Introduction
Since last century, the prevalence of asthma has
increased worldwide, which has resulted in substantial morbidity
and healthcare costs (1). Patients
with different phenotypes have different outcomes and prognoses.
The most effective treatment strategy to effectively control
asthma, as suggested by the Global Initiative for Asthma (GINA), is
to implement personalized treatment according to the different
phenotypes of asthma. However, it is difficult to correctly and
rapidly identify the phenotype using simple clinical features,
without complicated or invasive examinations.
Genetic susceptibility is critical in the
development of asthma; therefore, it is often used to identify the
phenotypes of asthma. Interleukin-4 receptor (IL-4R) is important
in regulating T helper (Th)2 cell development and immunoglobulin E
(IgE) production via its response to IL-4 or IL-13 (2). The loci of IL-4R, located on genomic
region 16p12, are linked to asthma phenotypes with increased airway
mast cells and IgE (+) cells. Numerous single nucleotide
polymorphisms (SNPs) in IL-4R gene have been associated with a
phenotype of severe asthma (3,4). However, sequencing the IL-4R gene for
every patient is not considered a feasible method of
differentiating the phenotypes of asthma.
In the past two decades, the value of palm
dermatoglyphic or fingerprint patterns has been described in the
detection of bronchial asthma by different studies; however, the
underlying mechanisms have not been clarified (5–8). In our
previous study, a distinctive palm pattern was identified, which
was characterized by deep grids in the thenar area that facilitated
the diagnosis of asthma, with a close association to two a
disintegrin and metalloprotein-33 (ADAM33) gene polymorphisms
(9). The palm pattern appears to be a
potential biomarker for endotypes of asthma, although the
association between distinctive palm patterns and other SNPs
associated with asthma have yet to be investigated.
Thus, in the present study, 11 SNPs of the IL-4R
gene were analyzed in a population of East Chinese Han adults to
clarify the link between a particular palm pattern and IL-4R gene
polymorphisms.
Materials and methods
Subjects
A total of 400 asthma patients were recruited from
the pulmonary clinics of two teaching hospitals, Qingdao Municipal
Hospital (Qingdao, China) and Qingdao Haici Hospital (Qingdao,
China) from January 2011 to January 2012 successively. Asthma was
diagnosed and evaluated based on symptoms and on spirometry
assessments by the criteria of the Global Initiative for Asthma
(version: 2010 update) (10).
Two-hundred healthy adults were recruited successively from the
Health check-up centers of Qingdao Municipal Hospital and Qingdao
Haici Hospital to serve as healthy controls. All control subjects
were asymptomatic for asthma and devoid of any atopic or pulmonary
diseases. Pregnant or lactating female subjects were excluded. The
age range for all of the study subjects was 18–70 years old.
Smoking status and education history data were collected for all
subjects. The study was performed in accordance with the Helsinki
Declaration and approved by the Ethics Committee of Qingdao
Municipal Hospital and Qingdao Haici Hospital. In addition, all
subjects provided written informed consent prior to the study.
Dermatoglyphic palm patterns
The palms of each subject were observed, after
washing clean with soap and water, by two researchers under natural
light. The palm patterns were determined by the dermatoglyphic
variables in the thenar area, including number and shape of the
ridges, as described in our previous study (9). A positive palm pattern typically
exhibited an increased ridge count (≥10) and deep grid patterns in
the thenar area, while a negative palm pattern demonstrated normal
ridge count (<10) and light grid patterns in the thenar area.
All subjects were sub-categorized into two groups; a positive palm
pattern group and a negative palm pattern group according to the
above standard.
Polymorphism genotyping
Whole blood (10 ml) was taken in lithium
heparin-coated test tubes from each subject and immediately
centrifuged at 1,600 × g. Buffy coat (peripheral white cells) was
separated and stored at −70°C on the day of enrollment by a nurse.
Genomic DNA was isolated from the peripheral blood leukocytes using
a DNA extraction kit (Tiangen Biotech Co., Ltd., Beijing, China).
The DNA was genotyped for the SNPs of the IL-4R gene at the Center
for Human Genetics Research, Shanghai Genesky Bio-Tech Co., Ltd.
(Shangai, China). Three SNPs of the IL-4R gene, rs1805010,
rs1805012 and rs1801275, were selected according to the published
literatures regarding SNP associations with asthma (11,12). Eight
tag SNPs, rs3024608, rs1110470, rs3024685, rs3024619, rs2057768,
rs3024585, rs12925861, and rs3024613, were then selected according
to the frequency information for Chinese populations from two
public databases the International HapMap Project (http://www.hapmap.org/) and the NCBI database
(http://www.ncbi.nlm.nih.gov/). The PCR
primers were designed by the authors and are presented in Table I.
 | Table I.Oligonucleotide primers used for
resequencing interleukin-4 receptor. |
Table I.
Oligonucleotide primers used for
resequencing interleukin-4 receptor.
|
| Oligonucleotide
primers |
|---|
|
|
|
|---|
| SNP | Forward | Reverse |
|---|
| rs1805010 |
CAGCCAGCCTACAGGTGACCA |
CTGACCACGTCATCCATGAGCA |
| rs1805012 |
GGAGAGGAGAATGGGGGCTTTT |
ACTTGGCTCCAGGTGGAGAG |
| rs1801275 |
AGATCCTCCGCCGAAATGTCCT |
ACCCTGCTCCACCGCATGTA |
| rs3024608 |
CAGCCAGGAAGTGGTAGTAGGGACT |
TCGTTAGCTGACCCCACCATGT |
| rs1110470 |
AGCCTTCACTGGCTCCCCACT |
GGAGAAGGACTGGCTGGGATG |
| rs3024685 |
ATGCCCTAACCTCCCAGGAATG |
TACCCCAGCTCCCTCTCCTTTG |
| rs3024619 |
AGAACTACAGAGGAAACTAATTGTATTGAAATG |
TCCTGTCCCCAGCAAAACAAAA |
| rs2057768 |
CCCTAGATGGGGGAACAGAGGTT |
GCATTGTTCTCGGGTGCAAGAG |
| rs3024585 |
CCACCTTCAGAGTCCAAAGATATGTTATTT |
CATGAGGGAAGAGCCTGCCTAAA |
| rs12925861 |
AAGCTGCCCACTGCTTAGAGGA |
TCATGGGTCTTAAATCCAGCACTCA |
| rs3024613 |
CAGACACTTCCCCTGGCTGAGT |
CAGGGAGGGAAACCACCTACAA |
Polymerase chain reaction (PCR) amplification of the
corresponding genomic region surrounding each SNP locus was
performed in a Takara PCR thermal cycler (Takara TP600; Takara
Biotechnology Co., Ltd., Dalian, China). The reaction was performed
in a final volume of 10 µl, including 3.0 mM Mg2+, 0.3
mM dNTP, 1 unit HotStarTaq polymerase (Qiagen Inc., Valencia, CA,
USA), 1 µl of each primer, and 1 µl (10 ng) of genomic DNA. The
cycling conditions were as follows: 1 Cycle at 95°C for 2 min, 11
cycles at 94°C for 20 sec, 65–0.5°C for 40 sec and 72°C for 1.5
min, and 24 cycles at 94°C for 20 sec, 59°C for 30 sec and 72°C for
1.5 min, and a final extension at 72°C for 2 min. The PCR products
were purified using a PCR purification kit containing 1unit Shrimp
Alkaline Phosphatase (SAP) and 1 unit Exonuclease I (Qiagen GmbH,
Hilden, Germany) and were used as DNA templates for the cycle
sequencing. Direct DNA sequencing was performed using 5 µl SNaPshot
Multiplex kit (Applied Biosystems; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) in 10-µl volumes containing 2 µl primer and 2 µl
DNA template, and were subjected to 1 cycle at 96°C for 1 min, 28
cycles of denaturation at 96°C for 10 sec, annealing at 52°C for 5
sec, and extension at 60°C for 30 sec. Sequencing products were
purified using 1 unit SAP at 37°C for 1 h and annealing at 75°C for
15 min. All SNPs were detected with an ABI 3130xl, and the data
were analyzed with GeneMapper 4.0 (Applied Biosystems; Thermo
Fisher Scientific, Inc.). The association analysis between a single
SNP and phenotype were conducted under five different genetic
models (inheritance patterns) as follows: Codominant, dominant,
recessive, overdominant and log-additive.
Statistical analysis
The differences in age, gender, body mass index
(BMI), smoking status and education history between patients and
control subjects were compared using the χ2 test or a
t-test accordingly. The correlation between palm patterns and
asthma severity was evaluated by Spearman analysis. The odds ratios
(ORs) and 95% confidence interval (CI) of asthma risk of
individuals with various genetic polymorphisms were calculated
using logistic regression analysis adjusting for differences in
gender. Hardy-Weinberg equilibrium and linkage disequilibrium was
estimated using SNPAnalyzer version 2.0 (Istech Corp., Korea;
http://istech21.com/). Statistical analyses were
conducted using the SPSS for Windows version 17.5, statistical
package (SPSS, Inc, Chicago, IL, USA) and P<0.05 was considered
to indicate a statistically significant difference.
Results
Demographics
The demographics of the asthma group and the control
group are presented in Table II. No
significant differences were identified between the age, gender,
BMI, smoking status, and education history of the asthma and
control groups (all P>0.05).
 | Table II.Demographics of asthma patients and
healthy control subjects (presented as means ± standard
deviation). |
Table II.
Demographics of asthma patients and
healthy control subjects (presented as means ± standard
deviation).
| Variable | Asthma (n=400) | Control (n=200) | t | χ2 | P-value |
|---|
| Age, years |
40.11±14.54 |
45.42±16.28 | 0.35 | – | >0.05 |
| Body mass index,
kg/m2 | 24.78±3.22 | 24.51±3.40 | 0.09 | – | >0.05 |
| Gender |
|
| – | 1.98 | >0.05 |
| Male | 172 | 74 |
|
|
|
|
Female | 228 | 126 |
|
|
|
| Smoking |
|
| – | 0.50 | >0.05 |
|
Smokera | 115 | 52 |
|
|
|
|
Non-smoker | 285 | 148 |
|
|
|
| Education, years | 15.24±8.13 | 15.89±8.81 | 0.08 | – | >0.05 |
Polymorphisms with asthma
All genotype frequencies were consistent with
Hardy-Weinberg equilibrium (P>0.05). The genotype distributions
of all 11 IL-4R SNPs in the asthma and control groups are listed in
Table III. There are two SNPs,
rs1805012 and rs3024608, which are associated with asthma in
different models as follows: rs1805012, dominant model (P=0.03) and
rs3024608, codominant model (P=0.029).
 | Table III.Genotype of interleukin-4 receptor
SNPs in the asthma and control groups (adjusted for gender and
age). |
Table III.
Genotype of interleukin-4 receptor
SNPs in the asthma and control groups (adjusted for gender and
age).
| SNP | Genotype | Control, n (%) | Asthma, n (%) | Odds ratio (95%
CI) | P-value |
|---|
| rs2057768 | T/T | 63 (31.50 | 115 (28.97) | 1 | 0.96 |
|
| C/T | 93 (46.50) | 195 (49.11) | 1.06 (0.68–1.65) |
|
|
| C/C | 44 (22.00) | 87
(21.91) | 1.07 (0.62–1.84) |
|
| rs1110470 | G/G | 85 (42.50) | 161 (40.25) | 1 | 0.59 |
|
| G/A | 93 (46.50) | 183 (45.75) | 0.97 (0.65–1.47) |
|
|
| A/A | 22 (11.00) | 56
(14.00) | 1.34 (0.71–2.50) |
|
| rs12925861 | A/A | 58 (29.00) | 111 (27.80) | 1 | 0.94 |
|
| A/T | 95 (47.50) | 201 (50.20) | 1.03 (0.66–1.62) |
|
|
| T/T | 47 (23.50) | 88
(22,00) | 0.95 (0.55–1.62) |
|
| rs1805010 | G/G | 54 (27.14) | 112 (28.14) | 1 | 0.75 |
|
| G/A | 99 (49.75) | 201 (50.50) | 0.88
(0.56–1.38) |
|
|
| A/A | 46 (23.12) | 85
(21.36) | 0.82
(0.48–1.41) |
|
| rs3024585 | A/A | 88 (44.22) | 173 (43.57) | 1 | 0.78 |
|
| G/A | 89 (44.72) | 182 (45.84) | 0.91
(0.60–1.36) |
|
|
| G/G | 23 (11.56) | 42
(10.57) | 0.81
(0.43–1.53) |
|
|
| SNP | Genotype | Control, n
(%)+ | Asthma, n (%) | Odds ratio (95%
CI) | P-value |
|
| rs3024608 | C/C | 161 (80.50) | 340 (85.56) | 1 | 0.03 |
|
| C/G | 39 (19.50) | 53 (13.35) | 0.56
(0.33–0.94) |
|
|
| G/G | 0 (0) | 4 (1.01) | NA (0.00-NA) |
|
| rs3024613 | C/C | 56 (28.28) | 99 (25.00) | 1 | 0.39 |
|
| C/T | 95 (47.98) | 208 (52.53) | 1.37
(0.86–2.18) |
|
|
| T/T | 47 (23.73) | 89 (22.47) | 1.15
(0.67–1.99) |
|
| rs3024619 | G/G | 68 (34.00) | 122 (30.73) | 1 | 0.51 |
|
| G/A | 95 (47.50) | 205 (51.64) | 1.26
(0.82–1.95) |
|
|
| A/A | 37 (18.50) | 70 (17.63) | 1.02
(0.58–1.79) |
|
| rs1805012 | T/T | 169 (84.50) | 361 (90.93) | 1 | 0.03 |
|
| C/T | 31 (15.50) | 35 (8.82) | 0.50
(0.27–0.90) |
|
|
| C/C | 0 (0) | 1 (0.25) | NA (0.00-NA) |
|
| rs1801275 | A/A | 141 (70.50) | 281 (70.78) | 1 | 0.47 |
|
| G/A | 51 (25.50) | 108 (27.20) | 0.97
(0.62–1.51) |
|
|
| G/G | 8 (4.00) | 8 (2.05) | 0.48
(0.15–1.53) |
|
| rs3024685 | C/C | 67 (33.50) | 133 (33.25) | 1 | 0.87 |
|
| C/T | 97 (48.50) | 194 (48.50) | 0.90
(0.59–1.38) |
|
|
| T/T | 36 (18.00) | 73 (18.25) | 0.90
(0.51–1.58) |
|
Polymorphisms with palm patterns
The genotype distribution of all 11 IL-4R SNPs in
the negative and positive palm groups are presented in Table IV. Two SNPs, rs1805010 and rs3024608,
were associated with the positive palm pattern in different models
as follows: rs1805010, log-additive model (P=0.031) and rs3024608,
codominant model (P=0.016). Notably, rs3024608 is associated with
asthma and the positive palm pattern. The rs1805010, rs1805012 and
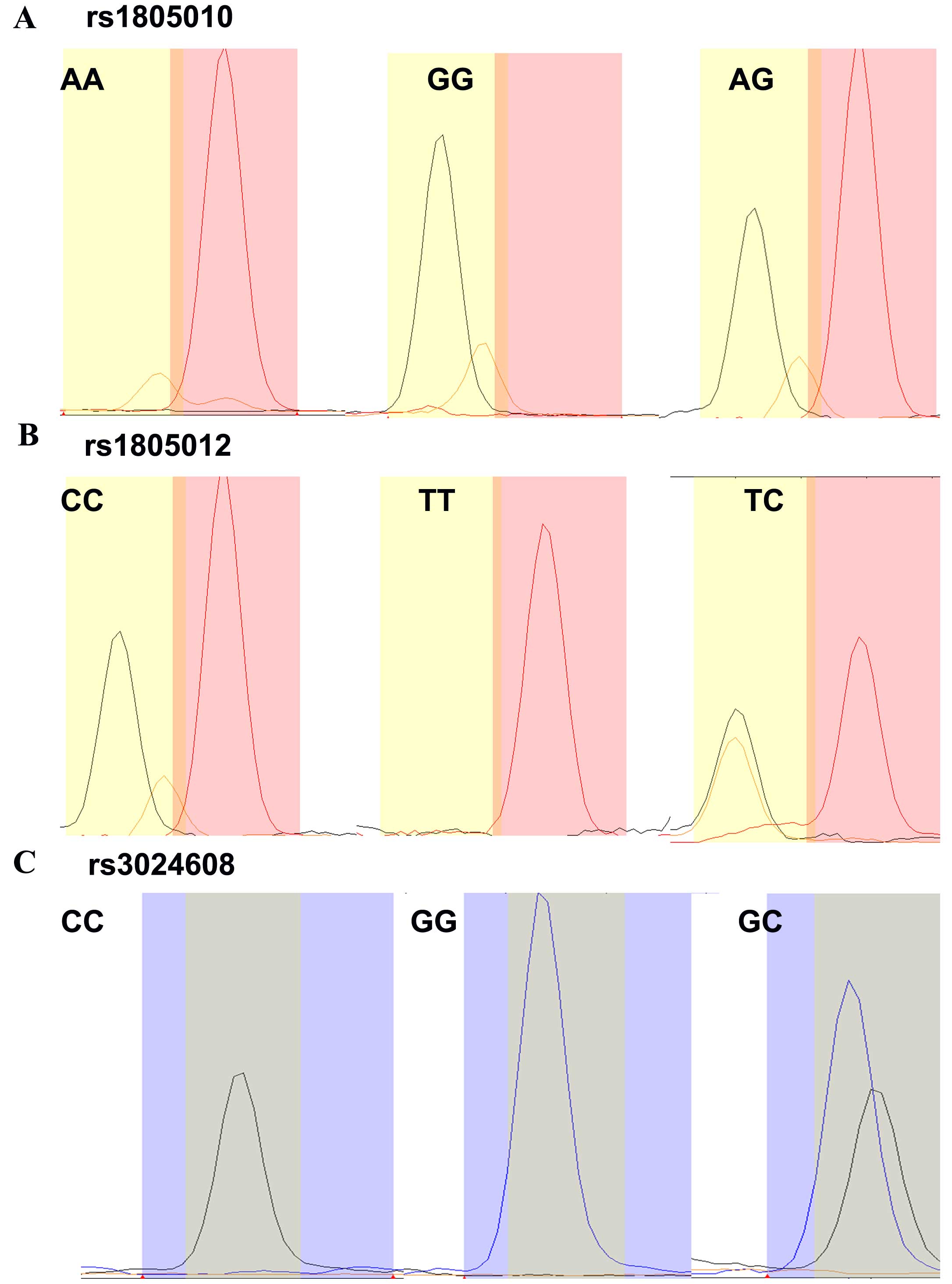
rs3024608 with different genotypes are presented in Fig. 1.
 | Table IV.Genotype of interleukin-4 receptor
SNPs in the negative and positive palm pattern groups (adjusted for
gender and age). |
Table IV.
Genotype of interleukin-4 receptor
SNPs in the negative and positive palm pattern groups (adjusted for
gender and age).
| SNP | Genotype | Negative palm, n
(%) | Positive palm, n
(%) | Odds ratio (95%
CI) | P-value |
|---|
| rs1805010 | G/G | 81 (25.55) | 85 (30.4) | 1 | 0.031 |
|
| G/A | 157 (49.53) | 143 (51.1) | 0.79
(0.52–1.19) |
|
|
| A/A | 79 (24.92) | 52 (18.6) | 0.58
(0.35–0.95) |
|
| rs1805012 | T/T | 282 (88.68) | 248 (88.9) | 1 | 0.47 |
|
| C/T | 36 (11.32) | 30 (10.8) | 1.10
(0.62–1.94) |
|
|
| C/C | 0 (0) | 1 (0.4) | NA (0.00-NA) |
|
| rs1801275 | A/A | 228 (71.69) | 194 (69.53) | 1 | 0.42 |
|
| G/A | 83 (26.10) | 76 (27.24) | 1.04
(0.70–1.55) |
|
|
| G/G | 7 (2.21) | 9 (3.23) | 2.15
(0.67–6.84) |
|
| rs3024608 | C/C | 264 (83.02) | 237 (84.95) | 1 | 0.02 |
|
| C/G | 54 (16.93) | 38 (13.62) | 0.73
(0.44–1.19) |
|
|
| G/G | 0 (0) | 4 (1.43) | NA (0.00-NA) |
|
| rs1110470 | G/G | 121 (37.93) | 125 (44.48) | 1 | 0.2 |
|
| G/A | 155 (48.58) | 121 (43.06) | 0.71
(0.49–1.04) |
|
|
| A/A | 43 (13.48) | 35 (12.45) | 0.79
(0.46–1.38) |
|
| rs3024685 | C/C | 106 (33.22) | 94 (33.45) | 1 | 0.96 |
|
| C/T | 154 (48.27) | 137 (48.75) | 0.97
(0.66–1.44) |
|
|
| T/T | 59 (18.50) | 50 (17.79) | 0.93
(0.56–1.54) |
|
| rs3024619 | G/G | 105 (33.02) | 85 (30.46) | 1 | 0.49 |
|
| G/A | 161 (50.63) | 139 (49.82) | 1.10
(0.74–1.64) |
|
|
| A/A | 52 (16.45) | 55 (19.71) | 1.36
(0.82–2.28) |
|
| rs2057768 | T/T | 89 (27.99) | 89 (31.90) | 1 | 0.44 |
|
| C/T | 153 (48.11) | 135 (48.38) | 0.85
(0.57–1.28) |
|
|
| C/C | 76 (23.90) | 55 (19.71) | 0.73
(0.44–1.19) |
|
| rs3024585 | A/A | 131 (41.19) | 130 (46.59) | 1 | 0.19 |
|
| G/A | 150 (47.17) | 121 (43.37) | 0.74
(0.51–1.08) |
|
|
| G/G | 37 (11.64) | 28 (10.04) | 0.66
(0.37–1.19) |
|
| rs12925861 | A/A | 84 (26.33) | 85 (30.25) | 1 | 0.14 |
|
| A/T | 153 (47.96) | 143 (50.89) | 0.88
(0.58–1.33) |
|
|
| T/T | 82 (25.71) | 53 (18.86) | 0.62
(0.37–1.01) |
|
| rs3024613 | C/C | 78 (24.68) | 77 (27.70) | 1 | 0.65 |
|
| C/T | 163 (51.58) | 140 (50.36) | 0.86
(0.56–1.31) |
|
|
| T/T | 75 (23.73) | 61 (21.44) | 0.80
(0.48–1.32) |
|
Discussion
In the present study, gene segments of 11 SNPs of
the IL-4R gene were amplified to identify the genetic basis of the
association between a distinctive palm dermatoglyphic pattern and
asthma in a Chinese population.
As the results of SNP studies are not always
consistent in different populations, the present study evaluated
the role of SNPs of IL-4R in asthma development in the Chinese
population. Although three SNPs, rs1801275 (−1902G/A), rs1805012
(1291T/C) and rs1805010 (−223G/A) demonstrated associations with
asthma in previous studies (11–13), only
rs1805012 exhibited an association with asthma (dominant model;
P=0.03) in the current study. To further investigate the effect of
IL-4R SNPs in asthma, eight tag SNPs of the IL-4R region were
investigated for an association with asthma, to the best of our
knowledge, for the first time. Of these SNPs, two were associated
with asthma in a different model: rs1805010, log-additive model
(P=0.031) and rs3024608, codominant model (P=0.016). Thus,
polymorphisms of the IL-4R gene may be the genetic basis of asthma
in this particular population.
Dermatoglyphs are formed in the 10th to 17th weeks
of the embryological phase, when the neurologic and immunity
systems are developing. Generally dermatoglyphs remain unchanged
throughout an individual's life, except in cases of serious
injuries that scar the dermis. Thus, a series of studies have
identified the association of dermatoglyphic patterns with certain
congenital defects or gene-associated diseases, such as Down's
syndrome (14–17). However, few practical associations have
been recognized in humans. Palm patterns are affected by many
complex factors, for example age-associated changes, gender and
ethnic differences. A distinctive palm pattern was identified in
asthma patients using theories of Chinese Traditional Medicine
(5). However, it is difficult to
confirm the clinical significance of these dermatoglyph changes in
clinical practice.
As asthma is a disorder associated with multiple
genetic factors, establishing the gene polymorphisms associated
with asthma is considered to be a convictive method to elucidate
the implications of the association between a distinctive palm
pattern and asthma. In our previous study, two SNPs of ADAM33,
rs44707 and rs2787094, were identified to be associated with a
positive palm pattern (6). In the
current study, of 11 analyzed SNPs of IL-4R, two SNPs were found to
be associated with the distinctive palm pattern in different models
(rs1805010: Log-additive model, P=0.031; rs3024608: Codominant
model, P=0.016). Notably, rs3024608 was associated with the
positive palm pattern and asthma in the same population; thus,
IL-4R polymorphisms may be the genetic basis of the association of
the distinctive palm pattern and asthma.
Recently Chavarri-Guerra and Soto-Perez-de-Celis
(14) described a 65-year-old woman
with stage IV breast cancer, who lost her fingerprints following
chemotherapy with capecitabine and bevacizumab (18). This indicated that dermatoglyphs may
change as a condition of the disease (19,20). The
clinical significance of dermatoglyphs requires further
clarification using well-designed studies.
In conclusion, the genetic variation in IL-4R may be
the basis of the association between asthma and a distinctive palm
pattern. Considering the genetic variant, further studies with a
prospective design in an unselected population are required to
validate the association between a distinctive palm pattern and
asthma, in order that a distinctive palm pattern may be considered
as a biomarker for asthma development or phenotypes (21).
Acknowledgements
The authors would like to thank all participants of
the study, and the Center for Human Genetics Research, Shanghai
Genesky Bio-Tech Co., Ltd. for performing the SNP analysis. The
current study was supported by a research grant from the National
Natural Science Foundation of China (grant nos. 30873315 and
81400024).
Glossary
Abbreviations
Abbreviations:
|
SNP
|
single nucleotide polymorphisms
|
|
IL-4
|
interleukin-4
|
|
IL-13
|
interleukin-13
|
|
IL-4R
|
interleukin-4 receptor
|
|
GINA
|
Global Initiative for Asthma
|
|
ADAM33
|
a disintegrin and
metalloprotein-33
|
|
PCR
|
polymerase chain reaction
|
References
|
1
|
Global Initiative for Asthma, . Global
Strategy for Asthma Management and Prevention. http://www.ginasthma.org
|
|
2
|
Zhu J: T helper 2 (Th2) cell
differentiation, type 2 innate lymphoid cell (ILC2) development and
regulation of interleukin-4 (IL-4) and IL-13 production. Cytokine.
75:14–24. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhu N, Gong Y, Chen XD, Zhang J, Long F,
He J, Xia JW and Dong L: Association between the polymorphisms of
interleukin-4, the interleukin-4 receptor gene and asthma. Chin Med
J (Engl). 126:2943–2951. 2013.PubMed/NCBI
|
|
4
|
Soto-Ramírez N, Arshad SH, Holloway JW,
Zhang H, Schauberger E, Ewart S, Patil V and Karmaus W: The
interaction of genetic variants and DNA methylation of the
interleukin-4 receptor gene increase the risk of asthma at age 18
years. Clin Epigenetics. 5:1–5. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhou ZS, Ji ZQ, Zhu XJ, Wang YQ, Xue WL,
Jiang HY and Liang WH: Study on objective and quantitative atopy of
thenar eminence print and its correlation with asthma. Shanghai
Journal of Traditional Chinese Medicine. 48:14–15. 2014.(In
Chinese).
|
|
6
|
Mahajan AA, Gour KK and Thakare AE: The
dermatoglyphic patterns in patients of bronchial asthma - a
qualitative study. Int J Biol Med Res. 2:806–807. 2011.
|
|
7
|
Pakhale SV, Borole BS, Doshi MA and More
VP: Study of the fingertip pattern as a tool for the identification
of the dermatoglyphic trait in bronchial asthma. J Clin Diagn Res.
6:1397–1400. 2012.PubMed/NCBI
|
|
8
|
Singh S, Khurana AK, Harode HA, Tripathi
A, Pakhare A and Chaware P: Study of fingerprint patterns to
evaluate the role of dermatoglyphics in early detection of
bronchial asthma. J Nat Sci Biol Med. 7:43–46. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xue W, Han W and Zhou ZS: ADAM33
polymorphisms are associated with asthma and a distinctive palm
dermatoglyphic pattern. Mol Med Rep. 8:1795–1800. 2013.PubMed/NCBI
|
|
10
|
Cazzoletti L, Marcon A, Corsico A, Janson
C, Jarvis D, Pin I, Accordini S, Bugiani M, Cerveri I, Gislason D,
et al: Therapy and Health Economics Group of the European Community
Respiratory Health Survey: Asthma severity according to Global
Initiative for Asthma and its determinants: An international study.
Int Arch Allergy Immunol. 151:70–79. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wenzel SE, Balzar S, Ampleford E, Hawkins
GA, Busse WW, Calhoun WJ, Castro M, Chung KF, Erzurum S, Gaston B,
et al: IL4R alpha mutations are associated with asthma
exacerbations and mast cell/IgE expression. Am J Respir Crit Care
Med. 175:570–576. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Narożna B, Hoffmann A, Sobkowiak P,
Schoneich N, Bręborowicz A and Szczepankiewicz A: Polymorphisms in
the interleukin 4, interleukin 4 receptor and interleukin 13 genes
and allergic phenotype: A case control study. Adv Med Sci.
61:40–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Murk W, Walsh K, Hsu LI, Zhao L, Bracken
MB and Dewan AT: Attempted replication of 50 reported asthma risk
genes identifies a SNP in RAD50 as associated with childhood atopic
asthma. Hum Hered. 71:97–105. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chavarri-Guerra Y and Soto-Perez-de-Celis
E: Images in clinical medicine. Loss of fingerprints. N Engl J Med.
372:e222015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kolgeci S, Kolgeci J, Azemi M, Daka A,
Shala-Beqiraj R, Kurtishi I and Sopjani M: Dermatoglyphics and
Reproductive Risk in a Family with Robertsonian Translocation
14q;21q. Acta Inform Med. 23:178–183. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zvi Shamir E, Levy A, Cassan S Morris,
Lifshitz T, Shefler G and Tarrasch R: Do biometric parameters of
the hand differentiate schizophrenia from other psychiatric
disorders? A comparative evaluation using three mental health
modules. Psychiatry Res. 228:425–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ezzati A, Batoei F, Jafari SA, Kiyani MA,
Mahdavi-Shahri N, Ahanchian H, Tehranian S and Kianifar HR:
Dermatoglyphic patterns in cystic fibrosis children. Iran J
Pediatr. 24:609–616. 2014.PubMed/NCBI
|
|
18
|
Sen J, Kanchan T and Mondal N: A
comparison of palmar dermatoglyphics in two ethnic Indian
populations of north Bengal, India. J Forensic Sci. 56:109–117.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Navit S, Chadha D, Khan SA, Singh RK,
Johri N, Navit P, Sharma A and Bahuguna R: The mystery of
handprints: Assesment and correlation of dermatoglyphics with early
childhood caries a case-control study. J Clin Diagn Res.
9:ZC44–ZC48. 2015.PubMed/NCBI
|
|
20
|
Eslami N, Jahanbin A, Ezzati A,
Banihashemi E and Kianifar H: Can dermatoglyphics be used as a
marker for predicting future malocclusions? Electron Physician.
8:1927–1932. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kumasaka N, Yamaguchi-Kabata Y, Takahashi
A, Kubo M, Nakamura Y and Kamatani N: Establishment of a
standardized system to perform population structure analyses with
limited sample size or with different sets of SNP genotypes. J Hum
Genet. 55:525–533. 2010. View Article : Google Scholar : PubMed/NCBI
|