Introduction
Portal vein system thrombosis (PVST) refers to the
blood clots in portal vein, splenic and superior mesenteric veins
or intrahepatic portal vein branches, as they form an interactive
vascular system without valves (1).
The clinical manifestations of PVST include asymptomatic to
symptomatic fever, abdominal pain, nausea, vomiting and ileus
(2). If not treated appropriately and
in a timely fashion, PVST is potentially fatal by further
increasing portal vein pressure and deteriorating liver function,
which may increase the risk of upper gastrointestinal bleeding,
hepatic coma or even fatal intestinal necrosis (3). With the introduction of advanced image
devices, increasing numbers of studies have shown that the
incidence of PVST secondary to splenectomy is significantly higher
than previously reported (4). The
majority of previous studies of PVST have predominantly focused on
splenectomy to treat hematologic and metabolic disorders (5–7). A previous
study found that cirrhotic patients also have a high risk of
developing PVST after splenectomy, despite thrombocytopenia and a
prolonged prothrombin time (8).
Therefore, early prevention of PVST is considered to be important
for avoiding adverse consequence in these patients. However, the
role of early prophylactic anticoagulation in preventing PVST
remains controversial, due to concerns regarding the risk of
inducing bleeding, particularly in cirrhotic patients (9). By contrast, previous studies demonstrated
that both pro- and anticoagulation elements were concomitantly
reduced in liver cirrhosis, and an intricate balance of coagulation
was thereby maintained (10,11). Under such circumstances, the occurrence
of bleeding in cirrhotic patients was primarily due to the severity
of portal pressure, endothelial dysfunction and bacterial
infections, but not the disturbed hemostasis (12). Accordingly, the prophylactic
application of anticoagulation might be theoretically feasible for
patients subsequent to splenectomy, including cirrhotic patients.
There are previous studies that have identified that prophylactic
anticoagulation therapy effectively prevents PVST after
splenectomy. However, no standard regimen for PSVT prophylaxis has
been developed and, to the best of our knowledge, there have been
no systematic evaluations of the efficacy and safety of early
prophylactic anticoagulation for the prevention of PVST following
splenectomy.
Therefore, a systematic review and meta-analysis of
the available studies was conducted. The impact of prophylactic
anticoagulation on rates of PVST and anticoagulation-associated
complications following splenectomy was assessed. These data may
enable clinicians to establish effective methods to avoid this
potential, lethal complication.
Materials and methods
Data sources and searches
A search of the PubMed (http://www.ncbi.nlm.nih.gov/pubmed/), EMBASE
(www.embase.com), Springer (http://link.springer.com/), and Cochrane Library
databases (http://www.cochranelibrary.com/) was performed with
the following search terms as free-text terms, as well as MeSH
terms: ‘Portal vein thrombosis’, ‘portal venous thrombosis’,
‘splenectomy’, ‘anticoagulation’ and ‘anticoagulant’. The search
was performed on March 2016. A manual search of the references of
the relevant publications was also performed. No language
restrictions were imposed.
Selection criteria
Studies reporting the outcome of prophylactic
anticoagulation for the prevention of PVST subsequent to
splenectomy were potentially eligible. The exclusion criteria were
as follows: i) Duplicates; ii) review articles; iii) Case reports;
iv) animal studies; v) studies in which PVST developed in malignant
patients; and vi) studies not associated with the topics of
interest.
Data extraction
The data extracted include: The first author,
publication year, institution, study design, study period, study
population, type of surgery, number of patients receiving or not
receiving prophylactic anticoagulation, anticoagulation regimen
(type of anticoagulants, dose of anticoagulants and duration of
treatment), number of patients developing or not developing PVST
and number of patients developing complications associated with
anticoagulation.
Quality assessment
Two investigators independently read the titles and
abstracts of potential studies, then read the full texts to
identify eligible studies. The methodological quality of each
randomized controlled trial (RCT) was assessed according to the
Jadad score (13), including the
following there aspects: Randomization, double blinding, and
withdrawals and dropouts. Total scores of 0–2 were considered low
quality, whereas studies with total scores ≥3 were defined as high
quality. For each non-randomized study, the Newcastle-Ottawa Scale
(NOS) (14) was used for quality
assessment. A star system was used in which each study is judged on
three broad perspectives: Selection, comparability and exposure for
case-control studies. Studies with total stars ≥7 were defined as
high quality, 4–6 as medium and ≤4 as low for quality (15).
Statistical analysis
The meta-analysis was performed using the
statistical software RevMan 5.1 (The Cochrane Collaboration,
http://tech.cochrane.org/revman). Pooled
odds ratios (ORs) with 95% confidence interval (CI) were used as
the effect indicator for the dichotomous variables. P<0.05 was
considered to indicate a statistically significant difference
between the two groups. Heterogeneity in all of the included
studies was evaluated by χ2 and I2
statistical tests. A random effects model was adopted when
P<0.05 or I2>50%. Otherwise, the
fixed-effect model was used. Taking into account the presence of
non-randomized controlled trials (RCTs), a sensitivity analysis was
performed to compare the incidence of PVST formation and
anticoagulation-associated complications between patients receiving
and not receiving prophylactic anticoagulation. A funnel plot was
designed to establish the existence of publication bias.
Results
Search results and included
studies
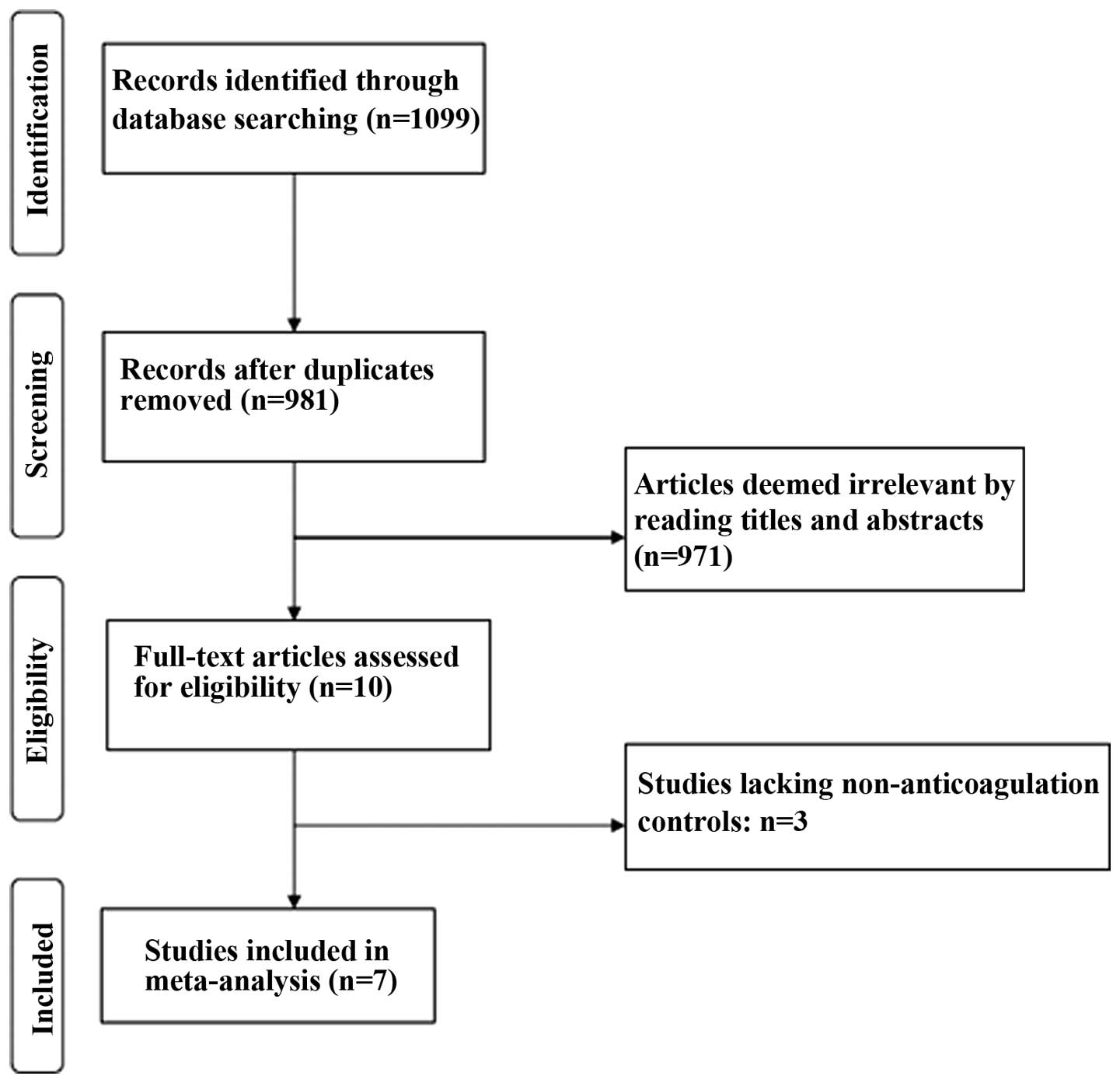
Overall, 1,099 potentially relevant articles were
retrieved according to the search strategy. Among these, 118 were
excluded due to duplication and 971 were excluded after reading the
title and abstract. Thus, 10 studies were potentially eligible for
this systematic review (Fig. 1).
However, three studies were further excluded due to insufficient
clinical data (lacking non-anticoagulation controls). Finally,
seven studies were included in this review involving 383 and 283
patients with and without regular prophylactic anticoagulation,
respectively (16–22) (Table I).
Although two of the studies (16,19) are from
a single institution, they are not duplicates.
 | Table I.Characteristics of the included
studies. |
Table I.
Characteristics of the included
studies.
|
| Subjects (n) | Anticoagulation
regimen |
|
|
|---|
|
|
|
|
|
|
|---|
| Author, year
(Refs.) | Institution | Study design | Study period | Study
population | Surgery | Prophylactic
anticoagulation | Control | Prophylactic
anticoagulation | Control | Jadad score | NOS |
|---|
| Kawanaka 2010
(16) | Japan,
single-center | Prospective | Jan 2005-July
2006 | Cirrhotic patients
with hypersplenism | LS | 25 | 25 | An intravenous
infusion of 1500 units of AT-III concentrates on POD 1, 2 and
3 | No prophylactic
therapy | – | 7 |
| Wang 2011 (17) | Canada,
single-center | RCT | Nov 2006-Nov
2008 | Patients with
hematological diseases or cirrhosis and hypersplenism | LS | 15 | 14 | 40 mg enoxaparin
subcutaneously once a day from POD 1 to 21 | No prophylactic
therapy | 5 | – |
| Lai 2012 (18) | China,
single-center | Retrospective | April 2004-July
2010 | Cirrhotic patients
with hypersplenism | Splenectomy with
gastroesophageal devascularization | 148 | 153 | Subcutaneous
injection of LMWH, 0.3 ml per 12 h from POD 1 to 5 and then
maintained by oral therapy with warfarin for one month | Irregular aspirin
or warfarin monotherapy for an undesignated time period | – | 6 |
| Kawanaka 2014
(19) | Japan,
single-center | Prospective | April 2008-March
2011 | Cirrhotic patients
with portal hypertension | LS | 37 | 16 | An intravenous
infusion of 1500 units of AT-III concentrates on POD 1, 2, and
3 | No prophylactic
therapy | – | 7 |
| Hongwei 2015
(20) | China,
single-center | Retrospective | Jan 2010-Dec
2013 | Cirrhotic patients
with portal hypertension | Splenectomy with or
without gastroesophageal devascularization | 90 | 46 | Within 12 h
postoperatively subcutaneous injection of 4,000 IU LMWH twice daily
for two weeks | No prophylactic
therapy | – | 6 |
| Svensson 2006
(21) | Sweden,
single-center | Retrospective | Jan 1999-Dec
2003 | Patients with
hematological diseases | LS or OS | 54 | 15 | Subcutaneous
injection of dalteparin 5,000 IU once or 2,500 IU twice daily,
median 7 d (range 1-30) | No prophylactic
therapy | – | 6 |
| Kakinoki 2013
(22) | Japan,
single-center | Prospective | Feb 2008-Apri
2010 | Cirrhotic patients
with portal hypertension | Hand-assisted
LS | 14 | 14 | An intravenous
infusion of Heparin 10,000 U/day, followed by warfarin started on
POD 1 or 2 | No prophylactic
therapy | – | 6 |
Of these seven studies, four were prospective,
including one RCT, and three were retrospective studies. The years
of publication spanned from 2006 to 2015. The included studies were
conducted in four countries: Three in Japan, two in China, one in
the Canada and one in Sweden. None of the seven studies was
considered low quality and the study characteristics are presented
in Table I.
Outcomes
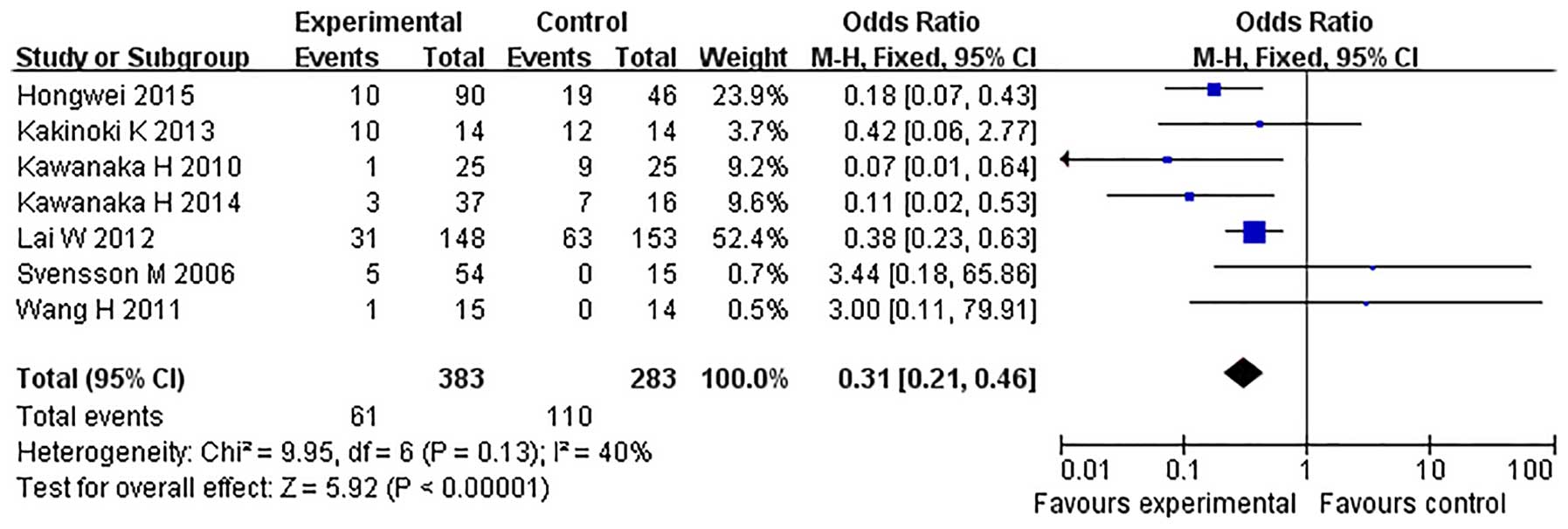
All seven studies reported the incidence of PVST
(16–22)
(Table II). PVST was diagnosed by
Doppler ultrasonography (US) in three studies and by Doppler US and
contrast-enhanced CT in the other four studies. The fixed-effects
model was adopted, as the heterogeneity analysis had not shown a
significant difference. The incidence of PVST was found to be
significantly reduced in the prophylactic anticoagulation group
compared with the no prophylactic therapy/irregular anticoagulation
group (OR=0.31, 95% CI, 0.21–0.46; P<0.00001) (Fig. 2). Due to heterogeneity in the study
design, sensitivity analyses were conducted using the four
prospective studies and the three retrospective studies. Evaluating
the four prospective studies (14,15,17,20), an
anticoagulation effect was also found, with an OR of 0.21 (95% CI,
0.08–0.52; P=0.0008) and no heterogeneity (χ2=4.53,
P=0.21, I2=34%) with the fixed effect model. For
the three retrospective studies (18,20,21), heterogeneity was identified
(χ2=4.65, P=0.10, I2=57%), thus a
random effects model was adopted and the anticoagulation effect was
also found, with an OR of 0.34 (95% CI, 0.14–0.82; P=0.02).
 | Table II.Primary outcomes of the included
studies. |
Table II.
Primary outcomes of the included
studies.
|
| Patients developing
PVST, n (%) | Patients developing
anticoagulation-associated complications, n (%) |
|---|
|
|
|
|
|---|
| Author, year
(ref.) | Prophylactic
anticoagulation | Control | Prophylactic
anticoagulation | Control |
|---|
| Kawanaka 2010
(16) | 1 (4%) | 9 (36%) | 0 (0%) | 0 (0%) |
| Wang 2011 (17) | 1 (6.67%) | 0 (0%) | 1 (6.67%) | 1 (7.14%) |
| Lai 2012 (18) | 31 (20.94%) | 63 (41.17%) | 2 (1.35%) | 1 (0.65%) |
| Kawanaka 2014
(19) | 3 (8.11%) | 7 (43.75%) | 0 (0%) | 0 (0%) |
| Hongwei 2015
(20) | 10 (11.11%) | 19 (41.30%) | 6 (6.67%) | 7 (15.22%) |
| Svensson 2006
(21) | 5 (9.26%) | 0 (0%) | N/A | N/A |
| Kakinoki 2013
(22) | 10 (71.43%) | 12 (85.71%) | N/A | N/A |
Sensitivity analyses were performed according to the
type of study population. In the subgroup analysis of two studies
predominantly including the patients with hematological diseases
(17,20), the incidence of PVST was not
significantly different between the prophylactic anticoagulation
group and the control group with an OR of 3.27 (95% CI, 0.36–29.57;
P=0.29) and no heterogeneity (χ2=0.00, P=0.95,
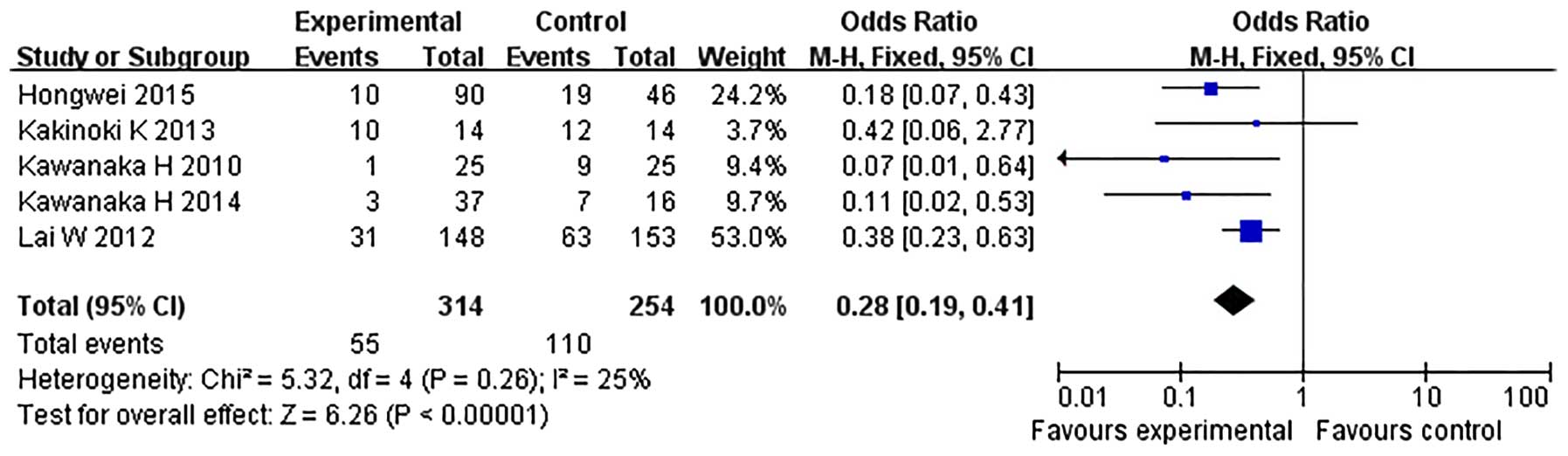
I2=0%) with the fixed-effect model. In the
subgroup analysis of five studies including cirrhotic patients with
hypersplenism, no heterogeneity was identified (χ2=5.32,
P=0.26, I2=25%), thus a fixed-effect model was
adopted and an anticoagulation effect was identified, with an OR of
0.28 (95% CI, 0.19–0.41; P<0.00001) (Fig. 3).
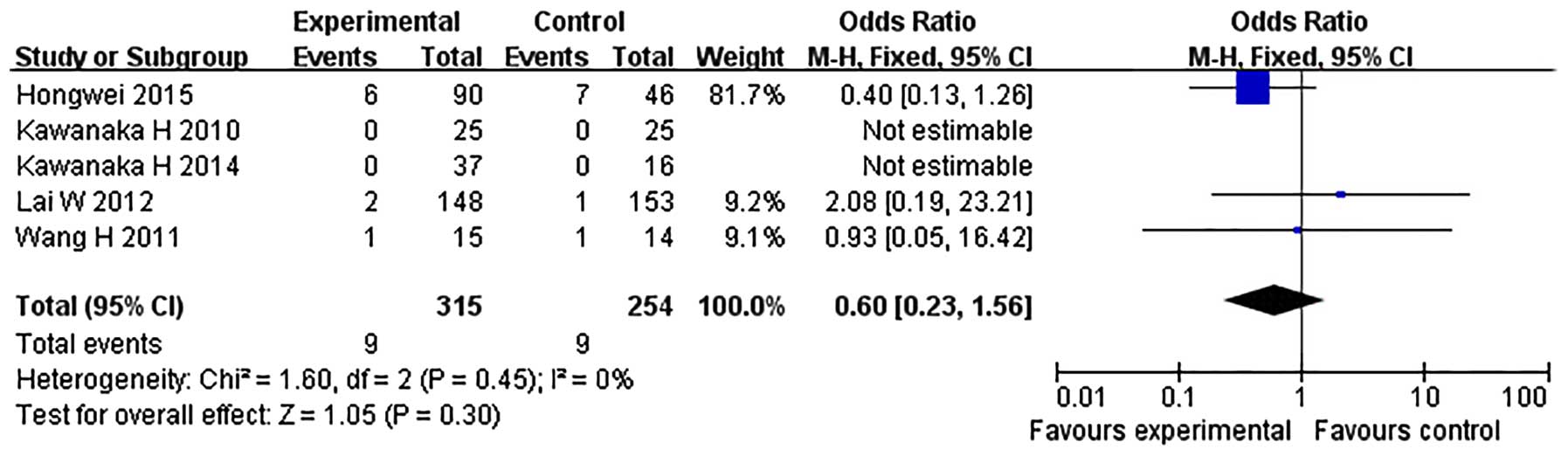
Among the seven studies, five provided the data
regarding the incidence of anticoagulation-associated complications
(Table II). During the anticoagulant
therapy, complications were observed in three studies (17,18,20), while no bleeding complications and
hepatic damage were observed in the other two (16,19). There
was no heterogeneity (χ2=1.60, P=0.45,
I2=0%) and a fixed-effect model was therefore
adopted. The meta-analysis showed that the incidence of
anticoagulation-associated complications was not significantly
different between the prophylactic anticoagulation group and the no
prophylactic therapy/irregular anticoagulation group (OR=0.60, 95%
CI, 0.23–1.56; P=0.30) (Fig. 4). Due
to heterogeneity in study design, sensitivity analyses were
conducted. Again, the complication rates were not identified to be
significantly different between the two groups using either
prospective studies or retrospective studies (data not shown).
Publication bias
Given the limited number of studies (n=7) in the
current meta-analysis, no formal steps were taken, such as Egger's
test, to determine publication bias.
Discussion
The reported incidence of PVST subsequent to
splenectomy differs markedly, ranging from 0.36% (4) to 80% (23).
There are various potential reasons for the inconsistency: i) The
lack of typical symptoms in a large majority of PVST patients
(24) results in diagnostic
difficulties; ii) the detection rate of PVST differs depending on
the examination methods (25); iii)
the reported incidence of PVST varies greatly between different
types of study. Previous studies, predominantly retrospective
studies, may inaccurately report the incidence of PVST. By
contrast, a number of prospective studies have reported a
relatively higher incidence of PVST following splenectomy (24–26); iv) the
incidence of PVST is also associated with the time and frequency of
postoperative examinations; v) underlying diseases are also an
important factor affecting the incidence. Patients with
hematological diseases have relatively higher risk of PVST
formation (25), whereas the risk is
minimal for autoimmune disease and traumatic splenectomy (26,27).
The detailed mechanisms for the formation of PVST
following splenectomy remain unclear. However, it is generally
hypothesized to be associated with local hypercoagulability
occurring in the portal vein system subsequent to surgery, which
may be attributed to soaring count and augmented aggregation
competence of platelets post-surgery (28). Hemodynamic changes of the portal venous
system may be another important reason for the formation of PVST.
In the splenic vein stump induced by ligation (25), blood turbulence or stasis results in
the deposition of blood cellular elements, which leads to the
development of splenic vein thrombosis and subsequently, the
development of portal and superior mesenteric vein thrombosis
(2). In addition, operative
manipulation, which may lead to serious damage of vascular
endothelial cells and trigger the coagulation system (3), and irrational use of coagulants (29) also contributes to the development of
PVST.
Although the prophylaxis of pulmonary embolism and
deep vein thrombosis has been relatively well established, the
prophylaxis of PVST following splenectomy remains controversial.
The primary concern is that prophylactic anticoagulation may induce
the anticoagulation-associated complications, typically associated
with bleeding. Currently, the management of PVST following
splenectomy is predominantly based on individual experience
(30) and a number of pilot studies
demonstrated the feasibility, safety, and efficacy of prophylactic
anticoagulation with decreased incidence of PVST and low rates of
bleeding complications when compared with controls (16–20).
However, due to the small number of cases in the individual
studies, more results with adequate power are required to confirm
these observations.
Recently, two meta-analyses regarding the role of
anticoagulation in PVST have been performed (31,32).
However, one meta-analysis (31)
focused on anticoagulation for the treatment of PVST, but not
prevention of PVST. In the other meta-analysis (32), certain studies that were included may
have been problematic: In the study by Ma et al (33), the authors compared different
anticoagulation methods (radix Salviae miliorrhazae plus
alprostadil vsersus radix Salviae miliorrhazae plus
aspirin), instead comparing anticoagulation with
non-anticoagulation. In the study by Xue et al (34), certain patients with high platelet
counts in the control group also received anticoagulation.
Therefore, it was considered necessary to perform the present
meta-analysis comparing early prophylactic anticoagulation with no
use of anticoagulation/irregular anticoagulation for the prevention
of PVST following splenectomy, using the latest data.
According to the inclusion criteria, seven clinical
studies were included in the present meta-analysis. In this
meta-analysis, the incidence of PVST was identified to be
significantly reduced in the prophylactic anticoagulation group
when compared with a no prophylactic therapy/irregular
anticoagulation group. In addition, the sensitivity analysis showed
the same effect in the prospective and retrospective studies,
respectively.
However, no significant difference was identified in
the only RCT study (17). One
explanation may be that this RCT is an underpowered study due to
the small sample size. Notably, the study population in this RCT
was predominantly patients with hematological diseases (25/29;
86.21%). Therefore, sensitivity analyses were conducted according
to the type of study population. The present results demonstrated
that the use of anticoagulants did not affect the incidence of PVST
subsequent to splenectomy in patients with hematological diseases.
However, the implementation of prophylactic anticoagulation
significantly reduced the incidence of PVST following splenectomy
in cirrhotic patients with hypersplenis, which indicates that
patients with hematological diseases may have a relatively lower
risk of PVST formation compared with cirrhotic patients, and
further multicenter clinical trials are required.
Another significant finding is that following
surgery, the incidence of anticoagulation-associated complications
was not significantly different between the prophylactic
anticoagulation group and the no prophylactic therapy/irregular
anticoagulation group. Wang et al (17) reported two patients, one in each group,
experienced bleeding complications and the two patients were
appropriately resuscitated. Anticoagulation was withheld
temporarily in the patient in the anticoagulation group, and there
were no long-term consequences. Lai et al (18) reported mild gastrointestinal bleeding
in one patient in the anticoagulation group and in two in the
control group. The anticoagulant therapy was terminated immediately
and hemostatic agents were administered. Bleeding was successfully
controlled and all patients recovered well. Hongwei et al
(20) reported two cases of pancreatic
leakage, three instances of subphrentic infection, and one case of
surgical site bleeding in the anticoagulation group, whereas in the
control group there were three, two and two cases, respectively.
However, the authors did not describe methods to treat the
complications. In the other four studies, no
anticoagulation-associated complications were observed (16,19) or there
was no relevant data available (21,22). These
results strengthened the confidence that prophylactic
anticoagulation may be safely administered subsequent to
splenectomy, even for cirrhotic patients.
There are potential limitations of the current
meta-analysis, which may increase the possibility of publication
bias and affect the final result. First, this meta-analysis
contained only seven studies and the number of cases was limited
for this particular subject. In addition, only one RCT was included
in this meta-analysis and the other non-RCT studies may have
resulted in the unbalanced selection of patients. Furthermore, the
study population, study design, surgical procedure, and
anticoagulation regimens were variable and the heterogeneities were
correlated with the habits and preferences of individual
institutions. Finally, whether the selection of patients diagnosed
with liver cirrhosis were treated or not treated with anticoagulant
therapy was influenced by other factors, including severity of
liver disease, esophageal varices and previous bleeding.
In conclusion, this meta-analysis demonstrates that
early prophylactic anticoagulation is associated with a reduced
incidence of PVST following splenectomy and is not associated with
the incidence of anticoagulation-associated complications. However,
due to the limitations of this analysis, further large multicenter
RCTs are required to confirm this conclusion.
References
|
1
|
Parikh S, Shah R and Kapoor P: Portal vein
thrombosis. Am J Med. 123:111–119. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rattner DW, Ellman L and Warshaw AL:
Portal vein thrombosis after elective splenectomy. An
underappreciated, potentially lethal syndrome. Arch Surg.
128:565–570. 1993.
|
|
3
|
Stamou KM, Toutouzas KG, Kekis PB, Nakos
S, Gafou A, Manouras A, Krespis E, Katsaragakis S and Bramis J:
Prospective study of the incidence and risk factors of
postsplenectomy thrombosis of the portal, mesenteric and splenic
veins. Arch Surg. 141:663–669. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Delaitre B, Champault G, Barrat C, Gossot
D, Bresler L, Meyer C, Collet D and Samama G: Laparoscopic
splenectomy for hematologic diseases. Study of 275 cases. French
society of laparoscopic surgery. Ann Chir. 125:522–529. 2000.(In
French).
|
|
5
|
Mesa RA, Nagorney DS, Schwager S, Allred J
and Tefferi A: Palliative goals, patient selection and
perioperative platelet management: Outcomes and lessons from 3
decades of splenectomy for myelofibrosis with myeloid metaplasia at
the Mayo Clinic. Cancer. 107:361–370. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
van't Riet M, Burger JW, van Muiswinkel
JM, Kazemier G, Schipperus MR and Bonjer HJ: Diagnosis and
treatment of portal vein thrombosis following splenectomy. Br J
Surg. 87:1229–1233. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Winslow ER, Brunt LM, Drebin JA, Soper NJ
and Klingensmith ME: Portal vein thrombosis after splenectomy. Am J
Surg. 184:631–636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kinjo N, Kawanaka H, Akahoshi T, Tomikawa
M, Yamashita N, Konishi K, Tanoue K, Shirabe K, Hashizume M and
Maehara Y: Risk factors for portal venous thrombosis after
splenectomy in patients with cirrhosis and portal hypertension. Br
J Surg. 97:910–916. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Northup PG and Intagliata NM:
Anticoagulation in cirrhosis patients: What don't we know? Liver
Int. 31:4–6. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tripodi A, Primignani M, Chantarangkul V,
Dell'Era A, Clerici M, de Franchis R, Colombo M and Mannucci PM: An
imbalance of pro- vs anti-coagulation factors in plasma from
patients with cirrhosis. Gastroenterology. 137:2105–2111. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tripodi A and Mannucci PM: The
coagulopathy of chronic liver disease. N Engl J Med. 365:147–156.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tripodi A: The coagulopathy of chronic
liver disease: Is there a causal relationship with bleeding? No.
Eur J Intern Med. 21:65–69. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jadad AR, Moore RA, Carroll D, Jenkinson
C, Reynolds DJ, Gavaghan DJ and McQuay HJ: Assessing the quality of
reports of randomized clinical trials: Is blinding necessary?
Control Clin Trials. 17:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wells G, Shea B, O'Connell D, et al: The
Newcastle-Ottawa Scale (NOS) for assessing the quality of
nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.aspJanuary
26–2016
|
|
15
|
Wang JZ, Liu Y, Wang JL, Lu L, Zhang YF,
Lu HW and Li YM: Sequential vs simultaneous revascularization in
patients undergoing liver transplantation: A meta-analysis. World J
Gastroenterol. 21:7036–7046. 2015.PubMed/NCBI
|
|
16
|
Kawanaka H, Akahoshi T, Kinjo N, Konishi
K, Yoshida D, Anegawa G, Yamaguchi S, Uehara H, Hashimoto N,
Tsutsumi N, et al: Impact of antithrombin III concentrates on
portal vein thrombosis after splenectomy in patients with liver
cirrhosis and hypersplenism. Ann Surg. 251:76–83. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Kopac D, Brisebois R, Sample C and
Shapiro AM: Randomized controlled trial to investigate the impact
of anticoagulation on the incidence of splenic or portal vein
thrombosis after laparoscopic splenectomy. Can J Surg. 54:227–231.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lai W, Lu SC, Li GY, Li CY, Wu JS, Guo QL,
Wang ML and Li N: Anticoagulation therapy prevents portal-splenic
vein thrombosis after splenectomy with gastroesophageal
devascularization. World J Gastroenterol. 18:3443–3450. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kawanaka H, Akahoshi T, Itoh S, Iguchi T,
Harimoto N, Uchiyama H, Yoshizumi T, Shirabe K, Takenaka K and
Maehara Y: Optimizing risk stratification in portal vein thrombosis
after splenectomy and its primary prophylaxis with antithrombin III
concentrates and danaparoid sodium in liver cirrhosis with portal
hypertension. J Am Coll Surg. 219:865–874. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hongwei C, Zhang L, Maoping L, Yong Z,
Chengyou D and Dewei L: Era of liver transplantation: Combined
anatomic splenectomy and anticoagulant therapy in prevention of
portal vein thrombosis after splenectomy. Hepatogastroenterology.
62:405–409. 2015.PubMed/NCBI
|
|
21
|
Svensson M, Wirén M, Kimby E and Hägglund
H: Portal vein thrombosis is a common complication following
splenectomy in patients with malignant haematological diseases. Eur
J Haematol. 77:203–209. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kakinoki K, Okano K, Suto H, Oshima M,
Hagiike M, Usuki H, Deguchi A, Masaki T and Suzuki Y: Hand-assisted
laparoscopic splenectomy for thrombocytopenia in patients with
cirrhosis. Surg Today. 4:883–888. 2013. View Article : Google Scholar
|
|
23
|
Romano F, Caprotti R, Conti M, Piacentini
MG and Uggeri F, Motta V, Pogliani EM and Uggeri F: Thrombosis of
the splenoportal axis after splenectomy. Langenbecks Arch Surg.
391:483–488. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Krauth MT, Lechner K, Neugebauer EA and
Pabinger I: The postoperative splenic/portal vein thrombosis after
splenectomy and its prevention-an unresolved issue. Haematologica.
93:1227–1232. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ikeda M, Sekimoto M, Takiguchi S, Kubota
M, Ikenaga M, Yamamoto H, Fujiwara Y, Ohue M, Yasuda T, Imamura H,
et al: High incidence of thrombosis of the portal venous system
after laparoscopic splenectomy: A prospective study with
contrast-enhanced CT scan. Ann Surg. 241:208–216. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chaffanjon PC, Brichon PY, Ranchoup Y,
Gressin R and Sotto JJ: Portal vein thrombosis following
splenectomy for hematologic disease: Prospective study with Doppler
color flow imaging. World J Surg. 22:1082–1086. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bach AM, Hann LE, Brown KT, Getrajdman GI,
Herman SK, Fong Y and Blumgart LH: Portal vein evaluation with US:
Comparison to angiography combined with CT arterial portography.
Radiology. 201:149–154. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Francoz C, Valla D and Durand F: Portal
vein thrombosis, cirrhosis, and liver transplantation. J Hepatol.
57:203–212. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Soyer T, Ciftci AO, Tanyel FC, Senocak ME
and Büyükpamukçu N: Portal vein thrombosis after splenectomy in
pediatric hematologic disease: Risk factors, clinical features, and
outcome. J Pediatr Surg. 41:1899–1902. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li MX, Zhang XF, Liu ZW and Lv Y: Risk
factors and clinical characteristics of portal vein thrombosis
after splenectomy in patients with liver cirrhosis. Hepatobiliary
Pancreat Dis Int. 12:512–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Qi X, De Stefano V, Li H, Dai J, Guo X and
Fan D: Anticoagulation for the treatment of portal vein thrombosis
in liver cirrhosis: A systematic review and meta-analysis of
observational studies. Eur J Intern Med. 26:23–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Qi X, Bai M, Guo X and Fan D:
Pharmacologic prophylaxis of portal venous system thrombosis after
splenectomy: A meta-analysis. Gastroenterol Res Pract.
2014:2926892014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ma JC, Zhao J, Su QH, Zhang DH, Guo Y and
Ji ZZ: Effects of alprostadil in prevention of portal vein
thrombogenesis after splenectomy and devascularization: A clinical
observation of 76 patients with portal hypertension. Zhonghua Yi
Xue Za Zhi. 88:524–526. 2008.(In Chinese). PubMed/NCBI
|
|
34
|
Xue H, Zhang H, Zhang Y and Jiang Q:
Portal anticoagulation in preventing thrombosis after porta-azygous
devascularization for portal hypertension. Zhonghua Wai Ke Za Zhi.
38:855–857. 2000.(In Chinese). PubMed/NCBI
|