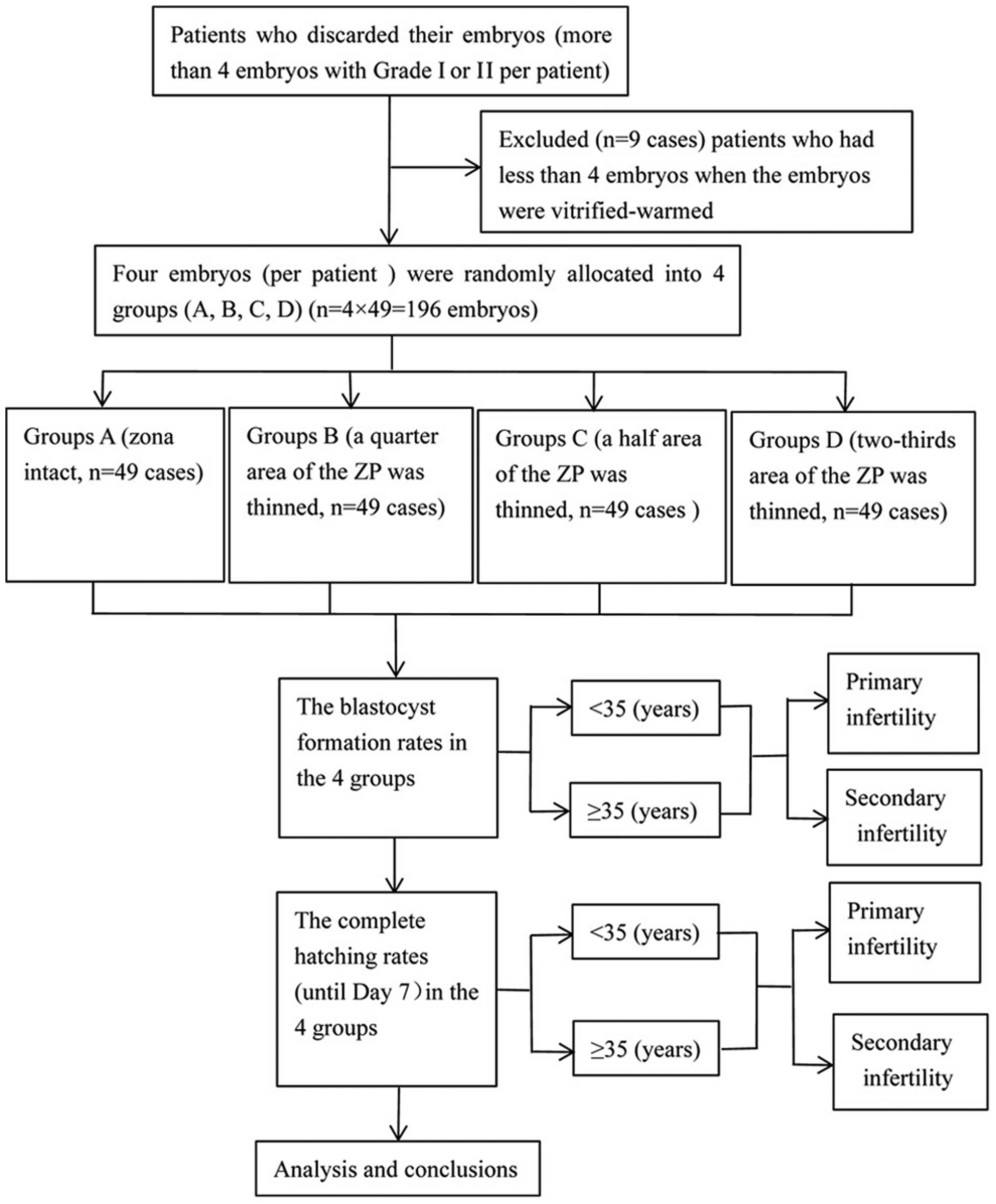
Patients and embryos
The present study originally included 58 infertility
patients who were admitted to the IVF unit of Linyi People's
Hospital (Linyi, China) from May 1, 2011 to November 31, 2011,
while 9 patients with <4 frozen-thawed embryos (grade І or II)
were excluded. Among them, there were 26 women aged <35 years
(13 in primarily infertility and 13 in secondary infertility) and
23 women aged ≥35 years (11 in primarily infertility and 12 in
secondary infertility). All of the patients with frozen-thawed
embryos had >4 high quality sister embryos surviving, and each
couple had succeeded in delivering at least one child. Any unused
embryos were discarded. The embryonic blastomeres selected
dissolved ≤2 (from 49 IVF patients) frozen-thawed blastomeres on
day 3, and grade I or II embryos at the 7–10 cell stage were
selected. The embryos were scored as indicated in a previous study
(18): grade I, uniform blastomere
size; regular morphology; intact zona; homogeneous cytoplasm,
clear, with no particle phenomenon; and embryo fragmentation of
<5%. Grade II, slightly uneven blastomere size; slightly
irregular morphology; cytoplasmic visible particle phenomenon; and
debris of 6–20%. The sister embryos from the same patient were
randomly divided into four groups: a control group of embryos that
were not zona-manipulated (zona intact, group A); one experimental
group of embryos in which a quarter area (1/4) of the ZP was
thinned using laser-assisted ZP thinning (group B); a second
experimental group of embryos in which a half area (1/2) of the ZP
was thinned (group C); and a third group in which a two-thirds area
(2/3) of the ZP was thinned (group D). Subsequent blastocyst
development was assessed (Fig. 1). The
[patients were included in the study if they met the following
requirements: 24–39 years of age and a body mass index of 18–28
kg/m2. The fecundities of the male partners of the
patients were normal according to the World Health Organization
criteria (19).
The present study was conducted at the Linyi
People's Hospital (Shandong, China) and was approved by the Ethics
Committee of Linyi People's Hospital. A written informed consent
form was obtained from all patients.
The embryos, which were all at the 7–10 cell stage,
were pooled and divided into four groups by defocusing the
microscope to prevent any bias in the selection.
Warming of embryos and AH
Vitrification was performed on embryos derived from
IVF or ICSI cycles. It has been shown that these two sources of
embryos have no effect on their cultivation (20–22).
Over 50% of the blastomeres in one embryo survived
and were continuously cultured. Otherwise, the embryos were
discarded. Vitrified embryos considered grade I or II embryos were
warmed in the following manner: the protective cover was warmed in
liquid nitrogen, and the end of the polypropylene strip was
immersed directly into 1 ml of 37°C 1.0 mol/l sucrose solution for
1 min. The embryos were then transferred into 1 ml of 37°C 0.5
mol/l sucrose solution for 3 min and washed twice in the base
medium for 5 min. AH was performed using a previously described
method (23). The average thickness of
the zona pellucida was calculated by the numerical values measured
at the 12, 3, 6, and 8 o'clock positions from the inside to the
outside.
The laser system consisted of a Fertilase 1.48-µm
laser, 100 mW, operated by an Octax Eyeware digital interface (MTG,
Bruckberg, Germany) and positioned on a Nikon Eclipse TE2000-U
inverted microscope (Nikon Instruments Europe B.V., Badhoevedorp,
The Netherlands).
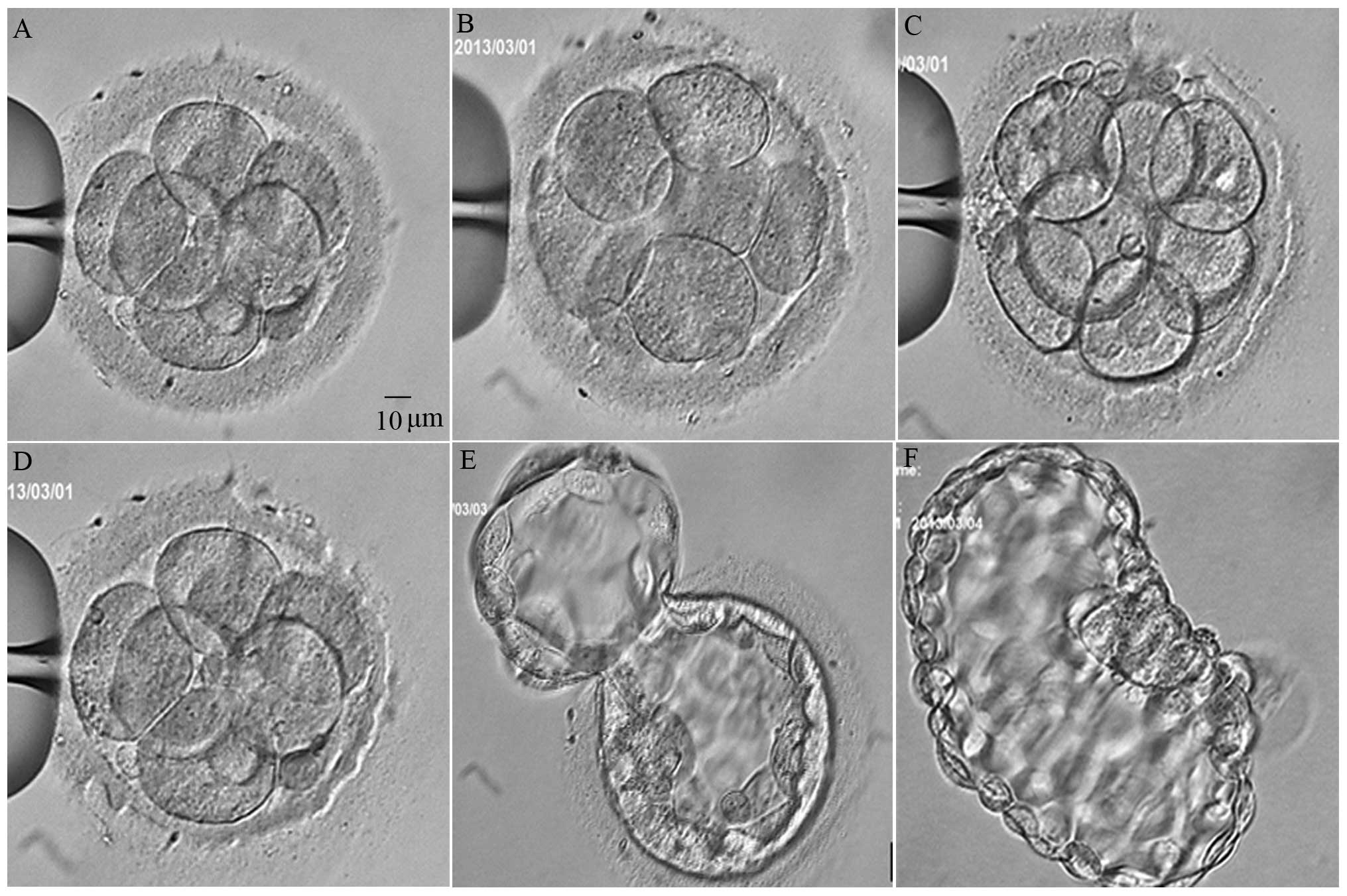
Embryos were randomly divided into four groups, and
the embryos were thawed immediately after LAH. The frozen-thawed
embryos were placed in the inverted microscope at 37°C, and the
embryos were fixed with a holding pipette at the 9 o'clock
position. The thinning procedure was standardized during pilot
experiments to establish a smooth lasered area. A 5-µm hole was
formed in the zona pellucida using one laser shot. The laser
thinning was initiated at the 12 o'clock position, and consecutive
irradiations were performed untill the 3 o'clock position (quarter
thinning, group B), the 6 o'clock position (half thinning, group C)
or the 8 o'clock position (two-thirds thinning, group D) at a depth
of 60–80% of the zona pellucida thickness (Fig. 2).
After LAH, the embryos were washed several times and
transferred to G2 medium. The embryos were then cultured in an
atmosphere of 6% CO2, 5% O2 and balance
N2, pH 7.32 at 37°C using a Labotect C200 incubator
(Labotect Labor-Technik-Göttingen GmbH, Göttingen, Germany). In the
control group, the same conditions were performed directly after
the embryos were thawed. Any manipulations were performed at room
temperature (22–23°C). In the present study, observations were
performed on day 5, at 6:00 a.m. and in the afternoon. Complete
blastocysts spreading (beyond a score of 4BB) was recorded four
times. Observation was continually performed on day 6, at 7:00 a.m.
and in the afternoon in order to record whether the blastocyst had
completely hatched.
Statistical analysis
SPSS 23.0 software for Windows was used for
statistical analysis (SPSS, Inc., Chicago, IL, USA). The data were
reported as the mean ± standard deviation (SD). The differences in
the variables between the groups were statistically analysed using
the Student's t-test or one-way ANOVA. For the purpose of analysis
of the categorical data (e.g., blastocyst formation rate, and
hatching rate), significant differences were evaluated using the
Chi-square test when appropriate. P<0.05 was considered
statistically significant.
Results
Patient information
Fifty-eight patients were initially included in the
study, and 9 patients with <4 frozen-thawed embryos (grade І or
II) were excluded. A total of 196 embryos from 49 patients were
eventually enrolled in the study.
The mean age for the <35-year women was
27.23±3.02 (mean ± SD) (95% CI, 26.00–28.45) and the mean age of
the ≥35-year women was 36±1.17 (95% CI, 35.50–36.50) for the
groups. The mean zona were 17.46±0.58, 17.35±0.51, 17.36±0.54 and
17.58±0.42 µm for groups A-D, respectively. There was no
significant difference in the thickness of the four groups
(P=0.088).
Rate of blastocyst formation and
complete hatching
The blastocyst formation rates were 71.43% (35/49)
in group A, 67.35% (33/49) in group B, 65.31% (32/49) in group C,
and 51.02% (25/49) in group D [overall group (groups B-D)
comparison with group A, P=0.661, P=0.515, P=0.038, respectively]
(Table I). The rates of complete
hatching were 30.61% (15/49) in group A, 38.78% (19/49) in group B,
61.22% (30/49) in group C, and 48.98% (24/49) in group D
[comparison with group A, P=0.396 (group B vs. group A), P=0.002
(group C vs. group A), P=0.063 (group D vs. group A)] (Table II).
 | Table I.Rate of blastocyst formations in the
different groups. |
Table I.
Rate of blastocyst formations in the
different groups.
| Groups | No. of blastocyst
formation | No. of blastocyst
non-formation | Rate of blastocyst
formation | P-value |
|---|
| Group A | 35 | 14 | 71.43% | – |
| Group B | 33 | 16 | 67.35% | 0.661a |
| Group C | 32 | 17 | 65.31% | 0.515b |
| Group D | 25 | 24 | 51.02% | 0.038c |
 | Table II.Rate of complete hatching (until day
7) in the different groups. |
Table II.
Rate of complete hatching (until day
7) in the different groups.
| Groups | No. of complete
hatching | No. of no or partial
hatching | Rate of complete
hatching | P-value |
|---|
| Group A | 15 | 34 | 30.61% | – |
| Group B | 19 | 30 | 38.78% | 0.396a |
| Group C | 30 | 19 | 61.22% | 0.002b |
| Group D | 24 | 25 | 48.98% | 0.063c |
Blastocyst formation and complete
hatching in the subgroups
The blastocyst formation rates for the subgroup of
women aged <35 years were 73.08% (19/26) in group A, 69.23%
(18/26) in group B, 80.77% (21/26) in group C, and 53.85% (14/26)
in group D. There was no significant difference in the blastocyst
formation rates in the different groups among women aged <35
years (χ2=4.694, P=0.196). The blastocyst formation
rates for the subgroup of patients aged ≥35 years were 69.57%
(16/23) in group A, 65.22% (15/26) in group B, 47.83% (11/23) in
group C, and 47.83% (11/23) in group D. There was no significant
difference in the blastocyst formation rates in the different
groups in women aged ≥35 years (χ2=3.357, P=0.340)
(Table III).
 | Table III.Blastocyst formation in the different
subgroups. |
Table III.
Blastocyst formation in the different
subgroups.
|
|
| No. of blastocyst
formation | Failed to
blastocyst formation |
|
|---|
|
|
|
|
|
|
|---|
| Groups | Age (years) | Primary
infertility | Secondary
infertility | Primary
infertility | Secondary
infertility | Total |
|---|
| A | <35 | 8 | 11 | 5 | 2 | 26 |
|
| ≥35 | 6 | 10 | 5 | 2 | 23 |
| B | <35 | 8 | 10 | 5 | 3 | 26 |
|
| ≥35 | 5 | 10 | 6 | 2 | 23 |
| C | <35 | 9 | 12 | 4 | 1 | 26 |
|
| ≥35 | 4 | 7 | 7 | 5 | 23 |
| D | <35 | 5 | 9 | 8 | 4 | 26 |
|
| ≥35 | 3 | 8 | 8 | 4 | 23 |
In addition, the rates of complete hatching (women
aged <35 years) were 30.77% (8/26) in group A, 50% (13/26) in
group B, 76.92% (20/26) in group C, and 53.85% (14/26) in group D.
There was a significant difference in the complete hatching in the
different groups among women aged <35 years
(χ2=11.230, P=0.011). The rates of complete hatching
(women aged ≥35 years) were 30.43% (7/23) in group A, 26.09% (6/23)
in group B, 43.48% (10/23) in group C, and 43.48% (10/23) in group
D. There was no significant difference in the complete hatching in
the different groups among women aged ≥35 years
(χ2=2.410, P=0.492) (Table
IV).
 | Table IV.Complete hatching (until day 7) in
the different subgroups. |
Table IV.
Complete hatching (until day 7) in
the different subgroups.
|
|
| No. of complete
hatching | No. of no or
partial hatching |
|
|---|
|
|
|
|
|
|
|---|
| Groups | Age (years) | Primary
infertility | Secondary
infertility | Primary
infertility | Secondary
infertility | Total |
|---|
| A | <35 | 3 | 5 | 10 | 8 | 26 |
|
| ≥35 | 2 | 5 | 9 | 7 | 23 |
| B | <35 | 6 | 7 | 7 | 6 | 26 |
|
| ≥35 | 2 | 4 | 9 | 8 | 23 |
| C | <35 | 8 | 12 | 5 | 1 | 26 |
|
| ≥35 | 3 | 7 | 8 | 5 | 23 |
| D | <35 | 5 | 9 | 8 | 4 | 26 |
|
| ≥35 | 2 | 8 | 9 | 4 | 23 |
Blastocyst formation and complete
hatching in the subgroups of patients with primary and secondary
infertility
The blastocyst formation rates for the subgroup of
patients with primary infertility were 58.33% (14/24) in group A,
54.17% (13/24) in group B, 54.17% (13/24) in group C, and 33.33%
(8/24) in group D. There was no significant difference in the
blastocyst formation rates in the different groups with primary
infertility (χ2=3.667, P=0.300). The blastocyst
formation rates for the subgroup of patients with secondary
infertility was 84% (21/25) in group A, 80% (20/25) in group B, 76%
(19/25) in group C, and 68% (17/25) in group D. There was no
significant difference in the blastocyst formation rates in the
different groups with secondary infertility (χ2=1.976,
P=0.577) (Table III).
In addition, the rates of complete hatching (primary
infertility) were 20.83% (5/24) in group A, 33.33% (8/24) in group
B, 45.83% (11/24) in group C, and 29.17% (7/24) in group D. There
was no significant difference in the complete hatching in the
different groups with primary infertility women
(χ2=3.573, P=0.311). The rates of complete hatching
(secondary infertility) were 40% (10/25) in group A, 44% (11/25) in
group B, 76% (19/25) in group C, and 68% (17/25) in group D. There
was a significant difference in complete hatching in the different
groups among women with secondary infertility women
(χ2=9.588, P=0.022) (Table
IV).
Discussion
Despite the rapid development of IVF and ICSI, the
implantation rate of embryos remains relatively low. It has been
indicated that only 15% of embryos were successfully transplanted
into the uterine cavity in the 1990s (24). Even if a normal chromosome embryo has
good developmental potential, it may be unable to grow successfully
because of failure to hatch (25).
Embryo implantation is affected by numerous factors, including
uterine endometrial receptivity, operation of transplantation
technology, and embryo hatching ability (26). Selective application of LAH in ART may
enhance hatching ability. Since hatching ability plays an important
role in the process of embryonic development, the basic conditions
that ensure the success of a hatched embryo include that the ZP
exhibits good elasticity to become thin with the expansion of the
blastocyst (27). Thus, a potential
mechanism to improve embryo implantation ability may be
technological assurance of embryos at an earlier stage of hatching
and early contact with the endometriium. ZP thinning may accelerate
nutrient exchange between the liquid culture and embryo, and may
promote embryonic development and blastocyst formation (28,29).
Although vitrification technology has been
previously developed, it has not been promoted. Studies on the
effect of LAH on the cycle of FET have focused more on programme
freezing (9). The vitrification
process can increase embryo zona pellucida hardness and affect
hatching compared with programmed freezing. Thus, patients who use
frozen-thawed embryos benefit from LAH. Most of the current
literature regarding LAH measures compares the embryo implantation
and clinical pregnancy rates, and when the patients were grouped,
the results were inevitably affected by endometrial receptivity and
many other factors (30). Although
most of the potential factors that interfere with the implantation
of the embryo have been eliminated, the embryo implantation
mechanism is extremely complex. In the present study, we
established a control group and observed the effect of different ZP
circumferences of LAH on the potential of embryonic development in
order to select the optimal LAH method. The results showed that the
two-third zona pellucida thinned group (group D) demonstrated a
significantly decreased blastocyst formation rate compared with the
control group (group A). In addition, the fully hatched rates of
the blastocysts in group C (one-half zona thinning) were
significantly higher than that of group A, while the remaining
experimental groups showed no significant difference compared with
the control group. These results are consistent with those
demonstrated in previous studies (1).
In the subgroup of patients, there was a significant difference in
the complete hatching in the different groups for women aged <35
years (P=0.011), and there was a significant difference in the
complete hatching in the different groups of secondary infertility
women (P=0.022).
The LAH drilling method is less time-consuming than
the ZP thinning method. However, previous findings have shown that
if the drilling is extremely small, it causes blastocyst hatching
to be incarcerated. By contrast, when the drilling is extremely
large, some blastomeres in the embryos may be lost before they are
closely connected (31). Therefore,
many centres favour zona pellucida thinning with AH. However,
findings regarding the size of the thinned area of the zona
pellucida are inconclusive. The reasons for this include, the
difference in AH method and technology, the difference in
experimental design, the difference in freezing method (programmed
freezing or vitrification), and patient characteristics.
In the past, limited data were reported on the final
results of the blastocysts in vitro after AH (27). In the present study, we employed
vitrified-warmed sister embryos of grade I and II and performed
zona thinning by ablating one-quarter, one-half or two-thirds of
the ZP circumference. The embryos were cultured using an in
vitro method to observe the embryonic development potential.
The results of the present study showed that the blastocyst
formation rates of the four groups were 71.43% (control group),
67.35% (quarter area zona thinning), 65.31% (half area zona
thinning), and 51.02% (two-thirds area zona thinning). These
results showed that the blastocyst formation rate in two-thirds
area zona thinning group was lower than that in control group
(P=0.038). The random grouping of sister embryos in the same
patient was performed to eliminate the difference between
individuals and may reflect the effect of the laser-assisted ZP
thinning in the developmental potential of embryos. However,
whether the different thinning areas in the sister embryos of the
same patient influence the hatching rate remains to be determined.
The current study elaborated that the one-half zona pellucida
thinning method significantly improved the blastocyst completely
hatched rate compared to the control group, particularly with women
aged <35 years or in women with secondary infertility. This
result may have a high value in clinical application. The data of
the present study are small scale, and therefore more studies are
required to confirm the results.
Acknowledgements
The present study was supported by the Shandong
Provincial Medical and Health Science and Technology Development
Project, China (grant no. 2015WS0377) and the Shandong Provincial
Natural Science Foundation, China (grant no. ZR2014HP026).
References
|
1
|
Hiraoka K, Hiraoka K, Horiuchi T, Kusuda
T, Okano S, Kinutani M and Kinutani K: Impact of the size of zona
pellucida thinning area on vitrified-warmed cleavage-stage embryo
transfers: a prospective, randomized study. J Assist Reprod Genet.
26:515–521. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andersen AN, Goossens V, Ferraretti AP,
Bhattacharya S, Felberbaum R, de Mouzon J and Nygren KG: European
IVF-monitoring (EIM) Consortium; European Society of Human
Reproduction and Embryology (ESHRE): Assisted reproductive
technology in Europe, 2004: results generated from European
registers by ESHRE. Hum Reprod. 23:756–771. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carroll J, Depypere H and Matthews CD:
Freeze-thaw-induced changes of the zona pellucida explains
decreased rates of fertilization in frozen-thawed mouse oocytes. J
Reprod Fertil. 90:547–553. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hsieh YY, Huang CC, Cheng TC, Chang CC,
Tsai HD and Lee MS: Laser-assisted hatching of embryos is better
than the chemical method for enhancing the pregnancy rate in women
with advanced age. Fertil Steril. 78:179–182. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Makrakis E, Angeli I, Agapitou K, Pappas
K, Dafereras A and Pantos K: Laser versus mechanical assisted
hatching: a prospective study of clinical outcomes. Fertil Steril.
86:1596–1600. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Primi MP, Senn A, Montag M, Van der Ven H,
Mandelbaum J, Veiga A, Barri P and Germond M: A European
multicentre prospective randomized study to assess the use of
assisted hatching with a diode laser and the benefit of an
immunosuppressive/antibiotic treatment in different patient
populations. Hum Reprod. 19:2325–2333. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng EH, Naveed F, Lau EY, Yeung WS, Chan
CC, Tang OS and Ho PC: A randomized double-blind controlled study
of the efficacy of laser-assisted hatching on implantation and
pregnancy rates of frozen-thawed embryo transfer at the cleavage
stage. Hum Reprod. 20:979–985. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sifer C, Sellami A, Poncelet C, Kulski P,
Martin-Pont B, Bottero J, Porcher R, Cedrin-Durnerin I, Hugues JN
and Wolf JP: A prospective randomized study to assess the benefit
of partial zona pellucida digestion before frozen-thawed embryo
transfers. Hum Reprod. 21:2384–2389. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Petersen CG, Mauri AL, Baruffi RL,
Oliveira JB, Felipe V, Massaro FC and Franco JG Jr: Laser-assisted
hatching of cryopreserved-thawed embryos by thinning one quarter of
the zona. Reprod Biomed Online. 13:668–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gabrielsen A, Agerholm I, Toft B, Hald F,
Petersen K, Aagaard J, Feldinger B, Lindenberg S and Fedder J:
Assisted hatching improves implantation rates on
cryopreserved-thawed embryos. A randomized prospective study. Hum
Reprod. 19:2258–2262. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Balaban B, Urman B, Yakin K and Isiklar A:
Laser-assisted hatching increases pregnancy and implantation rates
in cryopreserved embryos that were allowed to cleave in vitro after
thawing: a prospective randomized study. Hum Reprod. 21:2136–2140.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ge HS, Zhou W, Zhang W and Lin JJ: Impact
of assisted hatching on fresh and frozen-thawed embryo transfer
cycles: a prospective, randomized study. Reprod Biomed Online.
16:589–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Valojerdi MR, Eftekhari-Yazdi P, Karimian
L and Ashtiani SK: Effect of laser zona pellucida opening on
clinical outcome of assisted reproduction technology in patients
with advanced female age, recurrent implantation failure, or
frozen-thawed embryos. Fertil Steril. 90:84–91. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Martins WP, Rocha IA, Ferriani RA and
Nastri CO: Assisted hatching of human embryos: a systematic review
and meta-analysis of randomized controlled trials. Hum Reprod
Update. 17:438–453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Blake DA, Forsberg AS, Johansson BR and
Wikland M: Laser zona pellucida thinning - an alternative approach
to assisted hatching. Hum Reprod. 16:1959–1964. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mantoudis E, Podsiadly BT, Gorgy A, Venkat
G and Craft IL: A comparison between quarter, partial and total
laser assisted hatching in selected infertility patients. Hum
Reprod. 16:2182–2186. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Debrock S, Peeraer K, Spiessens C,
Willemen D, De Loecker P and D'Hooghe TM: The effect of modified
quarter laser-assisted zona thinning on the implantation rate per
embryo in frozen/vitrified-thawed/warmed embryo transfer cycles: a
prospective randomized controlled trial. Hum Reprod. 26:1997–2007.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Puissant F, Van Rysselberge M, Barlow P,
Deweze J and Leroy F: Embryo scoring as a prognostic tool in IVF
treatment. Hum Reprod. 2:705–708. 1987.PubMed/NCBI
|
|
19
|
Cooper TG, Noonan E, von Eckardstein S,
Auger J, Baker HW, Behre HM, Haugen TB, Kruger T, Wang C, Mbizvo
MT, et al: World Health Organization reference values for human
semen characteristics. Hum Reprod Update. 16:231–245. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kowalik A, Palermo GD, Barmat L, Veeck L,
Rimarachin J and Rosenwaks Z: Comparison of clinical outcome after
cryopreservation of embryos obtained from intracytoplasmic sperm
injection and in-vitro fertilization. Hum Reprod. 13:2848–2851.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Aytoz A, Van den Abbeel E, Bonduelle M,
Camus M, Joris H, Van Steirteghem A and Devroey P: Obstetric
outcome of pregnancies after the transfer of cryopreserved and
fresh embryos obtained by conventional in-vitro fertilization and
intracytoplasmic sperm injection. Hum Reprod. 14:2619–2624. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu Y, Maxson WS, Hoffman DI, Ory SJ and
Eager S: A comparison of post-thaw results between cryopreserved
embryos derived from intracytoplasmic sperm injection and those
from conventional IVF. Fertil Steril. 72:1045–1048. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hiraoka K, Fuchiwaki M, Hiraoka K,
Horiuchi T, Murakami T, Kinutani M and Kinutani K: Zona pellucida
removal and vitrified blastocyst transfer outcome: a preliminary
study. Reprod Biomed Online. 15:68–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edwards RG: Clinical approaches to
increasing uterine receptivity during human implantation. Hum
Reprod. 10(Suppl 2): 60–66. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huisman GJ, Fauser BC, Eijkemans MJ and
Pieters MH: Implantation rates after in vitro fertilization and
transfer of a maximum of two embryos that have undergone three to
five days of culture. Fertil Steril. 73:117–122. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kutlu P, Atvar O and Vanlioglu OF: Laser
assisted zona thinning technique has no beneficial effect on the
ART outcomes of two different maternal age groups. J Assist Reprod
Genet. 27:457–461. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cohen J: Assisted hatching of human
embryos. J In Vitro Fert Embryo Transf. 8:179–190. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Malter HE and Cohen J: Blastocyst
formation and hatching in vitro following zona drilling of mouse
and human embryos. Gamete Res. 24:67–80. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hershlag A and Feng HL: Effect of
prefreeze assisted hatching on postthaw survival of mouse embryos.
Fertil Steril. 84:1752–1754. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Debrock S, Spiessens C, Peeraer K, De
Loecker P, Willemen D and D'Hooghe TM: Higher implantation rate
using modified quarter laser-assisted zona thinning in repeated
implantation failure. Gynecol Obstet Invest. 67:127–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Edi-Osagie EC, Hooper L, McGinlay P and
Seif MW: Effect(s) of assisted hatching on assisted conception (IVF
& ICSI). Cochrane Database Syst Rev (4): CD001894. 2003.
View Article : Google Scholar
|