Introduction
Calprotectin was first described in 1980 (1), with its name referring to its calcium
binding and anti-microbial actions (2,3). It is found
in the cytosol of inflammatory cells (4) accounting for 60% of cytosolic protein in
neutrophils (5), but is also present
at a lower concentration in monocytes and reactive macrophages
(6). Calprotectin is released
following activation of leukocytes, and due to its ability to
inhibit zinc-dependent enzyme systems (2), it exerts bacteriostatic and fungistatic
effects (6). In addition, calprotectin
induces apoptosis in normal and cancer cells (7). Depending on the organ affected by
inflammation, increased levels of calprotectin can be observed in
the plasma, cerebrospinal fluid, synovial fluid, urine or faeces
(8). It has been observed that the
concentration of calprotectin in the faeces of healthy subjects, is
approximately six times the concentration found in their plasma and
in the presence of calcium, it withstands proteolytic degradation;
in faeces, it is therefore stable at room temperature for up to
seven days (9). Calprotectin can be
reliably measured in faecal samples of <5 g. In addition, the
protein's properties allow for the collection of a sample at home
and potentially delayed transportation to the laboratory (9). Numerous studies have indicated that
faecal calprotectin may represent an alternative marker of
neutrophil influx into the bowel lumen (10). In line with this, increased faecal
levels of calprotectin have been observed in inflammatory bowel
diseases (IBD) (9,11), colon cancer (12) and non-steroidal anti-inflammatory drug
(NSAID) enteropathy (13,14), suggesting that calprotectin is a
sensitive, but non-specific marker of intestinal inflammation.
IBD
IBD are chronic intestinal disorders arising in the
setting of complex interactions between host-derived and external
elements, involving various aspects of the intestinal microbiota,
the immune system, the genetic composition of the host and specific
environmental factors (15), and which
typically have a relapsing course (16). The two major clinically defined types
of IBD are ulcerative colitis (UC) and Crohn's disease (CD), each
of which may affect the entire colonic mucosa and gastrointestinal
tract, respectively, and is associated with an increased risk of
colon cancer (15).
CD is a chronic inflammatory gastrointestinal tract
disorder (17,18). While no definitive therapy has been
established (6), the main goal of IBD
treatment is the lasting and effective suppression of the
inflammatory response with the aim of achieving and maintaining
clinical remission. However, sub-clinical inflammation of the
intestinal wall may persist even after successful treatment and
significantly contributes to the risk of relapse (16). It has been reported that among patients
with medically induced remission, 30–60% show a relapse within 1
year (19) and at five years following
diagnosis, ~50% require surgery (20).
The exact etiology of IBD has remained elusive
(16); however, IBD has been suggested
to have a genetic basis and likely involves a response of the
immune system to certain environmental agents (15). The role of environmental factors
besides genetics in the pathogenesis of IBD has been evidenced by
differences among monozygotic twins regarding their proneness
towards developing IBD (21), as well
as the development of IBD in immigrants in high-prevalence
countries (22) and in countries
undergoing rapid westernization (23).
Experimental studies have reported the development of IBD-like
enterocolitis in interleukin-2 (IL-2), IL-10 or T-cell
receptor-mutant mice (24–26), and blockade of tumor necrosis factor-α
was proven be an effective treatment for patients with CD, which
initiated a new field of research in the early 1990s (15). In addition, studies on the genetic
basis of IBD (27) and a sequencing
analysis of the intestinal microbiome (28) provided further insight into the
pathophysiology of these conditions. A number of molecular pathways
are involved in IBD, interfering both with genes of the immune
system and the microbial flora. Immunoglobulins, tumor necrosis
factors, and growth factors are among the factors involved in
molecular pathways associated with the immune system (15). The molecular pathways involved in IBD
are summarized in Fig. 1.
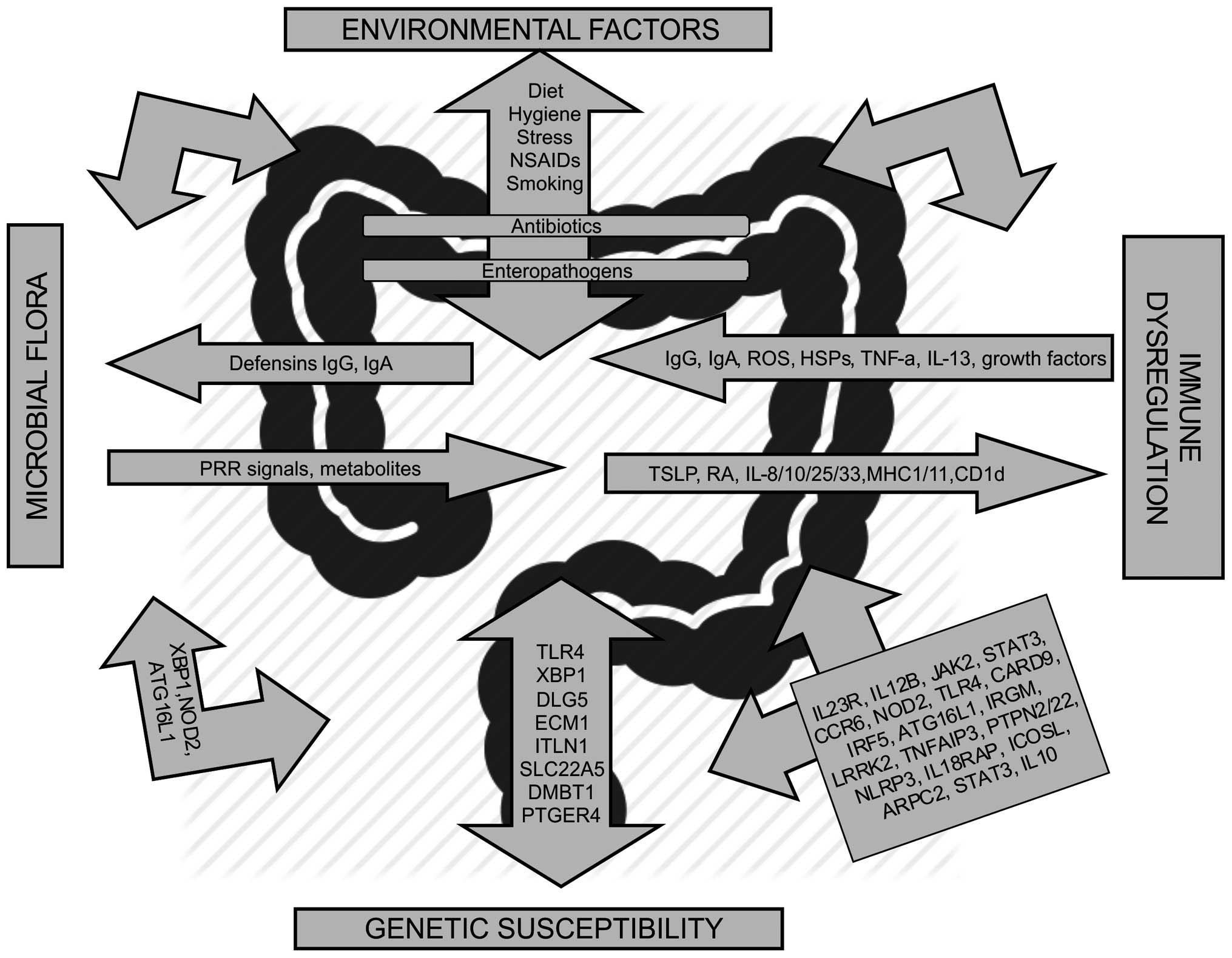 | Figure 1.Molecular pathways associated with
inflammatory bowel disease. Genes belonging to the same pathway are
grouped in the same arrow. Genetic associations in Crohn's disease
and ulcerative colitis are shown. Ig, immunoglobulin; TSLP, thymic
stromal lymphopoietin; RA, retinoic acid; ROS, reactive oxygen
species; NSAIDs, non-steroidal anti-inflammatory drugs; HSP, heat
shock protein; PRR, pattern-recognition receptor; XBP1, X-box
binding protein; NOD2, nucleotide-binding oligomerization
domain-containing protein; ATG16L1, autophagy-related protein 16-1;
TLR, Toll-like receptor; MHC, major histocompatibility complex;
DLG5, discs large homologue 5; ECM1, extracellular matrix protein
1; ITLN1, interlactin 1; SLC22A5, solute carrier family 22 member
5; DMBT1, deleted in malignant brain tumors 1; PTGER4,
prostaglandin E receptor 4; TNF-a, tumor necrosis factor alpha; IL,
interleukin; JAK2, Janus kinase 2; STAT3, signal transducer and
activator of transcription; CCR6, C-C-chemokine receptor type 6;
NOD2, nucleotide-binding oligomerization domain-containing protein
2; CARD9, caspase recruitment domain-containing protein 9; IRF5,
interferon regulatory factor 5; IRGM, immunity-related guanine
triphosphatase family M protein; LRRK2, leucine-rich repeat kinase
2; TNFAIP3, TNF-a-induced protein 3; PTPN2, protein tyrosine
phosphatase, non-receptor type 2; NLRP3, NACHT, LRR and PYD
domains-containing protein 3; IL18RAP, Interleukin 18 receptor
accessory protein; ICOSL, inducible T-cell co-stimulator ligand;
ARPC2, actin related protein 2/3 complex subunit 2 (15). |
There are, however, polymorphisms in the following
genes specific for CD: Nucleotide-binding oligomerization
domain-containing protein, autophagy-related protein 16-1,
interlactin 1, prostaglandin E receptor 4, C-C-chemokine receptor
type 6, immunity-related guanine triphosphatase family M protein,
NACHT, LRR and PYD domains-containing protein 3 and inducible
T-cell co-stimulator ligand, whereas those specific for UC are
extracellular matrix protein 1, actin related protein 2/3 complex
subunit 2 and IL10 (15) (Fig. 1).
Calprotectin concentration as a marker for
IBD
Numerous patients consider endoscopy for the direct
evaluation and diagnosis of IBD; however, the bowel preparation
required for this procedure is uncomfortable and expensive
(29). Furthermore, a large proportion
of patients with suspected IBD are negatively diagnosed by
endoscopy, and a non-invasive test was therefore required to
minimize the number of patients unnecessarily undergoing invasive
endoscopy (10). Specifically, one
third of all adults with bleeding-associated intestinal symptoms
have no abnormalities on endoscopy, whilst only half of those with
non-bleeding symptoms, such as diarrhoea, abdominal pain and weight
loss are diagnosed with IBD based on endoscopy results.
In an effort to identify novel diagnostic markers
for IBD, calprotectin was investigated. Early studies indicated an
increase of calprotectin levels in patients with IBD (9), which was also correlated with endoscopic
and histological evidence of inflammation (30–32). Since
2000, faecal calprotectin has been assessed in adult and paediatric
populations, including patients with known inflammatory bowel
disease on one side of the patient spectrum and healthy individuals
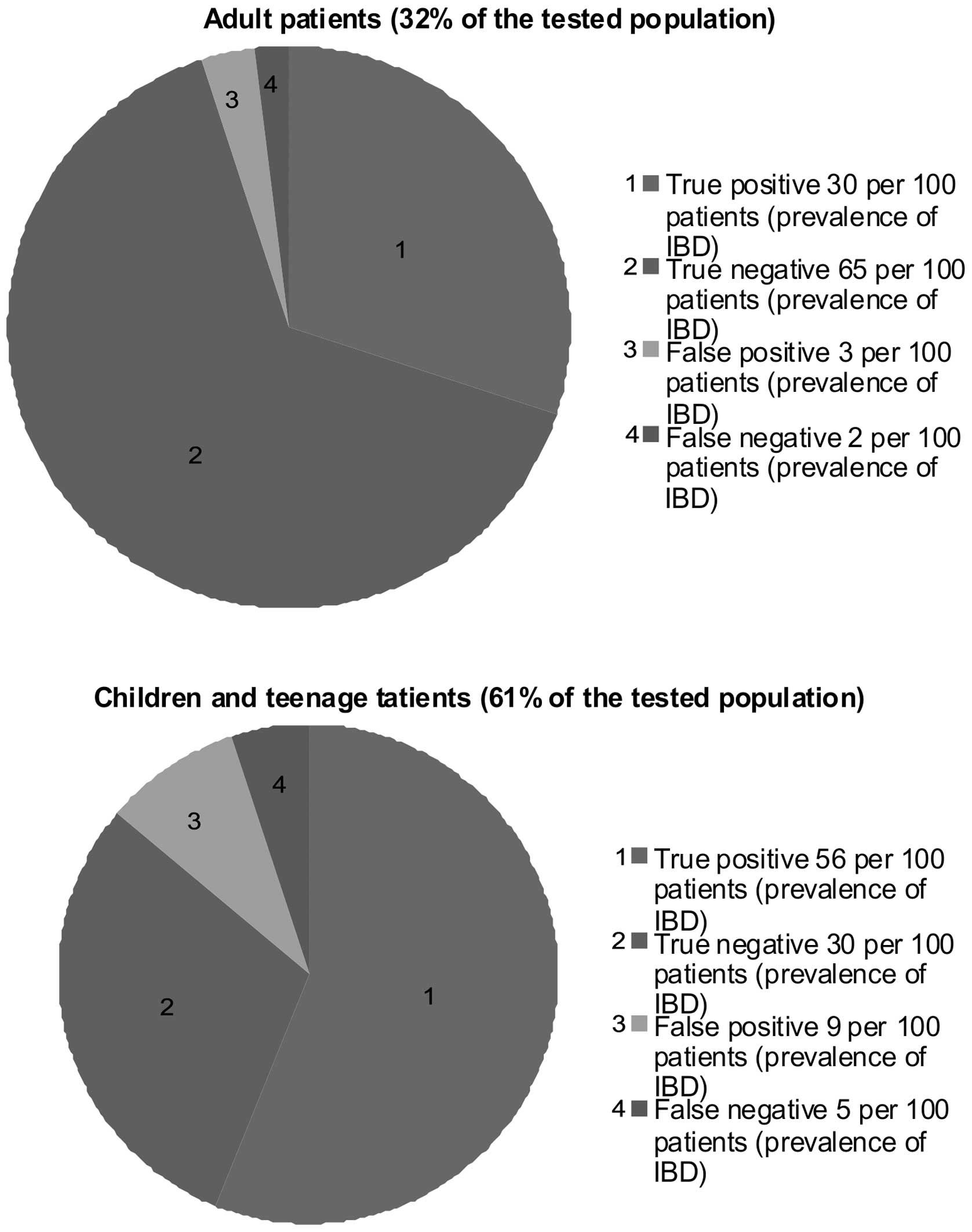
on the other (33). As shown in
Fig. 2, the calprotectin levels in 86%
of paediatric faecal samples and 95% of samples from adults
correlated with the endoscopic evidence, reflecting the high
sensitivity of the test in all age groups.
Studies have indicated that faecal calprotectin may
serve as a surrogate marker of neutrophil influx into the bowel
lumen and therefore as a marker of intestinal inflammation, with a
significant association with IBD (9,11), colon
cancer (12) and NSAID enteropathy
(13,14). These results suggested that
calprotectin serves as a sensitive but non-specific marker of
intestinal inflammation (34), a
predictor for the severity of IBS in adults (35) and children (11) as well as relapse of IBS (36), and may be used for monitoring treatment
responses (37).
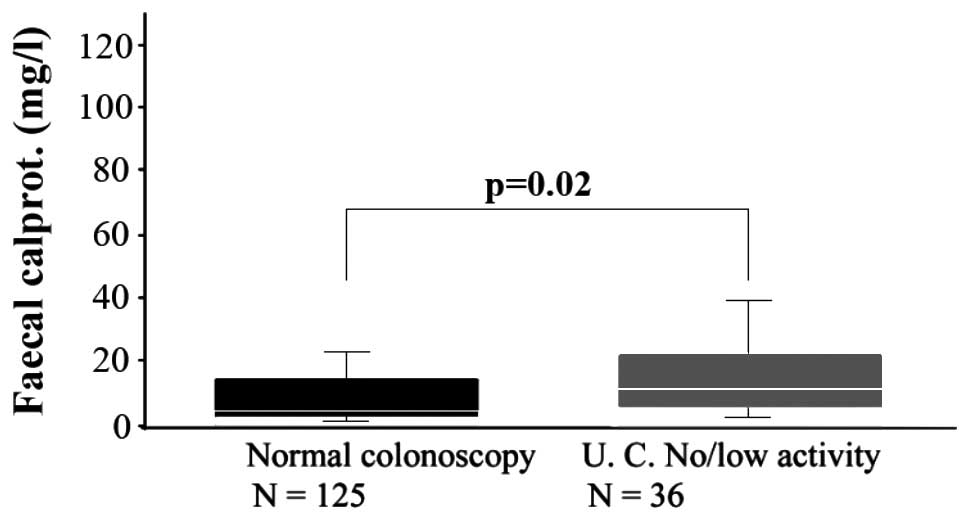
Regarding UC, increased levels of calprotectin have
been confirmed to be correlated with endoscopic results and
histological grading of disease activity (30,38). For
instance, in a study on 36 outpatients, whose endoscopic and
histological results showed low activity of UC, faecal calprotectin
was significantly higher than that in 125 patients who were normal
on colonoscopy (Fig. 3) (30).
Furthermore, due to the variable location and patchy
distribution of CD, it has been suggested that calprotectin as a
diagnostic marker is more sensitive than endoscopy. Unlike UC,
which exclusively affects the colon allowing for detection by
endoscopic examination and subsequent histological analysis, CD may
include areas of the small intestine, which are not always
accessible by endoscopic examination and may only be detectable by
capsule endoscopy (39). Therefore,
due to the lack of histological findings (6), detection of inflammation in CD is
difficult and laboratory parameters of inflammation are used.
However, C-reactive protein and the erythrocyte sedimentation rate
lack specificity or sensitivity and clinical indices of disease
activity, such as the CD activity index, the UC activity index and
the Harvey-Bradshaw activity index are associated with the
patient's quality of life and well-being rather than the degree of
mucosal inflammation (40–43).
Of note, faecal calprotectin can be utilized as an
ideal initial marker for IBD, as its detection is cost-efficient
and simple (6). A stool sample of
<5 g is required for analysis using a commercial enzyme-linked
immunosorbent assay kit. In addition, calprotectin in faecal
samples is stable at room temperature for up to seven days, which
allows for self-sampling by patients at home and as well as
convenience and reliability of laboratory detection.
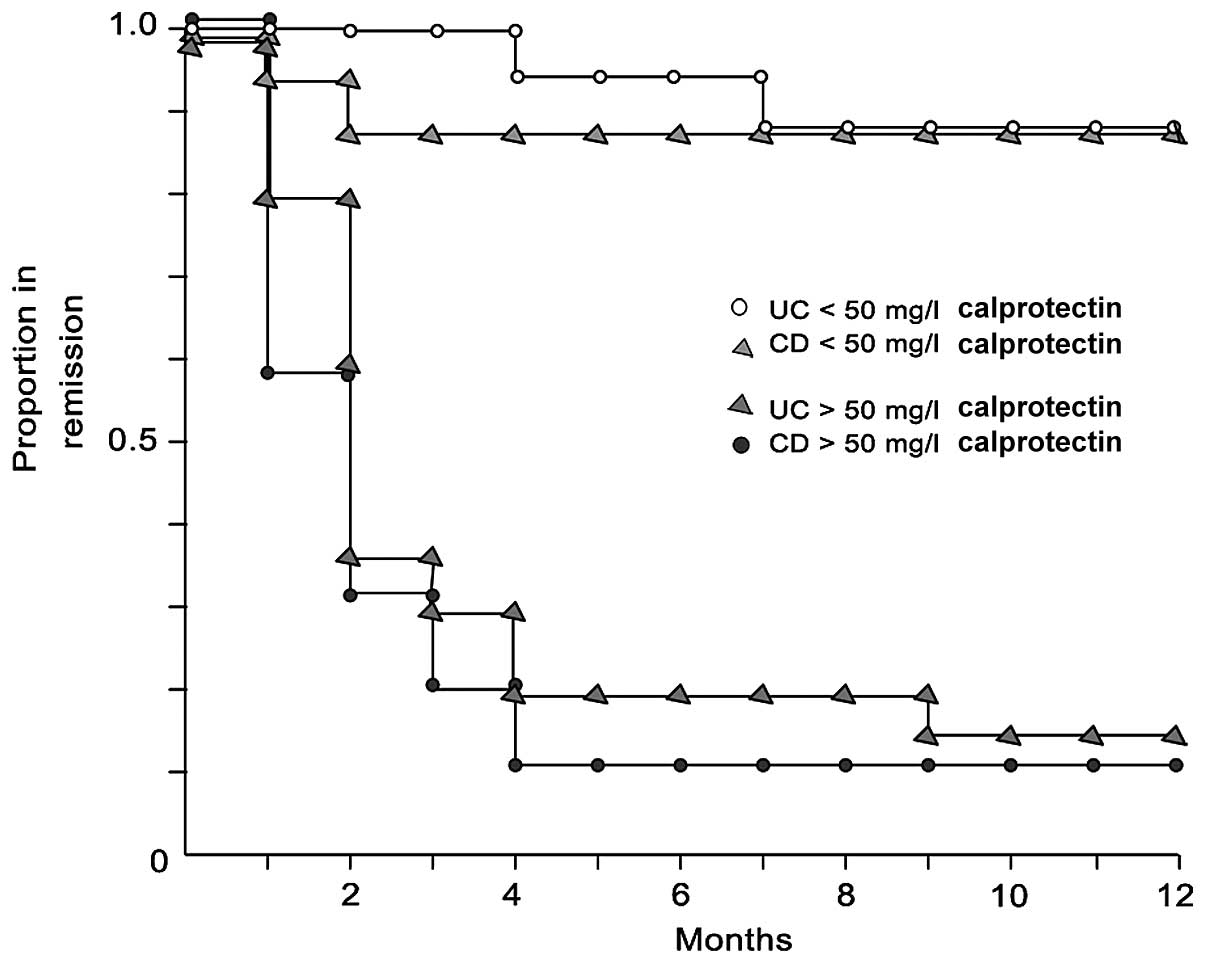
However, controversy remains regarding the
predictive value of faecal calprotectin in patients in remission. A
study by Tibble et al (44)
indicated that faecal calprotectin is associated with the risk of
early relapse, with no difference between UC and CD (16,45), as
shown by the Kaplan-Meier curves (Fig.
4).
Conclusion
Faecal calprotectin can be used as a cost-efficient
and non-invasive method for IBD diagnosis. While this preliminary
marker cannot replace endoscopy and histological analysis, it may
be a reliable tool to support the diagnosis of IBD, to predict
early relapse of IBD in patients with drug-induced remission and
may also help to spare patients from unnecessary endoscopy when
calprotectin levels are low.
Glossary
Abbreviations
Abbreviations:
|
IBD
|
inflammatory bowel disease
|
|
CD
|
Crohn's disease
|
|
UC
|
ulcerative colitis
|
|
IL-2
|
interleukin-2
|
|
TCR
|
T-cell receptor
|
|
TNF
|
tumor necrosis factor
|
|
ESR
|
erythrocyte sedimentation rate
|
|
CRP
|
C reactive protein
|
|
CDAI
|
Crohn's disease activity index
|
|
UCAI
|
ulcerative colitis activity index
|
References
|
1
|
Fagerhol MK, Dale I and Andersson T: A
radioimmunoassay for a granulocyte protein as a marker in studies
on the turnover of such cells. Bull Eur Physiopathol Respir.
16:273–282. 1980.PubMed/NCBI
|
|
2
|
Steinbakk M, Naess-Andresen CF, Lingaas E,
Dale I, Brandtzaeg P and Fagerhol MK: Antimicrobial actions of
calcium binding leucocyte L1 protein, calprotectin. Lancet.
336:763–765. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sohnle PG, Collins-Lech C and Wiessner JH:
Antimicrobial activity of an abundant calcium-binding protein in
the cytoplasm of human neutrophils. J Infect Dis. 163:187–192.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Røseth AG, Schmidt PN and Fagerhol MK:
Correlation between faecal excretion of indium-111-labelled
granulocytes and calprotectin, a granulocyte marker protein, in
patients with inflammatory bowel disease. Scand J Gastroenterol.
34:50–54. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fagerhol MK, Andersson KB, Andresen CF
Naess, Brandtzaeg P and Dale I: Calprotectin (the L1 leucoyte
protein)Stimulus Response Coupling: The Role of Intracellular
Calcium Binding Proteins. Smith VL and Dedman JR: CRC Press Inc.;
Boca Raton, FL: pp. 187–210. 1990
|
|
6
|
Gaya DR and Mackenzie JF: Faecal
calprotectin: A bright future for assessing diseaseactivity in
Crohn's disease. QJM. 95:557–558. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yiu S, Mikami M and Yamazaki M: Induction
of apoptoic cell death in mouse lymphoma and human leukaemia cell
lines by a calcium-binding protein complex, calprotectin, derived
from inflammatory peritoneal exudate cells. J Leukoc Biol.
336:763–765. 1995.
|
|
8
|
Johne B, Fagerhol MK, Lyberg T, Prydz H,
Brandtzaeg P, Naess-Andresen CF and Dale I: Functional and clinical
aspects of the myelomonocyte protein calprotectin. Mol Pathol.
50:113–123. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Røseth AG, Fagerhol MK, Aadland E and
Schjønsby H: Assessment of the neutrophil dominating protein
calprotectin in feces. A methodologic study. Scand J Gastroenterol.
27:793–798. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lasson A, Kilander A and Stotzer PO:
Diagnostic yield of colonoscopy based on symptoms. Scand J
Gastroenterol. 43:356–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bunn SK, Bisset WM, Main MJ and Golden BE:
Fecal calprotectin as a measure of disease activity in childhood
inflammatory bowel disease. J Pediatr Gastroenterol Nutr.
32:171–177. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Røseth AG, Kristinsson J, Fagerhol MK,
Schjønsby H, Aadland E, Nygaard K and Roald B: Faecal calprotectin:
A novel test for the diagnosis of colorectal cancer? Scand J
Gastroenterol. 28:1073–1076. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Meling TR, Aabakken L, Røseth A and Osnes
M: Faecal calprotectin shedding after short-term treatment with
non-steroidal anti-inflammatory drugs. Scand J Gastroenterol.
31:339–344. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tibble JA, Sigthorsson G, Foster R, Scott
D, Fagerhol MK, Roseth A and Bjarnason I: High prevalence of NSAID
enteropathy as shown by a simple faecal test. Gut. 45:362–366.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaser A, Zeissig S and Blumberg RS:
Inflammatory bowel disease. Annu Rev Immunol. 28:573–621. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Riley SA, Mani V, Goodman MJ, Dutt S and
Herd ME: Microscopic activity in ulcerative colitis: What does it
mean? Gut. 32:174–178. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Barton JR, Gillon S and Ferguson A:
Incidence of inflammatory bowel disease in Scottish children
between 1968 and 1983; marginal fall in ulcerative colitis,
three-fold rise in Crohn's disease. Gut. 30:618–622. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Armitage E, Drummond H, Ghosh S and
Ferguson A: Incidence of juvenile-onset Crohn's disease in
Scotland. Lancet. 353:1496–1497. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Greenberg GR, Feagan BG, Martin F,
Sutherland LR, Thomson AB, Williams CN, Nilsson LG and Persson T:
Canadian Inflammatory Bowel Disease Study Group: Oral budesonide as
maintenance treatment for Crohn's disease: A placebo-controlled,
dose-ranging study. Gastroenterology. 110:45–51. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rutgeerts PJ: Prevention of early
recurrence of Crohn's disease after ileal resection with ileocolic
anastomosis. Eur J Gastroenterol Hepatol. 6:113–116. 1994.
View Article : Google Scholar
|
|
21
|
Halme L, Paavola-Sakki P, Turunen U,
Lappalainen M, Farkkila M and Kontula K: Family and twin studies in
inflammatory bowel disease. World J Gastroenterol. 12:3668–3672.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Tsironi E, Feakins RM, Probert CS, Rampton
DS and Phil D: Incidence of inflammatory bowel disease is rising
and abdominal tuberculosis is falling in Bangladeshis in East
London, United Kingdom. Am J Gastroenterol. 99:1749–1755. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Thia KT, Loftus EV Jr, Sandborn WJ and
Yang SK: An update on the epidemiology of inflammatory bowel
disease in Asia. Am J Gastroenterol. 103:3167–3182. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sadlack B, Merz H, Schorle H, Schimpl A,
Feller AC and Horak I: Ulcerative colitis-like disease in mice with
a disrupted interleukin-2 gene. Cell. 75:253–261. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kühn R, Löhler J, Rennick D, Rajewsky K
and Muller W: Interleukin-10-deficient mice develop chronic
enterocolitis. Cell. 75:263–274. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mombaerts P, Mizoguchi E, Grusby MJ,
Glimcher LH, Bhan AK and Tonegawa S: Spontaneous development of
inflammatory bowel disease in T cell receptor mutant mice. Cell.
75:274–282. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gregersen PK and Olsson LM: Recent
advances in the genetics of autoimmune disease. Annu Rev Immunol.
27:363–391. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Peterson DA, Frank DN, Pace NR and Gordon
JI: Metagenomic approaches for defining the pathogenesis of
inflammatory bowel diseases. Cell Host Microbe. 3:417–427. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ristvedt SL, McFarland EG, Weinstock LB
and Thyssen EP: Patient preferences for CT colonography,
conventional colonoscopy, and bowel preparation. Am J
Gastroenterol. 98:578–585. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Røseth AG, Aadland E, Jahnsen J and
Raknerud N: Assessment of disease activity in ulcerative colitis by
faecal calprotectin, a novel granulocyte marker protein. Digestion.
58:176–180. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tibble J, Teahon K, Thjodleifsson B,
Roseth A, Sigthorsson G, Bridger S, Foster R, Sherwood R, Fagerhol
M and Bjarnason I: A simple method for assessing intestinal
inflammation in Crohn's disease. Gut. 47:506–513. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Costa F, Mumolo MG, Bellini M, Romano MR,
Ceccarelli L, Arpe P, Sterpi C, Marchi S and Maltinti G: Role of
faecal calprotectin as non-invasive marker of intestinal
inflammation. Dig Liver Dis. 35:642–647. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
van Rheenen PF, Van de Vijver E and Fidler
V: Faecal calprotectin for screening of patients with suspected
inflammatory bowel disease: Diagnostic meta-analysis. BMJ.
341:c33692010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
von Roon AC, Karamountzos L, Purkayastha
S, Reese GE, Darzi AW, Teare JP, Paraskeva P and Tekkis PP:
Diagnostic precision of fecal calprotectin for inflammatory bowel
disease and colorectal malignancy. Am J Gastroenterol. 102:803–813.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Limburg PJ, Ahlquist DA, Sandborn WJ,
Mahoney DW, Devens ME, Harrington JJ and Zinsmeister AR: Fecal
calprotectin levels predict colorectal inflammation among patients
with chronic diarrhea referred for colonoscopy. Am J Gastroenterol.
95:2831–2837. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Costa F, Mumolo MG, Ceccarelli L, Bellini
M, Romano MR, Sterpi C, Ricchiuti A, Marchi S and Bottai M:
Calprotectin is a stronger predictive marker of relapse in
ulcerative colitis than in Crohn's disease. Gut. 54:364–368. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Røseth AG, Aadland E and Grzyb K:
Normalization of faecal calprotectin: A predictor of mucosal
healing in patients with inflammatory bowel disease. Scand J
Gastroenterol. 39:1017–1020. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bunn SK, Bisset WM, Main MJ, Gray ES,
Olson S and Golden BE: Fecal calprotectin: Validation as a
noninvasive measure of bowel inflammation in childhood inflammatory
bowel disease. J Pediatr Gastroenterol Nutr. 33:14–22. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Fireman Z, Mahajna E, Broide E, Shapiro M,
Fich L, Sternberg A, Kopelman Y and Scapa E: Diagnosing small bowel
Crohn's disease with wireless capsule endoscopy. Gut. 52:390–392.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Crama-Bohbouth G, Pena AS, Biemond I,
Verspaget HW, Blok D, Arndt JW, Weterman IT, Pauwels EK and Lamers
CB: Are activity indices helpful in assessing active intestinal
inflammation in Crohn's disease? Gut. 30:1236–1240. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Modigliani R, Mary JY, Simon JF, Cortot A,
Soule JC, Gendre JP and Rene E: Clinical, biological, and
endoscopic picture of attacks of Crohn's disease. Evolution on
prednisolone. Groupe d'Etude Thérapeutique des Affections
Inflammatoires Digestives. Gastroenterology. 98:811–818. 1990.
|
|
42
|
Cellier C, Sahmoud T, Froguel E, Adenis A,
Belaiche J, Bretagne JF, Florent C, Bouvry M, Mary JY and
Modigliani R: Correlations between clinical activity, endoscopic
severity, and biological parameters in colonic or ileocolonic
Crohn's disease. A prospective multicentre study of 121 cases. The
Groupe d'Etudes Thérapeutiques des Affections Inflammatoires
Digestives. Gut. 35:231–235. 1994.
|
|
43
|
Biancone L, De Nigris F, Del Vecchio
Blanco G, Monteleone I, Vavassori P, Geremia A and Pallone F:
Review article: Monitoring the activity of Crohn's disease. Aliment
Pharmacol Ther. 16 Suppl 4:29–33. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Tibble JA, Sigthorsson G, Bridger S,
Fagerhol MK and Bjarnason I: Surrogate markers of intestinal
inflammation are predictive of relapse in patients with
inflammatory bowel disease. Gastroenterology. 119:15–22. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Modigliani R: Endoscopic management of
inflammatory bowel disease. Am J Gastroenterol. 89:S53–S65.
1994.PubMed/NCBI
|