Introduction
Simvastatin (Sim) is a drug widely used for the
treatment of cardiovascular disease (CVD) (1,2). Sim acts as
an inhibitor of 3-hydroxy-methylglutaryl coenzyme A reductase,
which is a rate-determining enzyme in the biosynthesis of
cholesterol, and reduces the plasma levels of low-density
lipoprotein (LDL) (3). Clinical
studies have shown that treatment with Sim markedly decreased the
incidence of cardiovascular events (4). While the lipid-lowering effect is a major
mechanism of action of Sim against CVD, increasing evidence has
demonstrated that other mechanisms are involved, including
reduction of oxidative stress and vascular inflammation,
improvement of endothelial function, and enhancement of the
stability of atherosclerotic plaques (5). In addition, independent of its
lipid-lowering properties, Sim induced vascular relaxation in the
aorta and inferior mesenteric artery of rats (2). Moreover, Sim protected the vascular
endothelium against damage induced by LDL or oxidized LDL, and
relaxed the thoracic aorta in rats (6). However, the underlying mechanisms have
remained to be fully elucidated.
Therefore, the present study was designed to explore
the mechanisms by which Sim induces relaxation in the superior
mesenteric artery of rats. It enhanced the current knowledge on the
underlying mechanisms to contribute to the further development of
cardiovascular drugs.
Materials and methods
Reagents
Phenylephrine hydrochloride (PE), acetylcholine
chloride (ACh), Nω-nitro-L-arginine methyl ester (L-NAME),
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one (ODQ), indomethacin
(Indo), 4-aminopyridine (4-AP), barium chloride dehydrate
(BaCl2), glibenclamide (Gli), tetraethylammonium
chloride (TEA), Triton X-100 and Sim were obtained from
Sigma-Aldrich (St. Louis, MO, USA). ODQ, TEA, Gli, 4-AP and Sim
were dissolved in Dimethylsulfoxide. All other compounds were
dissolved in distilled water.
In vitro pharmacology
Thirty Sprague-Dawley rats (male, 8 weeks old,
300–350 g), which were obtained from the Animal Center of Xi'an
Jiaotong University (Xi'an, China), were sacrificed by
CO2 inhalation. The superior mesenteric artery was
gently removed and freed from adhering tissue under a dissecting
microscope. The endothelium was denuded by perfusion of the vessel
with on Triton X-100 (0.1%, v/v) for 10 sec, followed by
physiologic saline solution (PSS; NaCl 119 mM, KCl 4.6 mM,
NaHCO3 15 mM, NaH2PO4 1.2 mM,
MgCl2 1.2 mM, CaCl2 1.5 mM and glucose 5.5
mM) for another 10 sec. The vessels were then cut into cylindrical
segments of 1–3 mm in length. The segments were immersed in
individual baths containing PSS (5 ml) in a temperature-controlled
(37°C) myograph (Danish Myo Technology A/S, Aarhus, Denmark). The
solution was continuously aerated with gas (containing 5%
CO2 and 95% O2), resulting in a pH of 7.4.
Following mounting of the arterial segments, isometric tension was
continuously recorded of using Chart software (ADInstruments,
Oxford, UK). The segments were allowed to stabilize at a resting
tone of 2 mN for at least 1.5 h, followed by immersion in a
K+-rich (60 mM) buffer solution with the same
composition as the standard solution, except for NaCl being
replaced by KCl to reach a final K+ concentration of 60
mM (KPSS). The potassium-induced contraction was used as a
reference for contractile capacity, and only the segments which
showed reproducible responses over 1.0 mN to potassium were used.
In another group, PE (10 µM) was used instead of KPSS. After a
sustained tension was obtained, Sim
(10−10-10−5 M) was cumulatively added to the
baths and concentration-response curves to Sim were
constructed.
In the experiment involving endothelium, Ach (10 µM)
was added after pre-contraction with KPSS to test the completeness
of endothelium denudation. An effective functional removal of the
endothelium was indicated by absence of relaxation in response to
Ach. The rings with endothelium showing <30% relaxation in
response to Ach were discarded (7).
Furthermore, the artery rings with endothelium were pre-incubated
with the cyclooxygenase inhibitor Indo (5 µM), the guanylate
cyclase inhibitor ODQ (10 µM), the endothelial nitric oxide (NO)
synthase (eNOS) inhibitor L-NAME (100 μM), or with the with the
K+ channel blockers 4-AP (100 µM), BaCl2 (10
µM), Gli (10 µM) or TEA (1 mM), respectively, for 20 min prior to
addition of KCl (60 mM), followed by cumulative addition of
Sim.
A further experiment was performed in the absence of
Ca2+, for which the rings were washed with
Ca2+-free PSS. After incubation with or without Sim (10
µM) for 20 min in Ca2+-free PSS, PE (10 µM) was added to
stimulate the release of intracellular Ca2+ and the
contraction was recorded (8). In
another experiment, the rings were washed with Ca2+-free
PSS containing ethylene glycol-bis(β-aminoethyl
ether)-N,N,N',N'-tetraacetic acid (EGTA; 100 µM; Sigma-Aldrich) and
then rinsed with Ca2+-free PSS (without EGTA) containing
KCl (60 mM K+). After incubation with or without Sim (10
µM) for 20 min, CaCl2 (2 mM) was added to contract the
artery rings (8).
All procedures involving animals were performed
according to the Guide for the Care and Use of Laboratory Animals
Published by the US National Institutes of Health (Publication no.
85–23, revised 1996) and the Guidelines for Animal Experimentation
of Xi'an Medical University (Xi'an, China). The experimental
protocols of the present study were approved by the Laboratory
Animal Administration Committee of Xi'an Medical University (Xi'an,
China).
Statistical analysis
Values are expressed as the mean ± standard error of
the mean. The effects of Sim are expressed as the percentage of
relaxation with regard to the pre-contraction. For each agent, the
negative logarithm of the concentration that caused 50% of the
maximum response (pD2) and the maximum relaxation
(Emax%) were calculated. The unpaired Student's t-test
was used to assess differences between groups. P<0.05 was
considered to indicate a statistically significant difference
between groups. The analysis was performed using the SPSS 16.0
software (SPSS Inc., Chicago, IL, USA).
Results
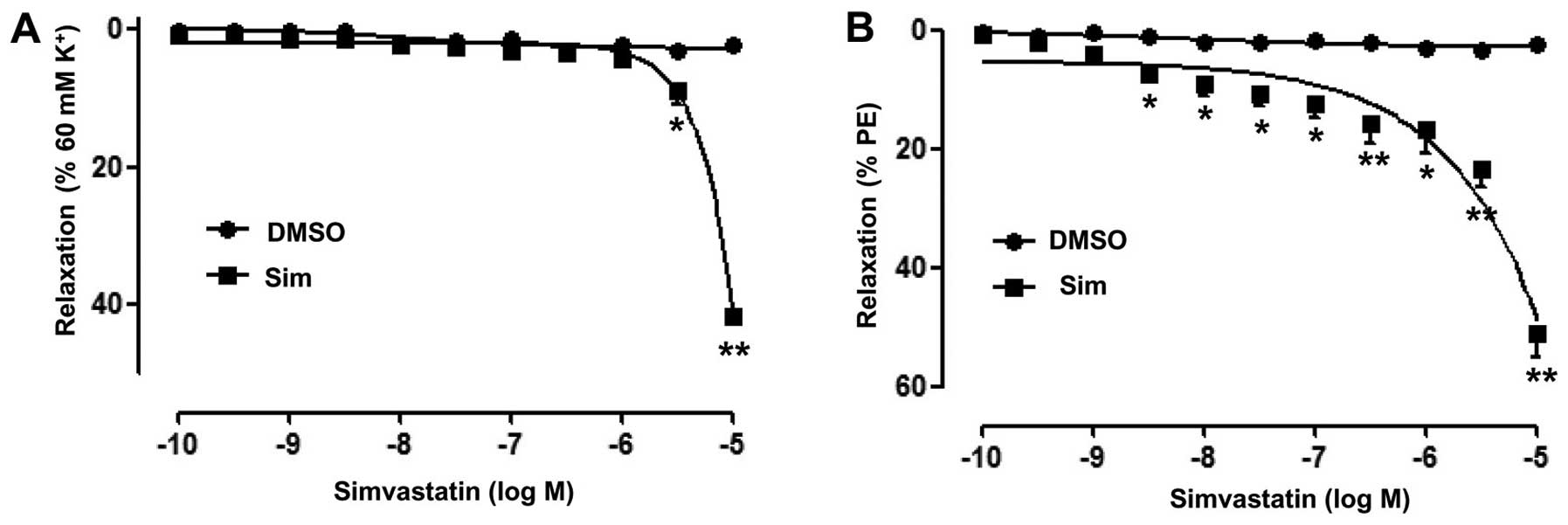
Simrelaxes rat superior mesenteric
arteries pre-constricted by PE or KCl
In order to evaluate the vasodilative effects of
Sim, the superior mesenteric artery rings of rats were
pre-contracted with PE (10 µM) or KCl (60 mM), and once a plateau
was attained, concentration-response curves were obtained by adding
cumulative doses of Sim to the bath. The results showed that Sim
concentration-dependently relaxed the superior mesenteric artery
rings with endothelium pre-contracted by PE
[Emax=51.05±4.09% (Sim, 10−5 M);
pD2=4.17±0.18] or KCl [Emax=41.65±1.32% (Sim,
10−5 M); pD2=3.55±0.10] (Fig. 1A and B).
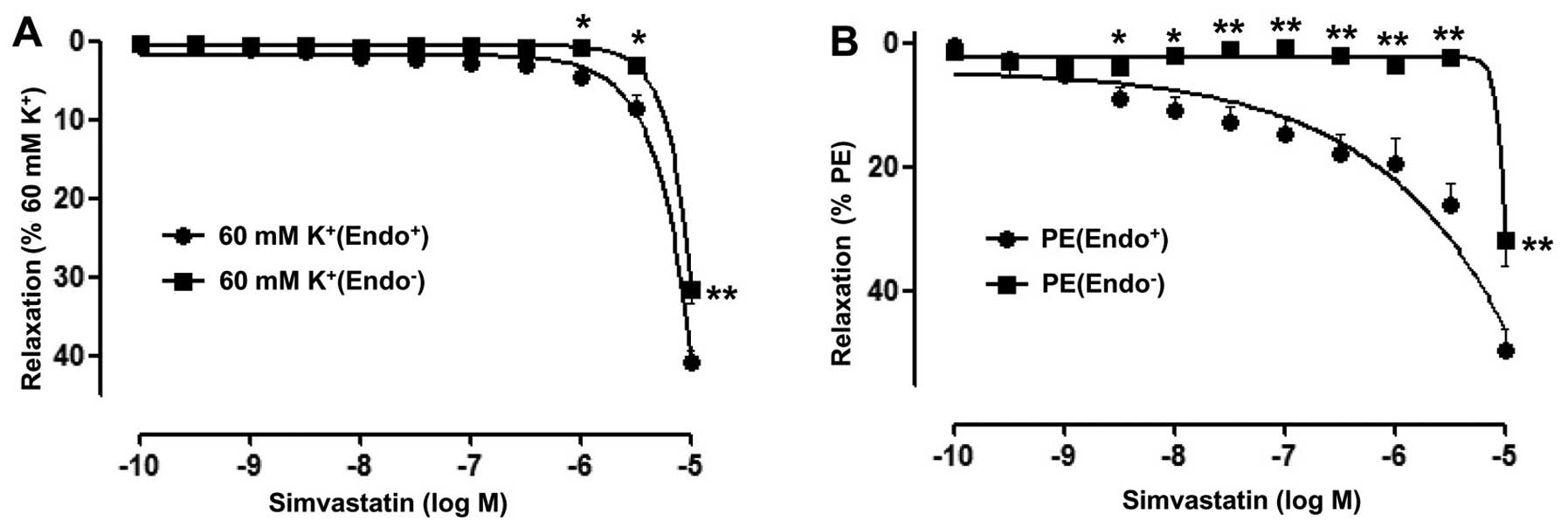
Role of the endothelium in Sim-induced
relaxation of rat superior mesenteric arteries
The vasorelaxant effects of Sim on superior
mesenteric artery rings with endothelium pre-contracted by PE (10
µM) were significantly stronger than those on artery rings without
endothelium, with Emax=49.55±3.67 vs. 31.82±4.02% and
pD2=4.21±0.15 vs. 0.35±0.15 (P<0.01). Moreover,
vasorelaxation induced by Sim in artery rings with endothelium
pre-contracted by KCl (60 mM) also was significantly stronger than
in artery rings without endothelium (Emax=40.79±1.49 vs.
31.68±1.76% and pD2=3.56±0.09 vs. 3.57±0.08; P<0.01),
while the effects were more marked in artery rings pre-contracted
by PE (Fig. 2A and B).
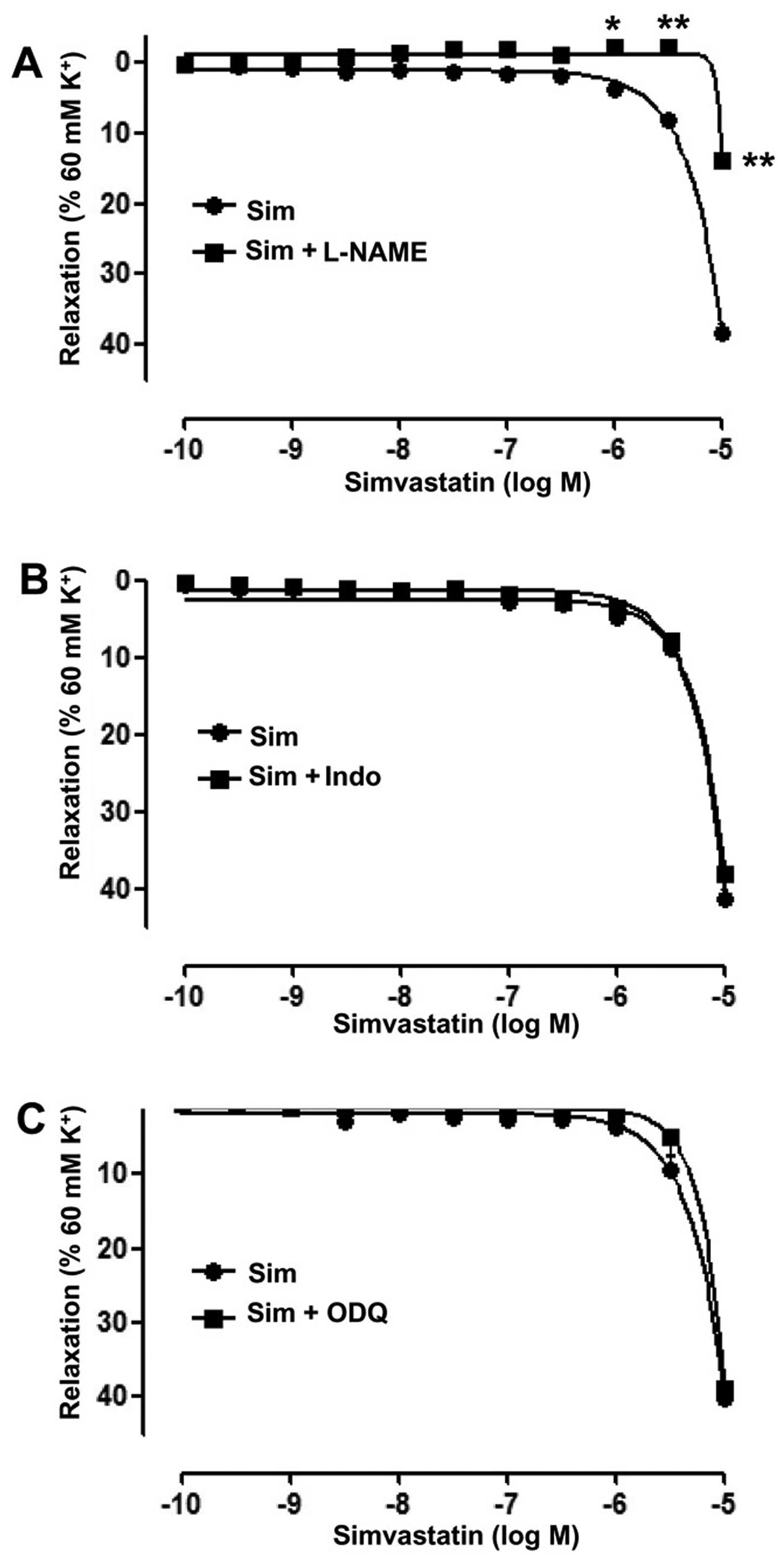
To identify endothelial mediators associated with
the vasodilative effects of Sim, the cyclooxygenase inhibitor Indo
(5 µM), the guanylate cyclase inhibitor ODQ (10 µM) and the eNOS
inhibitor L-NAME (100 µM) were used, respectively. The results
showed that in the artery rings with endothelium, L-NAME
significantly inhibited the vasodilative effect of Sim,
(Emax=13.72±1.12 vs. 38.46±1.36%;
pD2=1.22±0.18 vs. 3.72±0.09; P<0.01) (Fig. 3A). However, ODQ and Indo did not
significantly affect the relaxation induced by Sim in the artery
rings with endothelium (Fig. 3B and
C).
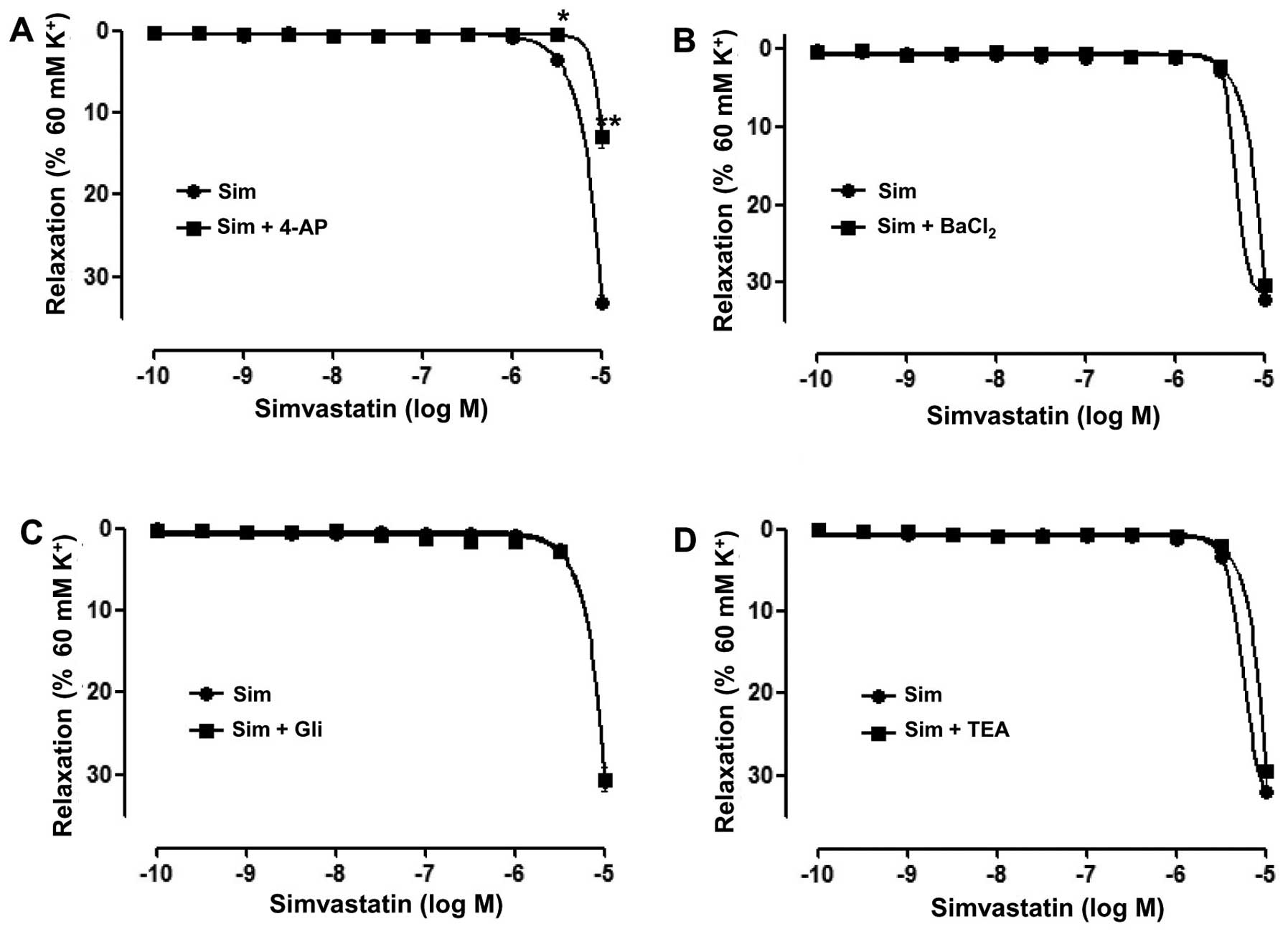
Role of K+ channels in
Sim-induced relaxation of rat superior mesenteric arteries
pre-constricted by KCl
To assess the role of K+ channels in
Sim-induced vasorelaxation, artery rings ithout endothelium were
pre-incubated with the K+ channel blockers 4-AP (100
µM), BaCl2 (10 µM), Gli (10 µM) or TEA (1 mM) for 20 min
prior to addition of KCl (60 mM), following which Sim was added
cumulatively. The results showed that 4-AP significantly reduced
the relaxation induced by Sim in the artery rings without
endothelium (Emax=13.02±1.24 vs. 33.08±0.91% and
pD2=1.36±0.28 vs. 3.77±0.28; P<0.01) (Fig. 4A). However, BaCl2, Gli and
TEA did not significantly affect the relaxation induced by Sim in
the artery rings without endothelium (Fig.
4B-D).
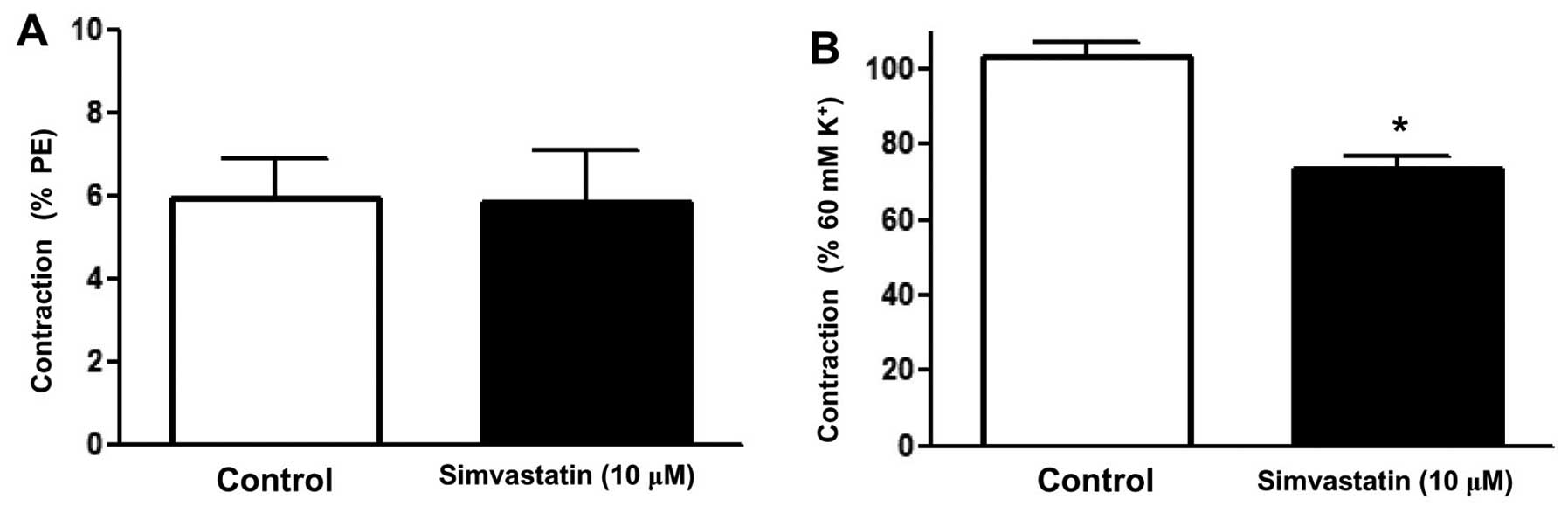
Effect of Sim on the calcium release
by the sarcoplasmic reticulum in rat superior mesenteric arteries
pre-constricted by PE
To clarify whether the relaxation induced by Sim was
associated with intracellular Ca2+ release, an
experiment in Ca2+-free PSS was performed. After
incubation with or without Sim (10 µM) for 20 min, PE (10 µM) was
added to stimulate the release of intracellular Ca2+ and
the contraction was recorded (8). The
results showed that PE induced a transient contraction due to the
release of intracellular Ca2+ into the
Ca2+-free solution, while Sim did not attenuate this
contraction (Emax=5.84±1.25 vs. 5.93±0.97%) (Fig. 5A).
Effect of Sim on extracellular
Ca2+-induced contraction activated in rat superior
mesenteric arteries pre-constricted by KCl
To determine whether the inhibition of extracellular
Ca2+ influx affected the relaxation induced by Sim, an
experiment was performed in Ca2+-free PSS. Following
immersion in Ca2+-free PSS containing KCl (60 mM), the
rings were incubated with or without Sim (10 µM) for 20 min,
followed by contraction of the artery rings by addition of
CaCl2 (2 mM) (8). The
results showed that Sim significantly attenuated the contraction
induced by addition of CaCl2 to the Ca2+-free
PSS plus KCl (Emax=73.77±2.8 vs. 102.94±3.98%) (Fig. 5B). It was therefore suggested that Sim
inhibits Ca2+ influx in the superior mesenteric
artery.
Discussion
The present study revealed that Sim
concentration-dependently relaxed the superior mesenteric artery
rings with or without endothelium pre-contracted by PE or KCl. The
results suggested that Sim exerted its vasorelaxation effects via
endothelium-dependent and -independent pathways. Moreover, the
vasorelaxation induced by Sim was inhibited by L-NAME, while it was
not affected by ODQ and Indo in artery rings with endothelium. In
addition, Sim-induced vasorelaxation was inhibited by 4-AP, while
it was not affected by Gli, BaCl2 and TEA in artery
rings without endothelium. Finally, the vasorelaxation induced by
Sim was shown to be mediated through blockade of Ca2+
influx from extracellular medium.
Vascular endothelium located between vascular smooth
muscle and circulating blood is known to be important in regulation
of vascular tone. Vasorelaxation is mediated by vasorelaxant
substances synthesized and released into the endothelium (9). In the present study, the relaxant effect
induced by Sim was attenuated in the superior mesenteric artery
rings without endothelium, suggesting that Sim also relaxes
arteries through an endothelium-dependent pathway. Furthermore, the
eNOS inhibitor L-NAME significantly reduced the vasorelaxation
induced by Sim. However, cyclooxygenase inhibitor Indo and
guanylate cyclase inhibitor ODQ did not affect the action of Sim.
These results suggested that NO is involved in the relaxation of
Sim in the superior mesenteric artery with endothelium, whereas the
effect was not attributed to or prostanoids (Indo inhibits
prostaglandin-endoperoxide synthase) or the cyclic guanosine
monophosphate pathway. In accordance with the results of the
present study, a previous study reported that in the aorta and
small mesenteric artery, the efficacy of Sim was closely associated
with the NO system in endothelial cells (2). However, the study also identified an
association with prostanoids, which may therefore require
clarification by further studies.
The present study further revealed that Sim also
exerted vasorelaxant effect in superior mesenteric arteries without
endothelium, suggesting that Sim has a direct effect on vascular
smooth muscle cells (VSMCs). The opening of K+ channels
in these cells causes hyperpolarization of the membrane potential
and a decreased Ca2+ influx through voltage-operated
Ca2+ channels, resulting in vasorelaxation (10,11). Several
types of K+ channel have been identified in vascular
smooth muscle, the most abundant ones being the large conductance
Ca2+-activated K+ channel, the
voltage-sensitive K+ channel, the adenosine triphosphate
(ATP)-sensitive K+ channel and inward-rectifyer
K+ channels. In order to detect the contribution of
different types of K+ channel to endothelium-independent
relaxation induced by Sim in superior mesenteric artery rings,
various K+ channel-blocking agents were used, including
the voltage-dependent K+ channel blocker 4-AP,
inward-rectifying potassium channel blocker BaCl2,
ATP-sensitive K+ channel blocker Gli and
Ca2+-activated K+ channel blocker TEA
(12,13). The results revealed that 4-AP
significantly inhibited the effect of Sim, indicating that the
voltage-dependent K+ channel was involved in the
mechanism of the vasorelaxant action of Sim. However,
BaCl2, Gli and TEA did not affect the
concentration-response curves of Sim, suggesting that the
inward-rectifyer, ATP-sensitive and Ca2+-activated
K+ channels were not involved in Sim-mediated
vasorelaxation.
Accumulation of intracellular calcium is associated
with vascular smooth muscle contraction. Moreover, intracellular
calcium levels may increase via extracellular Ca2+
influx through the receptor-operated or voltage-dependent calcium
channels, as well as intracellular Ca2+ release
(14). Contractions of VSMCs induced
by KCl almost exclusively rely on Ca2+ influx through
activation of voltage-sensitive channels (15), whereas PE-induced contractions are
mediated via increasing the Ca2+ influx through
receptor-operated (16) as well as
voltage-sensitive channels (17). The
results of the present study showed that Sim inhibited the
contractile effects induced by PE or KCl on the superior mesenteric
artery without endothelium, suggesting that Sim may interfere with
receptor-operated as well as voltage-sensitive potassium channels.
The release of intracellular stored Ca2+ is mainly
regulated by the ryanodine and inositol triphosphate (IP3) receptor
systems. In Ca2+-free medium, PE induces vascular
contraction via inducing intracellular Ca2+ release
through sarcoplasmic reticulum Ca2+ channels activated
by IP3 (18). In the present study,
Sim was shown to significantly inhibit CaCl2-induced
contraction of superior mesenteric artery rings without endothelium
in Ca2+-free PSS containing KCl (60 mM), indicating that
Sim is able to block Ca2+ influx. However, Sim did not
inhibit the contraction triggered by PE in Ca2+-free
PSS, suggesting that Sim does not affect Ca2+
mobilization from intracellular stores. It can therefore be
concluded that in the superior mesenteric artery, Sim induces
vasorelaxation via inhibition of extracellular calcium influx into
VSMCs.
In conclusion, the results of the present study
suggested that Sim induced relaxation of superior mesenteric
arteries of rats through an endothelium-dependent pathway involving
NO release, as well as an endothelium-independent pathway, opening
of voltage-dependent K+ channels and blockade of
extracellular Ca2+ influx. These findings may support
the further development of treatments for CVD.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (no. 81500350), the China
Postdoctoral Science Foundation (no. 2015M582607) and the Shaanxi
Postdoctoral Sustentation Fund, China (2015, second fund, no.
59).
Glossary
Abbreviations
Abbreviations:
|
PE
|
phenylephrine hydrochloride
|
|
Ach
|
acetylcholine chloride
|
|
4-AP
|
4-aminopyridine
|
|
eNOS
|
endothelial nitric oxide synthase
|
|
Gli
|
glibenclamide
|
|
Indo
|
indomethacin
|
|
BaCl2
|
barium chloride dehydrate
|
|
L-NAME
|
Nω-nitro-L-arginine methyl ester
|
|
NO
|
nitric oxide
|
|
ODQ
|
1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin-1-one
|
|
Sim
|
simvastatin
|
|
TEA
|
tetraethylammonium chloride
|
|
VSMCs
|
vascular smooth muscle cells
|
References
|
1
|
Pedersen TR, Wilhelmsen L, Faergeman O,
Strandberg TE, Thorgeirsson G, Troedsson L, Kristianson J, Berg K,
Cook TJ, Haghfelt T, et al: Follow-up study of patients randomized
in the Scandinavian simvastatin survival study (4S) of cholesterol
lowering. Am J Cardiol. 86:257–262. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
De Sotomayor M Alvarez, Herrera MD,
Marhuenda E and Andriantsitohaina R: Characterization of
endothelial factors involved in the vasodilatory effect of
simvastatin in aorta and small mesenteric artery of the rat. Br J
Pharmacol. 131:1179–1187. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mauro VF and MacDonald JL: Simvastatin: A
review of its pharmacology and clinical use. DICP. 25:257–264.
1991.PubMed/NCBI
|
|
4
|
Gryn SE and Hegele RA: Ezetimibe plus
simvastatin for the treatment of hypercholesterolemia. Expert Opin
Pharmacother. 16:1255–1262. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson JG: Simvastatin: Present and
future perspectives. Expert Opin Pharmacother. 8:2159–27. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang JL, Jiang DJ, Tang YH, Li NS, Deng
HW and Li YJ: Effect of simvastatin on endothelium-dependent
vaso-relaxation and endogenous nitric oxide synthase inhibitor.
Acta Pharmacol Sin. 25:893–901. 2004.PubMed/NCBI
|
|
7
|
Cao YX, Zhang W, He JY, He LC and Xu CB:
Ligustilide induces vasodilatation via inhibiting voltage dependent
calcium channel and receptor-mediated Ca2+ influx and release.
Vascul Pharmacol. 45:171–176. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhu XM, Fang LH, Li YJ and Du GH:
Endothelium-dependent and -independent relaxation induced by
pinocembrin in rat aortic rings. Vascul Pharmacol. 46:160–165.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rubanyi GM: The role of endothelium in
cardiovascular homeostasis and diseases. J Cardiovasc Pharmacol. 22
Suppl 4:S1–S14. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nelson MT and Quayle JM: Physiological
roles and properties of potassium channels in arterial smooth
muscle. Am J Physiol. 268:C799–C822. 1995.PubMed/NCBI
|
|
11
|
Bolotina VM, Najibi S, Palacino JJ, Pagano
PJ and Cohen RA: Nitric oxide directly activates calcium-dependent
potassium channels in vascular smooth muscle. Nature. 368:850–853.
1994. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brayden JE: Potassium channels in vascular
smooth muscle. Clin Exp Pharmacol Physiol. 23:1069–1076. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vergara C, Latorre R, Marrion NV and
Adelman JP: Calcium-activated potassium channels. Curr Opin
Neurobiol. 8:321–329. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Horowitz A, Menice CB, Laporte R and
Morgan KG: Mechanisms of smooth muscle contraction. Physiol Rev.
76:967–1003. 1996.PubMed/NCBI
|
|
15
|
Hirata S, Enoki T, Kitamura R, Vinh VH,
Nakamura K and Mori K: Effects of isoflurane on receptor-operated
Ca2+ channels in rat aortic smooth muscle. Br J Anaesth.
81:578–583. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lee CH, Poburko D, Sahota P, Sandhu J,
Ruehlmann DO and van Breemen C: The mechanism of
phenylephrine-mediated [Ca(2+)](i) oscillations underlying tonic
contraction in the rabbit inferior vena cava. J Physiol.
534:641–650. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lee CN, Wong KL, Liu JC, Chen YJ, Cheng JT
and Chan P: Inhibitory effect of stevioside on calcium influx to
produce antihypertension. Planta Med. 67:796–799. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Eckert RE, Karsten AJ, Utz J and Ziegler
M: Regulation of renal artery smooth muscle tone by
alpha1-adrenoceptors: Role of voltage-gated calcium channels and
intracellular calcium stores. Urol Res. 28:122–127. 2000.
View Article : Google Scholar : PubMed/NCBI
|