Introduction
Transplantation and reconstruction of local skin
flaps is the common surgical therapeutic strategy for soft tissue
defects caused by trauma or tumor-resection. However, partial
necrosis of the skin flaps restricts the survival of local skin
flaps. These factors, including deficiency of blood perfusion,
ischemia-reperfusion injury and expression of inflammatory factors,
have been proven to contribute to partial skin necrosis (1). To enhance the survival of the local skin
flap, it is crucial to improve the tolerance of the tissue to
ischemia and inflammation, accelerate angiogenesis and alleviate
tissue edema (2–4).
Diammonium glycyrrhizinate (DG) is a substance
extracted from a traditional Chinese medical herb,
Glycyrrhiza. Currently, DG is widely administered to
patients with chronic hepatitis and human immunodeficiency virus
infection for its anti-inflammatory, anti-viral and
hepatoprotective effects (5,6). A previous study in a model of ulcerative
colitis indicated that DG was able to reduce inflammatory injury
via suppression of nuclear factor κ-light-chain-enhancer of
activated B cells (NF-κB), tumor necrosis factor-α and
intercellular adhesion molecule 1, which are thought to promote
inflammatory injury (7). Furthermore,
it was found that DG had neuroprotective potential against
ischemia-reperfusion injury in a model of focal cerebral
ischemic-reperfusion injury, and this effect was also likely
associated with the anti-inflammatory function of DG according to a
previous study (8). However, the
protective role of DG in random skin flap survival has not, to the
best of our knowledge, been clearly characterized. In the current
study, the effect of DG on random skin flap survival in rats was
investigated.
Materials and methods
Animal model and drug
administration
Ethics statement
The experiments of the current study were conducted
in strict accordance with the guidelines from the National
Institutes of Health and the Committee on Animal Research. Ketamine
hydrochloride and xylazine hydrochloride were used in all surgical
procedures. Animals were removed from the study and euthanized by
an overdose of ketamine, and all efforts were made to minimize
suffering.
Animals and materials
Sixty male Sprague-Dawley rats (weight, 250–300 g)
were obtained from Wenzhou Medical University, Wenzhou, China [SCXK
(Zhe) 2005–0019]. All rats were randomly divided into three groups,
including one control group (group I) and two experimental groups
(group II and group III). Each group contained 20 rats. A DG
injection (H10940190) was purchased from Zhengda Tianqing Pharmacy
Co., Ltd. (Lianyungang, China). Superoxide dismutase (SOD) and
malondialdehyde (MDA) testing kits were purchased from Nanjing
Jiancheng Biological Engineering Institute (Nanjing, China). Goat
serum (SL038) was purchased from Solarbio Life Sciences Company
(Beijing, China). Rat anti-vascular endothelial growth factor
(VEGF) antibody (cat no. sc-53462) was purchased from Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) and goat anti-rat IgG-R
(ZDR-5307) was purchased from Zhongshan Golden Bridge
Biotechnology, Co., Ltd. (Beijing, China).
Flap model and experimental design
The rats were anesthetized with an intraperitoneal
injection of 50 mg/kg ketamine hydrochloride (Ketalar, Eczacıbası,
Turkey) and 5 mg/kg xylazine (Rompun; Bayer AG, Berlin, Germany).
After anesthesia, rats were placed in the prone position and the
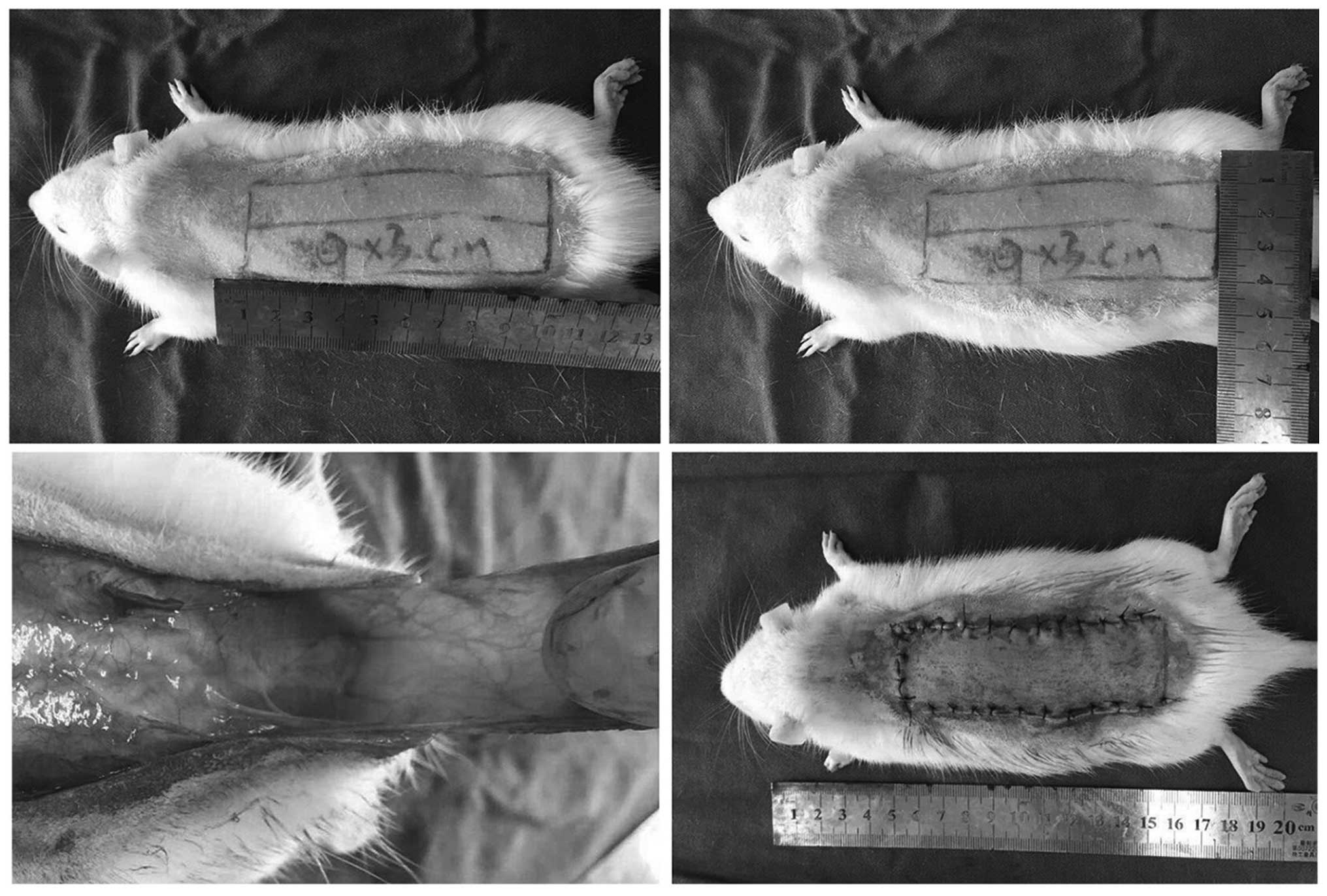
dorsal skin was shaved. A McFarlane flap (size, 9×3 cm) was created
at the dorsum of each rat (9). After
controlling any bleeding, the flap was immediately sutured to the
original position with 4-0 running nylon sutures and a wedged-on
cutting needle (Fig. 1) (10). The flap area was divided into three
distinct zones of equal size: The proximal area (area I); the
intermediate area (area II); and the distal area (area III). All
surgical procedures were performed by one researcher and no rats
died during surgery. Group II and group III received 10 ml/kg DG
via intraperitoneal injection once and twice per day, respectively,
for 7 days. The control group was injected with the same quantity
of saline solution in the same way once per day during the
experiment (11). All rats were housed
in a environmentally controlled room at a temperature of 20–22°C
under 12 h light/dark cycles. The rats were individually housed to
prevent cannibalism or injury caused by normal socialization
(12), and were fed standard rat chow
and water ad libitum. Seven days later, the rats were sacrificed
via ketamine overdose.
Macroscopic evaluation
On the seventh postoperative day, the survival area
of each flap was photographed and differences between the two
experimental groups and the control group in general appearance,
color, texture and hair condition, and any differences were
recorded. The images were analyzed using Image-Pro Plus v6.0
software (Media Cybernetics, Inc., Rockville, MD, USA).
Assessment of survival areas
To quantify the survival areas, the flaps were
measured by superimposition of photographs onto graph paper. All
the results were represented as a percentage of viable area
calculated using the following formula: Extent of viable area
(mm2) × 100/total area (viable and ischemic;
mm2).
Tissue edema measurements
The degree of tissue edema was evaluated by the
percentage of water content (13). On
the seventh postoperative day, the flap (taken immediately after
the rats had been sacrificed) was weighed and dehydrated in an
autoclave at 50°C. The samples were weighed daily until the weight
was constant for 2 days. The percentage water content of the tissue
was determined by the following equation: Tissue water content (%)
= [(wet weight - dry weight)/wet weight] × 100.
Histology
On day 7, subsequent to the rats being euthanized,
the flap tissues were harvested from each area and divided into
three parts of equal size (1×1 cm). All of the tissue specimens
were fixed in 10% paraformaldehyde for 24 h, embedded in paraffin
and sectioned into 4-µm slices. According to the standard protocol,
each section was stained with hematoxylin and eosin (H&E). The
microvessel number per unit area (/mm2) was then counted
under a light microscope at a magnification of ×200 to establish
the microvascular density (MVD). In addition, neutrophil
infiltration was counted under a light microscope at a
magnification of ×200.
VEGF expression
The VEGF expression level was evaluated
immunohistochemically by employing a streptavidin/peroxidase-based
protocol. Firstly, the slides were blocked with normal goat serum
at room temperature for 20 min, and immersed in 50 µl anti-VEGF
antibody solution (diluted 1:100) at 4°C overnight. All slides were
maintained at 37°C for 45 min and washed with phosphate-buffered
saline (PBS). Then 50 µl goat anti-rat antibody (diluted 1:50) was
added to the slides. All slides were incubated at 37°C for 1 h and
rinsed with PBS. The specimens were incubated in
3,3′-diaminobenzidine tetrahydrochloride solution for 5 min for
color development. Under low magnification, the positive expression
of VEGF-intensive regions was observed, and vessels in five fields
of view per slide were viewed at a higher magnification (×200). The
observation parameters (white balance, aperture, shutter speed and
time) were unchanged throughout. Image-Pro Plus software v6.0 was
used to save the images, and the integral absorbance (IA) value, as
an indicator of VEGF expression, was detected.
Analyses of SOD activity and MDA content
On day 2 postoperatively, 10 tissue specimens
(0.3×0.3 cm) were obtained from section II/III boundaries of each
group, weighed, homogenized using a Polytron homogenizer (Janke and
Kunkel; IKA, Staufen, Germany) followed by centrifugation at 845.2
× g for 15 min, and diluted with saline to 10% (vol/vol) in an ice
bath. SOD activity was determined using an oxidase enzymatic
method, and the MDA level was measured by a method based on the
reaction with thiobarbituric acid at 90–100°C, as previously
described (14).
Statistical analysis
The results are expressed as means ± standard
deviations. Statistical evaluation of the data was performed by
one-way analysis of variance followed by post hoc comparison test
using the least significant difference (equal variances assumed) or
Dunnett's T3 (equal variances not assumed) method. All data were
analyzed with SPSS software 20.0 (IBM SPSS, Armonk, NY, USA) and
graphs were constructed using GraphPad Prism v6.0 (GraphPad
Software, Inc., La Jolla, CA, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Macroscopic evaluation
On the first postoperative day, the flap pedicles of
the three groups were pale, and the distal area (III) was dull red
with some small spots exhibiting obvious necrosis. On the seventh
day, the necrotic region became darker and tended to fuse, scab and
shrink. The boundary between necrotic and surviving parts was
clearly visible on each flap. Surviving flap portions grew tiny
hairs. In addition, area I of all of the flaps survived, flaps in
area II survived partly, however, area III of all the rats became
necrotic. The range of necrosis in the two experimental groups was
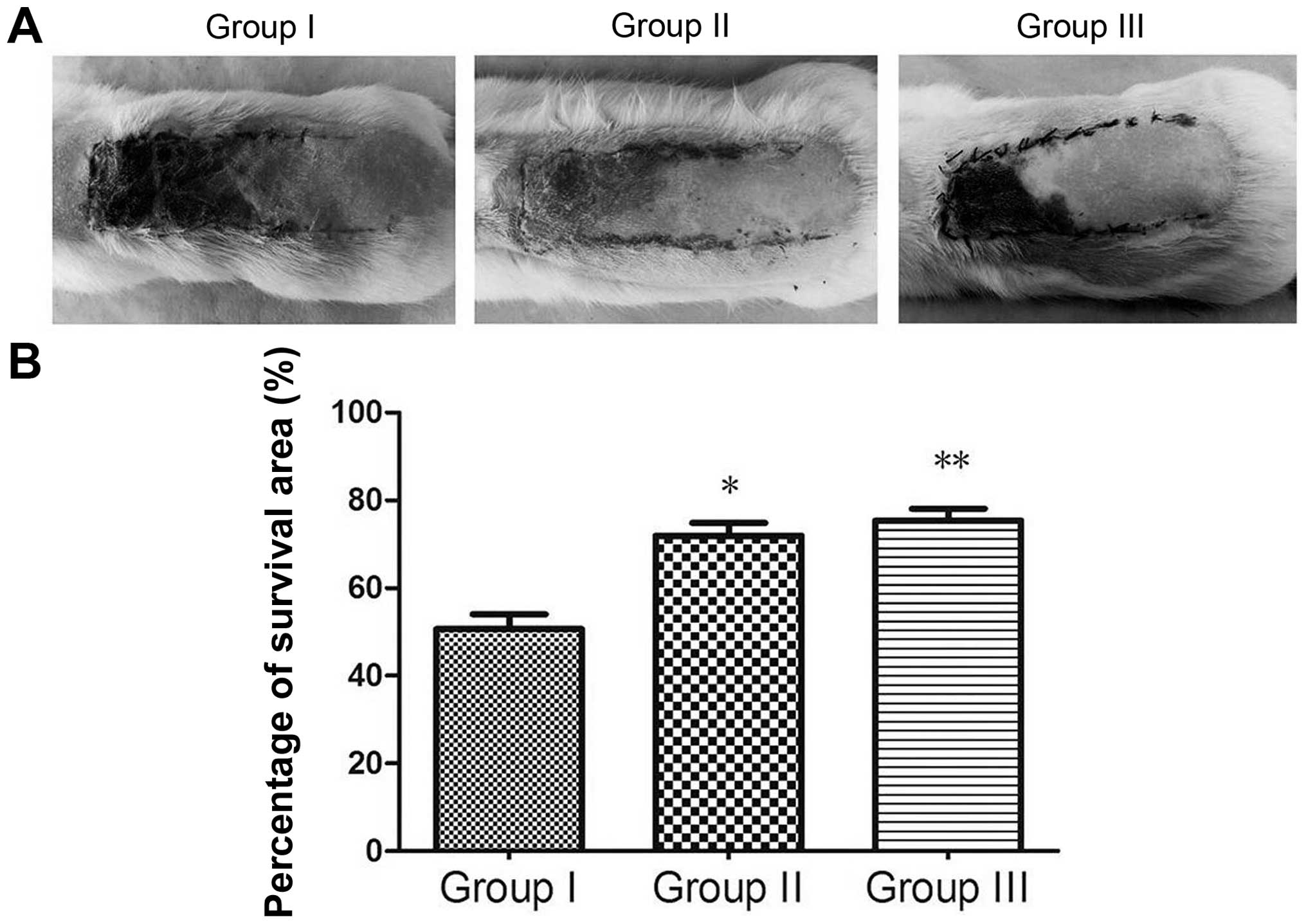
obviously smaller than that of the control group (Fig. 2A).
Percentage survival area
Seven days after surgery, the mean survival area
percentages were 71.983±7.084% in group II and 75.373±6.708% in
group III, which were significantly higher than those in the
control group (50.618±8.455%). There was a significant difference
in mean survival area between group I and II (P<0.05), and group
III exhibited a higher flap survival area than group II (P<0.05;
Fig. 2B).
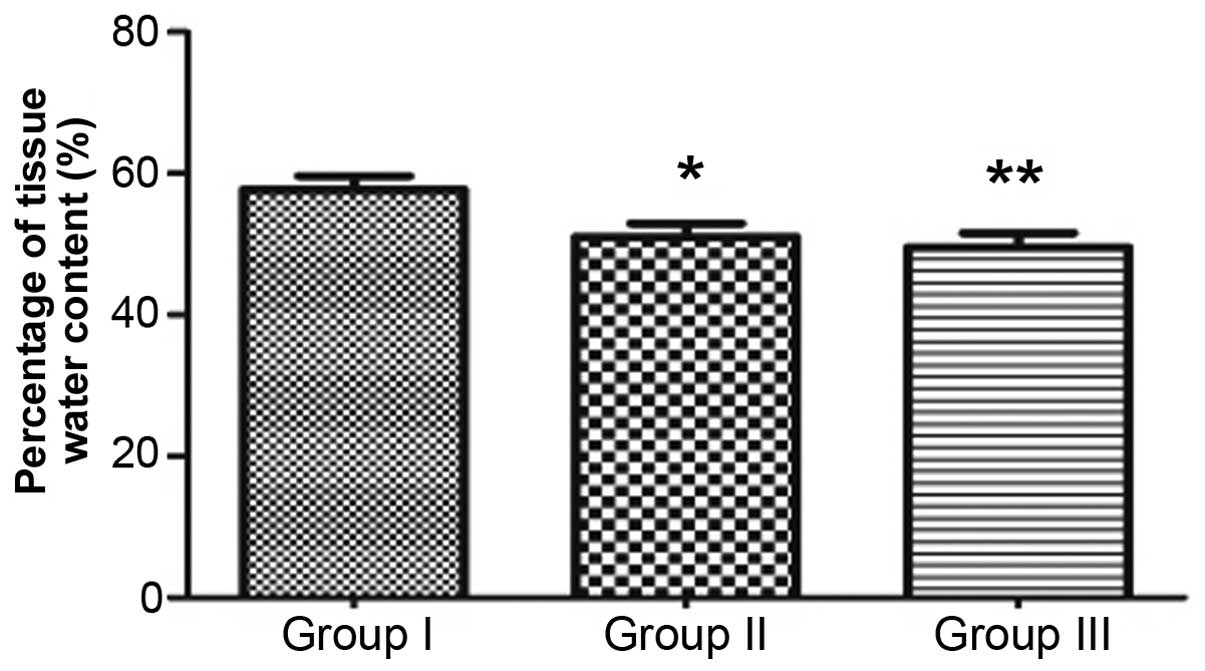
Tissue edema
Percentage tissue water contents in groups I, II and
III were 57.675±4.841, 51.036±4.519 and 49.554±4.994, respectively.
The percentage tissue water content in group II was significantly
lower than that in the control group (P<0.05). In addition, the
water content in group III was significantly lower than that in
group II (P<0.05), indicating that DG is able to reduce the
degree of tissue edema (Fig. 3).
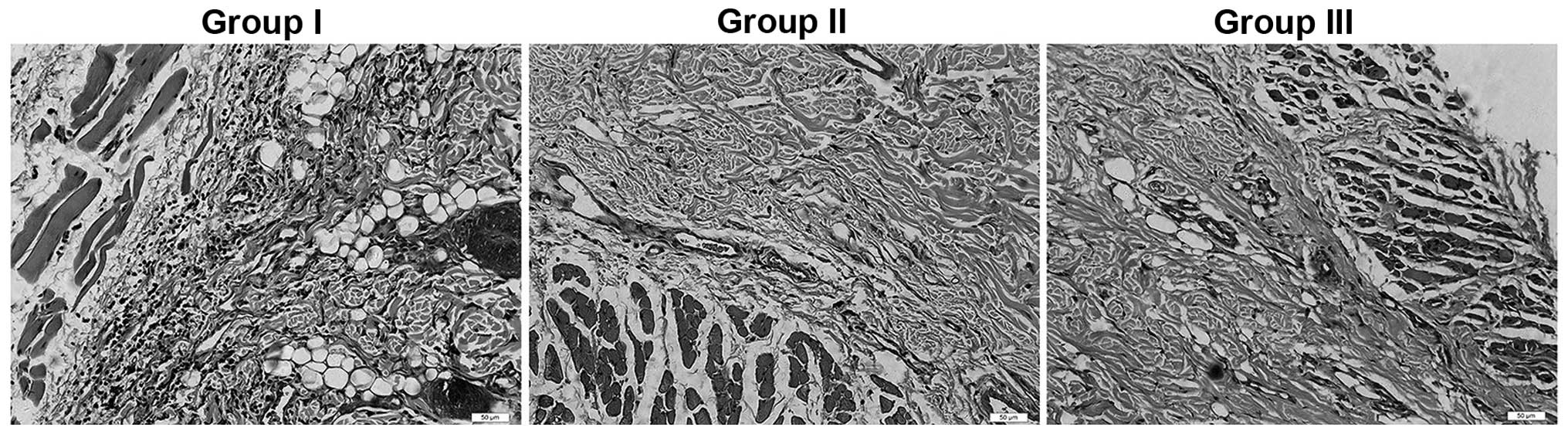
Histology
Seven days after surgery, the flap tissue specimens
in area II of the three groups presented differently under the
microscope: Acute inflammatory infiltration was apparent in all
groups. The infiltration was prominent; 90% of tissue in the images
showed degeneration and necrosis of muscle fibers. However,
inflammatory reactions were less severe in the two experimental
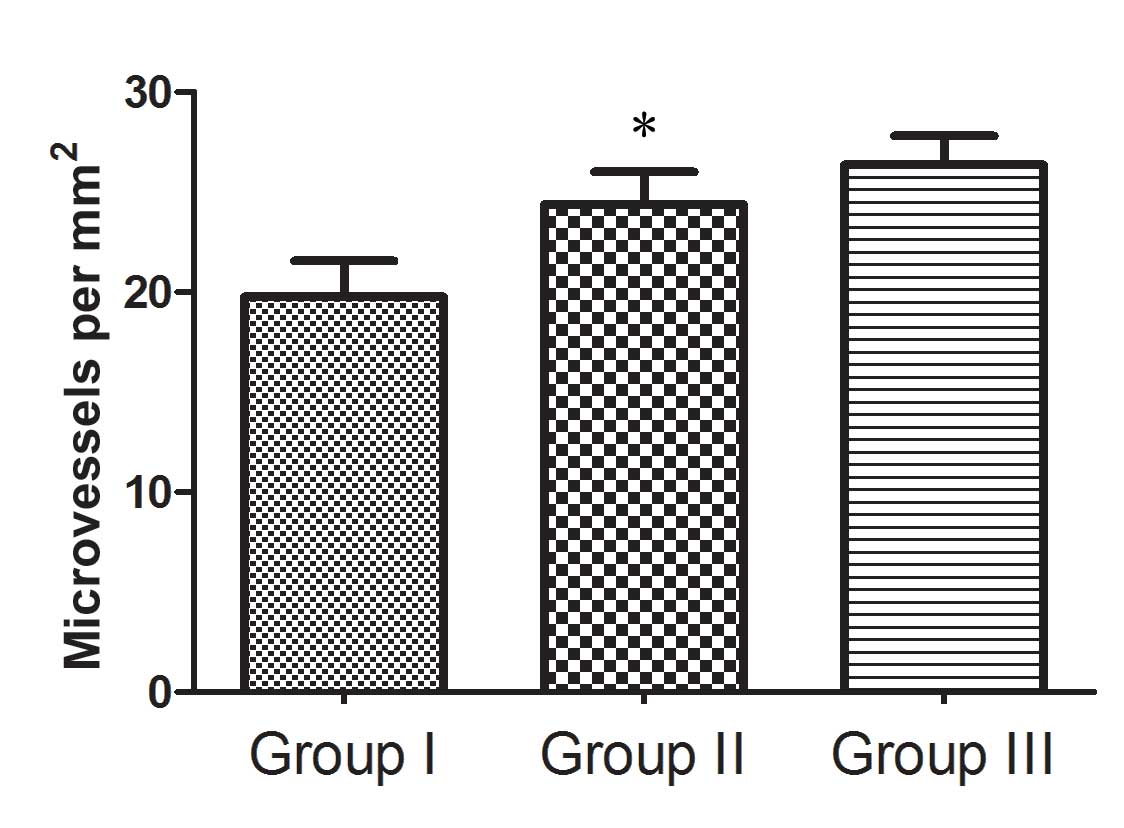
groups compared with those in the control group (Fig. 4). In area II, the MVD was
19.76±3.61/mm2, 24.39±3.21/mm2 and
26.36±2.89/mm2 for groups I, II and III, respectively
(Fig. 5). MVD in group II was
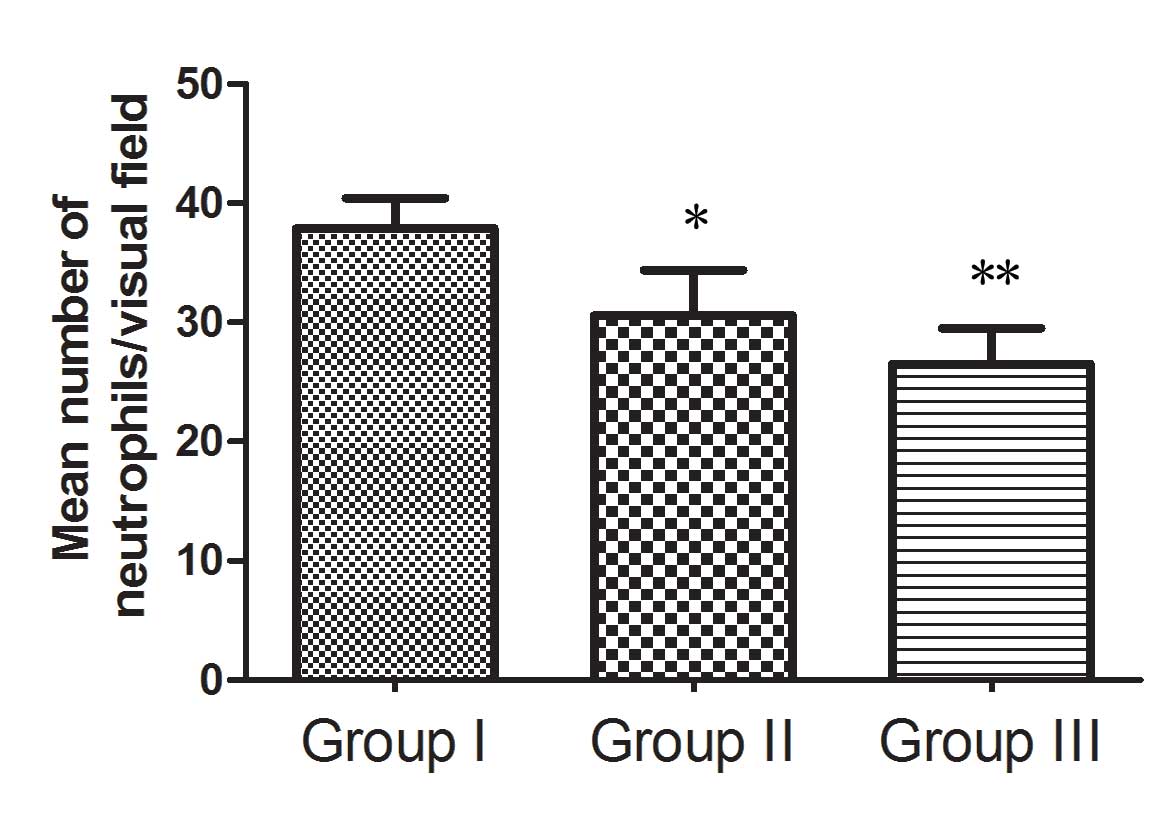
significantly higher than that in group I (P<0.05). Dense
neutrophil infiltration was observed in group I (37.91±3.54),
compared with which neutrophil infiltration was decreased
significantly in group II (30.59±5.39; P<0.05). Neutrophil
infiltration in group III (26.52±4.24) was also significantly less
than that in group II (P<0.05; Fig.
6). Inflammatory reactions were evaluated according to dense
neutrophil infiltration; therefore, the result indicated that the
experimental groups had less severe inflammatory reactions on the
skin flap when compared with the control group.
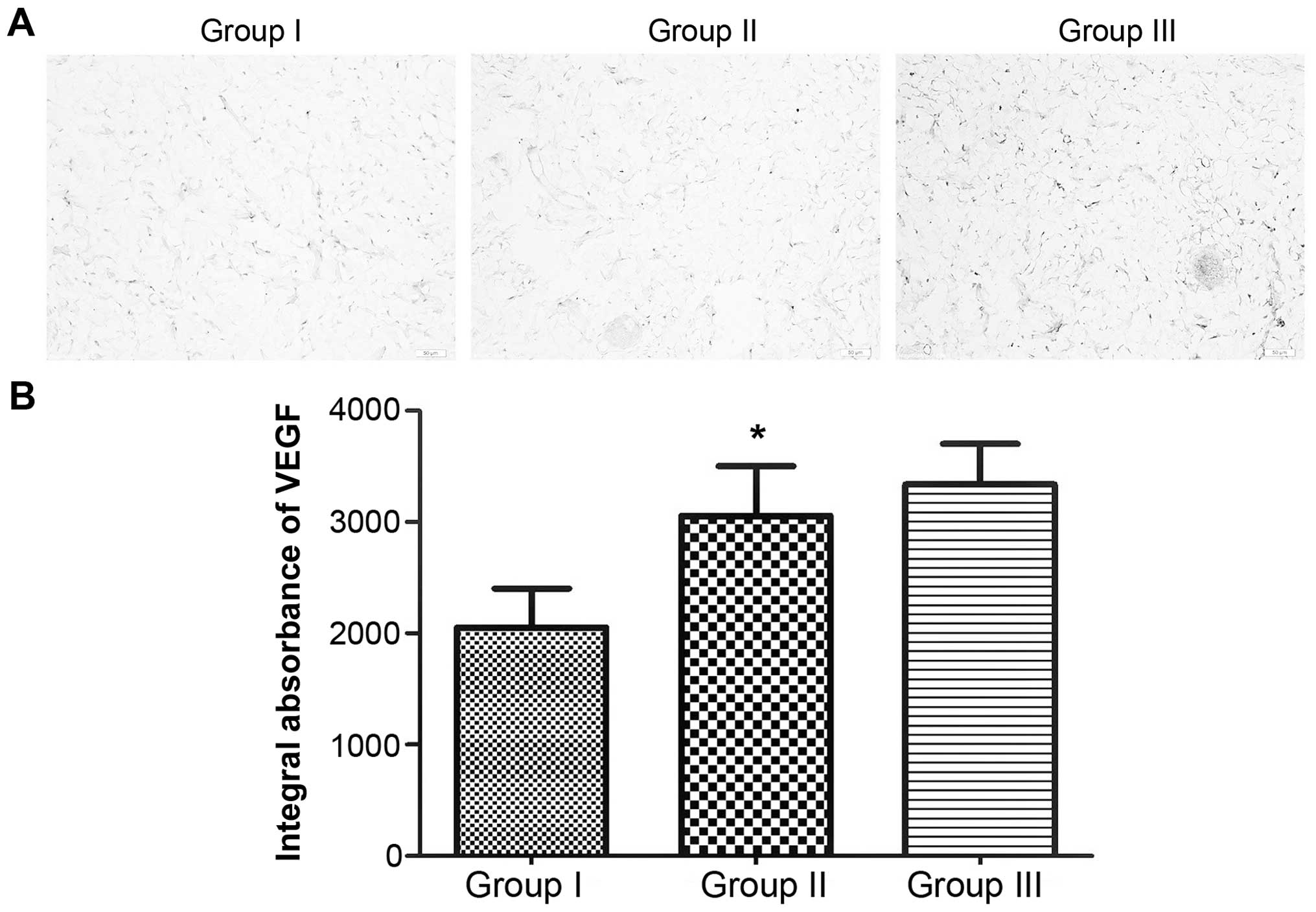
VEGF expression
By calculating IA values, the differences in VEGF
expression among the three groups were observed (Fig. 7A). The IA of VEGF in group II
(3,056.21±627.91) was higher than that in group I (2,050.14±494.97;
P<0.05). Group III (3,337.16±513.29) exhibited the highest IA of
VEGF among the three groups (Fig.
7B).
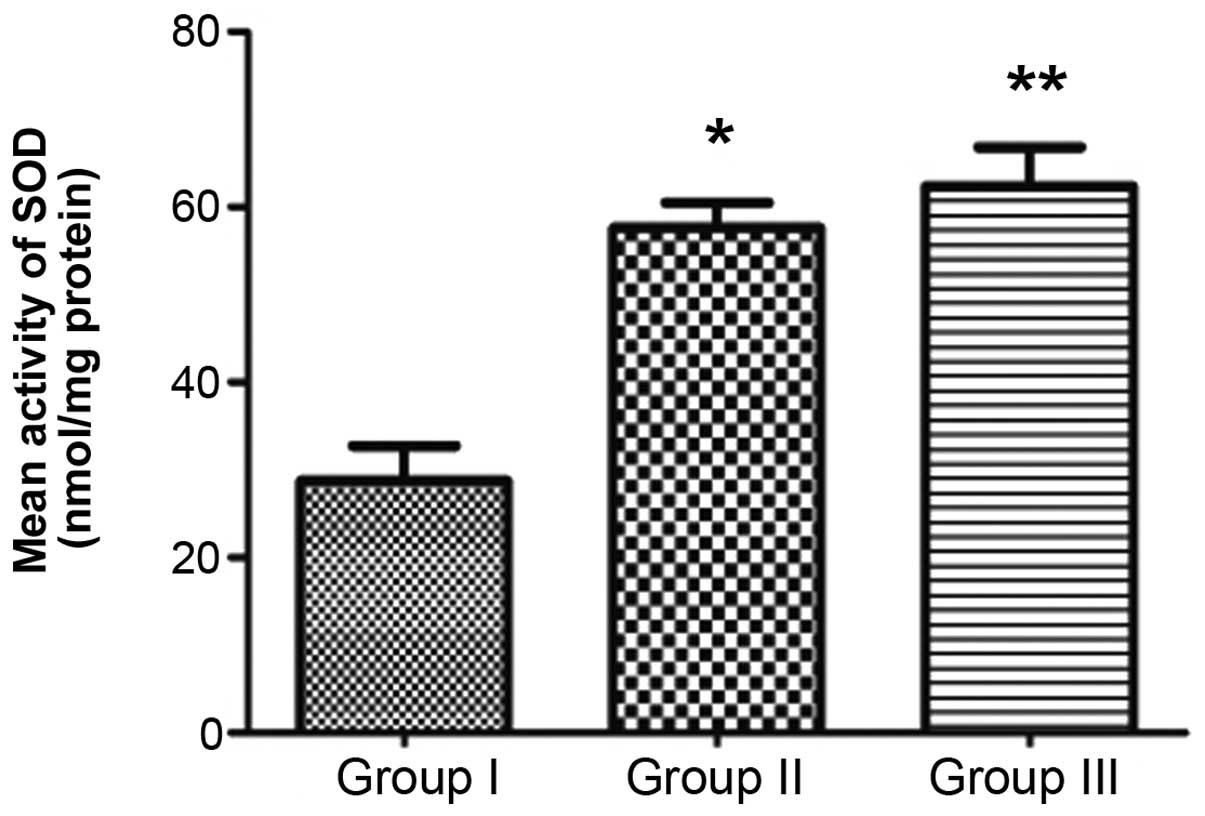
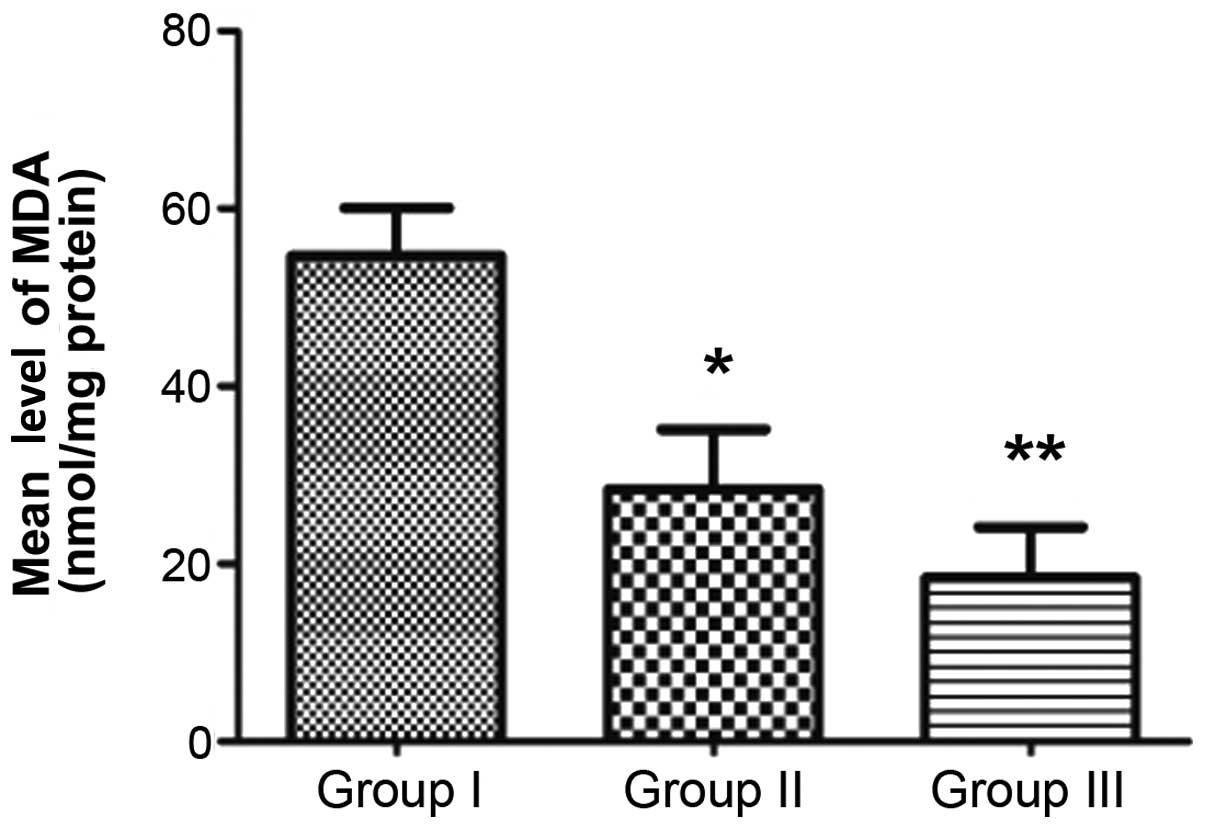
SOD activity and MDA content
Forty-eight hours after surgery, the mean SOD
activity in group II was 57.605±4.052 nmol/mg protein, which was
significantly higher than that in the control group 28.740±5.657
nmol/mg protein (P<0.05). The mean SOD of group III was
62.345±6.329 nmol/mg protein, significantly higher than that of
group II (P<0.05; Fig. 8). The mean
MDA level in group II (28.444±9.479 nmol/mg protein) was
significantly less than that in group I (54.717±7.644 nmol/mg
protein; P<0.05). The mean MDA level of group III was
18.446±8.062 nmol/mg protein, significantly lower than that of
group II (P<0.05; Fig. 9).
Discussion
Local random pattern skin flap is clinically used in
skin and soft issue reconstruction. However, partial necrosis of
skin flaps remains common in the postoperative period and causes
the surgery to fail. Therefore, it is crucial to prevent partial
necrosis in order to enhance survival of the skin flap. Previous
studies have found that ischemia-reperfusion injury and
inflammation are critical in partial necrosis (15,16). In
addition, certain studies have confirmed that VEGF promotes
revascularization (17,18), thus enhancing the survival of skin
flaps. Numerous studies have focused on herbal remedies to improve
the survival of skin flap by inhibiting ischemia-reperfusion
injury, reducing inflammatory reaction and accelerating
angiogenesis, with specific examples including Xuebijing,
Shuxuetong and Huangqi (3,19,20).
DG is the salt form of glycyrrhetinic acid, which
has been widely demonstrated to inhibit inflammation in mice models
of liver injury (21–25). Clinically, DG is widely used in the
therapy of chronic hepatitis for its effects of inhibiting
inflammatory reactions and ischemia-reperfusion injury. These
effects have been demonstrated to be beneficial in rat models of
ulcerative colitis and cerebral ischemia reperfusion (7,8). The present
study focused on its effect on random skin flap survival in
rats.
Evidence from the current study demonstrates that DG
may improve the survival of a random skin flap in rats. The mean
percentages of survival area in the two experimental groups were
significantly higher than that of the control group. From general
observations of the seventh postoperative day, necrotic regions
observed in all three groups had become fused, scabbed and
hardened. However, necrotic regions of the experimental groups only
existed in flap area III, compared with areas II and III in the
control group.
DG may potentially improve flap survival via various
mechanisms. It has been reported that venous crisis is more common
than arterial crisis following flap surgery (26). If this cannot be treated effectively,
flap necrosis is likely to occur. Venous congestion leads to skin
swelling and purple discoloration (26). According to the present study, the area
of swollen skin in the experimental groups was significantly less
than in the control group (by general observation). Furthermore,
the percentage tissue water content was identified to be
significantly lower in the experimental groups when compared with
that in the control group, indicating that DG reduces the degree of
tissue edema.
Inflammatory reactions produced by neutrophil
accumulation negatively affect random skin flap survival. Bächle
et al (27) found that
inflammation had an adverse effect on ischemic random-pattern
flaps. Previous works demonstrated that NF-κB is a key molecule in
the initiation and progression phase of the inflammatory reaction
(28,29). In a rat model of ulcerative colitis,
the expression of NF-κB was inhibited by DG (7). This result indicated that DG was able to
reduce neutrophil count and suppress the inflammatory reaction,
potentially by reducing the expression of NF-κB, thus inhibiting
the expression of proinflammatory cytokines, adhesive molecules and
chemokines. The present study demonstrated that neutrophil density
and inflammation were significantly lower in group II compared with
group I after observing H&E-stained slices. Furthermore,
neutrophil density in group III was significantly lower than that
in group II, indicating that a high dose of DG may be advantageous,
with further restriction of the inflammatory reaction.
In addition to the anti-inflammatory effect, the
current study determined that DG exerted angiogenesis effects that
contributed to increased flap survival. VEGF, an angiogenic growth
factor, was previously shown to be an effective agent in reducing
skin flap necrosis by increasing angiogenesis and blood supply to
the skin flap (30,31). In the present study, the expression
level of VEGF was higher in the experimental groups than in the
control group. Furthermore, it was found that the MVD in area II of
the experimental groups was significantly greater than that of the
control group. These results indicate that DG may promote
neovascularization and microcirculation in ischemic flaps by
increasing VEGF expression, thus enhancing the survival of random
skin flaps. However, the detailed mechanism by which DG regulates
VEGF expression requires further investigation.
Ischemia-reperfusion injury has been shown to be a
primary pathogenic factor causing the necrosis of random skin
flaps. During this process, oxygen-delivered free radicals attack
the cell membrane and cause lipid peroxidation within the first few
minutes of reperfusion (32).
Furthermore, accumulation of activated neutrophils in ischemic
tissue and activation of xanthine oxidase in endothelial cells
induced by reperfusion cause the damage of random skin flaps
(33,34). SOD, as an important antioxidase, is the
predecessor of H2O2 and OH−,
protecting tissue from injury caused by toxic oxygen-derived free
radicals. It is a sensitive indicator of antioxidant status
(35). As a product of lipid
peroxidation, MDA indirectly reflects the extent of tissue damage
due to ischemia-reperfusion injury (36). In the present study, mean SOD activity
in the experimental groups were significantly higher than those in
the control group. However, the mean MDA levels in the experimental
groups were lower than those in the control group. It was also
found that a higher dose of DG had greater beneficial effects on
the mean SOD activity and mean MDA level. These results indicated
that DG protects the tissue from damage caused by
ischemia-reperfusion injury in a dose-dependent manner.
In conclusion, in the present study, DG successfully
improved the survival of random skin flaps in rats. The areas of
necrosis were significantly smaller in the experimental groups
compared with the control group. The effects of DG correlated well
with the histological and immunohistochemical findings of increased
VEGF expression level and promoted neovascularization. Furthermore,
the anti-inflammatory and antioxidant effects of DG were associated
with improved skin flap survival. Additionally, DG was identified
to exert a dose-dependent effect on promoting the survival of
random skin flap in rats, which also demonstrated the beneficial
effect of DG. These results provide a novel therapeutic approach to
improve the survival of random skin flaps. However, further
clinical studies are required to fully understand the benefits and
limitations of DG in the treatment for humans.
Acknowledgements
The present study was supported by the Xinmiao
talent plan of Zhejiang Province (grant no. 2015R413005), Zhejiang
Province Chinese medicine scientific research fund (grant no.
2014ZB074), the National Natural Science Foundation of China (grant
no. 81503397), and the Zhejiang provincial medical and health
science and technology program (grant no. 2016KYB195).
References
|
1
|
Yang M, Sheng L, Li H, Weng R and Li QF:
Improvement of the skin flap survival with the bone marrow-derived
mononuclear cells transplantation in a rat model. Microsurgery.
30:275–281. 2010.PubMed/NCBI
|
|
2
|
Kim HJ, Xu L, Chang KC, Shin SC, Chung JI,
Kang D, Kim SH, Hur JA, Choi TH, Kim S, et al: Anti-inflammatory
effects of anthocyanins from black soybean seed coat on the
keratinocytes and ischemia-reperfusion injury in rat skin flaps.
Microsurgery. 32:563–570. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bin C, Dingsheng L, Leyi C, Bin L, Yuting
L, Liren W and Zhijie L: Beneficial effects of Xuebijing injection
on random skin flap survival in rats. J Surg Res. 196:421–426.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cao B, Wang L, Lin D, Cai L and Gao W:
Effects of lidocaine on random skin flap survival in rats. Dermatol
Surg. 41:53–58. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
vanRossum TG, Vulto AG, de Man RA, Brouwer
JT and Schalm SW: Review article: Glycyrrhizin as a potential
treatment for chronic hepatitis C. Aliment Pharmacol Ther.
12:199–205. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iino S, Tango T, Matsushima T, Toda G,
Miyake K, Hino K, Kumada H, Yasuda K, Kuroki T, Hirayama C, et al:
Therapeutic effects of stronger neo-minophagen C at different doses
on chronic hepatitis and liver cirrhosis. Hepatol Res. 19:31–40.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yuan H, Ji WS, Wu KX, Jiao JX, Sun LH and
Feng YT: Anti-inflammatory effect of Diammonium Glycyrrhizinate in
a rat model of ulcerative colitis. World J Gastroenterol.
12:4578–4581. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou SZ, Li Y, Zhu XL, Wang ZY, Wang X and
Xu Y: Ameliorative effects of diammonium glycyrrhizinate on
inflammation in focal cerebral ischemic-reperfusion injury. Brain
Res. 1447:20–27. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mandriota SJ, Pyke C, Di Sanza C, Quinodoz
P, Pittet B and Pepper MS: Hypoxia-inducible angiopoietin-2
expression is mimicked by iodonium compounds and occurs in the rat
brain and skin in response to systemic hypoxia and tissue ischemia.
Am J Pathol. 156:2077–2089. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kelly CP, Gupta A, Keskin M and Jackson
IT: A new design of a dorsal flap in the rat to study skin necrosis
and its prevention. J Plast Reconstr Aesthet Surg. 63:1553–1556.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren H, Lin D, Mou Z and Dong P: The
adverse effect of selective cyclooxygenase-2 inhibitor on random
skin flap survival in rats. PLoS One. 8:e828022013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen Y, Tong H, Zhang X, Tang L, Pan Z,
Liu Z, Duan P and Su L: Xuebijing injection alleviates liver injury
by inhibiting secretory function of Kupffer cells in heat stroke
rats. J Tradit Chin Med. 33:243–249. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yamaguchi S, Watanabe G, Tomita S and
Tabata S: Lidocaine-magnesium blood cardioplegia was equivalent to
potassium blood cardioplegia in left ventricular function of canine
heart. Interact Cardiovasc Thorac Surg. 6:172–176. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu L, Liu Y, Cui J, Liu H, Liu YB, Qiao
WL, Sun H and Yan CD: Oxidative stress induces gastric submucosal
arteriolar dysfunction in the elderly. World J Gastroenterol.
19:9439–9446. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Coskunfirat OK, Cinpolat A, Bektas G, Ogan
O and Taner T: Comparing different postconditioning cycles after
ischemia reperfusion injury in the rat skin flap. Ann Plast Surg.
72:104–107. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xu R, Ge J, Lei Y and Lu X: Improvement
effect of estrogen on flap reperfusion injury and blood supply.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 23:964–968. 2009.(In
Chinese). PubMed/NCBI
|
|
17
|
Zhang F and Lineaweaver W: Acute and
sustained effects of vascular endothelial growth factor on survival
of flaps and skin grafts. Ann Plast Surg. 66:581–582. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang F, Fischer K, KomorowskaTimek E, Guo
M, Cui D, DorsettMartin W, Buncke HJ and Lineaweaver WC:
Improvement of skin paddle survival by application of vascular
endothelial growth factor in a rat TRAM flap model. Ann Plast Surg.
46:314–319. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cai L, Cao B and Lin D: Effects of
Traditional Chinese Medicine Huangqi Injection (Radix astragali) on
Random Skin Flap Survival in Rats. J Reconstr Microsurg.
31:565–570. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cai L, Huang W and Lin D: Effects of
traditional Chinese medicine Shuxuetong injection on random skin
flap survival in rats. Sci World J. 2014:8165452014. View Article : Google Scholar
|
|
21
|
Tang B, Qiao H, Meng F and Sun X:
Glycyrrhizin attenuates endotoxin- induced acute liver injury after
partial hepatectomy in rats. Braz J Med Biol Res. 40:1637–1646.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Abe K, Ikeda T, Wake K, Sato T, Sato T and
Inoue H: Glycyrrhizin prevents of
lipopolysaccharide/D-galactosamine-induced liver injury through
down-regulation of matrix metalloproteinase-9 in mice. J Pharm
Pharmacol. 60:91–97. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tsuruoka N, Abe K, Wake K, Takata M, Hatta
A, Sato T and Inoue H: Hepatic protection by glycyrrhizin and
inhibition of iNOS expression in concanavalin A-induced liver
injury in mice. Inflamm Res. 58:593–599. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yoshida T, Abe K, Ikeda T, Matsushita T,
Wake K, Sato T, Sato T and Inoue H: Inhibitory effect of
glycyrrhizin on lipopolysaccharide and d-galactosamine-induced
mouse liver injury. Eur J Pharmacol. 576:136–142. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee CH, Park SW, Kim YS, Kang SS, Kim JA,
Lee SH and Lee SM: Protective mechanism of glycyrrhizin on acute
liver injury induced by carbon tetrachloride in mice. Biol Pharm
Bull. 30:1898–1904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yingxin G, Guoqian Y, Jiaquan L and Han X:
Effects of natural and recombinant hirudin on VEGF expression and
random skin flap survival in a venous congested rat model. Int
Surg. 98:82–87. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bächle AC, Mörsdorf P, Rezaeian F, Ong MF,
Harder Y and Menger MD: N-acetylcysteine attenuates leukocytic
inflammation and microvascular perfusion failure in critically
ischemic random pattern flaps. Microvasc Res. 82:28–34. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neurath MF, Fuss I, Schürmann G,
Pettersson S, Arnold K, Müller-Lobeck H, Strober W, Herfarth C and
Büschenfelde KH: Cytokine gene transcription by NF-kappa B family
members in patients with inflammatory bowel disease. Ann N Y Acad
Sci. 859:(1 INTESTINAL PL). 149–159. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Blackwell TS and Christman JW: The role of
nuclear factor-kappa B in cytokine gene regulation. Am J Respir
Cell Mol Biol. 17:3–9. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vourtsis SA, Spyriounis PK, Agrogiannis G,
Papalois A and Ionac M: Does VEGF have an effect on the survival of
a long random skin flap by its application at the recipient area?
Chirurgia (Bucur). 107:494–500. 2012.PubMed/NCBI
|
|
31
|
Fang T, Lineaweaver WC, Chen MB, Kisner C
and Zhang F: Effects of vascular endothelial growth factor on
survival of surgical flaps: A review of experimental studies. J
Reconstr Microsurg. 30:1–13. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Braunersreuther V and Jaquet V: Reactive
oxygen species in myocardial reperfusion injury: From
physiopathology to therapeutic approaches. Curr Pharm Biotechnol.
13:97–114. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ozkan F, Senayli Y, Ozyurt H, Erkorkmaz U
and Bostan B: Antioxidant effects of propofol on tourniquet-induced
ischemia-reperfusion injury: An experimental study. J Surg Res.
176:601–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Cui J, Liu L, Zou J, Qiao W, Liu H, Qi Y
and Yan C: Protective effect of endogenous hydrogen sulfide against
oxidative stress in gastric ischemia-reperfusion injury. Exp Ther
Med. 5:689–694. 2013.PubMed/NCBI
|
|
35
|
Sirota TV, Zakharchenko MV and Kondrashova
MN: Cytoplasmic superoxide dismutase activity is a sensitive
indicator of the antioxidant status of the rat liver and brain.
Biomed Khim. 60:63–71. 2014.(In Russian). View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sun L, Li Y, Shi J and Wang X and Wang X:
Protective effects of ligustrazine on ischemia-reperfusion injury
in rat kidneys. Microsurgery. 22:343–346. 2002. View Article : Google Scholar : PubMed/NCBI
|