Introduction
Colorectal cancer (CRC), one of most prevalent
malignancies, is considered to be the third most commonly diagnosed
cancer (1.36 million cases), and the fourth cause of
cancer-associated mortality (694,000 deaths) worldwide (1). Colorectal adenoma (Ad), also known as one
of the pre-cancerous lesion types, is closely associated with CRC,
and is present in the majority of cases of CRC (2). In spite of what has thus far been
accomplished in terms of diagnosing and treating CRC, the
diagnostic rate of CRC requires further improvement, and the
prognosis of patients with advanced CRC remains poor. Therefore, it
is crucial to explore the underlying mechanisms of carcinogenesis
in CRC and to identify novel, sensitive and specific diagnostic
biomarkers to improve diagnostic efficiency in cases of CRC.
MicroRNAs (miRNAs or miRs) are a class of small,
evolutionarily conserved, non-coding single-stranded nucleotide
molecules (20–24 nt), which are able to inhibit or promote gene
expression via binding to the 3′-untranslated region of the target
messenger RNA (mRNA) at the post-transcriptional level (3–5). A
burgeoning body of evidence has demonstrated that miRNAs serve a
crucial role in a variety of biological processes, including cell
growth (6), cell apoptosis (7) and development of the nervous system
(8). In oncological studies, a growing
number of miRNAs have been confirmed to have an association with a
large number of neoplasms (9–11). For example, miR-15a and miR-16 may be
closely associated with cancer pathogenesis (12,13).
miR-143, as a tumor suppressor, inhibits the growth, and induces
apoptosis of, gastric cancer cells through targeting
cyclooxygenase-2 (14). To date,
miRNAs have been detected and extracted not only in tissues, but
also in the plasma, serum and urine (15). Willeit et al (16) demonstrated that miR-122, a liver miRNA,
is able to function as a novel biomarker for cardiovascular and
metabolic diseases. Therefore, miRNAs, as regulators of gene
expression, are involved in the pathogenesis of numerous types of
tumors, and are able to be applied as potential biomarkers for
tumor diagnosis.
The miR-196 family, which is most strongly
correlated with malignancies, contains three members, miR-196a-1,
miR-196a-2 and miR-196b, which have been reported to be involved in
several biological processes, including embryonic development and
neoplasia (3,17,18).
Emerging evidence has indicated that the aberrant expression of
miR-196b is closely associated with leukemogenesis via increasing
the population of leukemic stem/progenitor cells, blocking cell
differentiation, promoting cell proliferation and diminishing cell
apoptosis (19). Furthermore, Ge et
al (20) demonstrated that
expression of miR-196b in tissues may have a significant
correlation with an aggressive progression of the disease and poor
clinical outcomes in patients with CRC. However, the association
between serum expression of miR-196b and CRC has yet to be fully
elucidated.
The present study, has sought to determine whether
the expression of serum miR-196b is upregulated in CRC, and to
evaluate the diagnostic value of serum miR-196b in CRC.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong
University (Shanghai, China). All examinations were performed after
obtaining written informed consent from patients and
volunteers.
Patients and serum
Between February 2010 and February 2015, 103
patients with primary CRC, comprising 62 males and 41 females, who
accepted surgical resection in Renji Hospital, School of Medicine,
Shanghai Jiao Tong University, were recruited in the present study.
All the patients were diagnosed with CRC by two experienced
pathologists, and did not receive chemotherapy or radiotherapy. In
addition, 51 patients with Ad and 100 healthy individuals were
enrolled as a middle group and the control group, respectively.
Baseline information of all the groups is shown in Table I. Blood samples (5 ml) from the 103
patients with CRC, 51 patients with Ad and 100 healthy controls
were collected in tubes containing ethylenediaminetetra-acetic acid
(EDTA) prior to surgical operation. The sera samples were separated
by centrifugation in two successive steps: A centrifugation at
1,600 × g for 10 min at 4°C, followed by a second centrifugation at
16,000 × g for 10 min at 4°C. Subsequently, the supernatant sera
were stored in liquid nitrogen at −80°C for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
 | Table I.Patient characteristics in the three
groups. |
Table I.
Patient characteristics in the three
groups.
| Characteristic | CRC group, n=103 | Ad group, n=51 | Control group,
n=100 | P-value |
|---|
| Age, years | 52.8±4.6 | 54.3±5.1 | 53.4±4.8 | 0.562 |
| Gender |
|
|
|
|
|
| Male | 62 (60.2%) | 29 (56.9%) | 52 (52.0%) | 0.712 |
|
Female | 41 (39.8%) | 22 (43.1%) | 48 (48.0%) |
|
| Nation |
|
|
|
|
|
| Han
Chinese | 95 (92.2%) | 49 (96.1%) | 94 (94.0%) | 0.664 |
|
Minority | 8 (7.2%) | 2 (3.9%) | 6 (6.0%) |
|
| Registered
residence |
|
|
|
|
|
|
Urban | 64 (62.1%) | 26 (51.0%) | 56 (56.0%) | 0.390 |
|
Rural | 39 (37.9%) | 25 (49.0%) | 44 (44.0%) |
|
| History of alcohol
consumption |
| Yes | 60
(58.3%) | 23 (45.1%) | 52 (52.0%) | 0.293 |
| No | 43 (41.7%) | 28 (54.9%) | 48 (48.0%) |
|
| History of
smoking |
| Yes | 47 (45.6%) | 27 (52.9%) | 48 (48.0%) | 0.694 |
| No | 56 (54.4%) | 24 (47.1%) | 52 (52.0%) |
|
RNA isolation and RT-qPCR
Total RNA was extracted from 100 µl serum using a
Qiagen miRNeasy Mini kit (Qiagen, Valencia, CA, USA). The cDNA of
miR-196b was reverse-transcribed from total RNA using a PrimeScript
RT-PCR kit (Takara Biotechnology Co., Ltd., Dalian, China). U6 RNA
was used as an internal control in the present study. The four
primers used in this study are listed in Table II. PCR for detecting the expression
levels of miR-196b was performed using an Applied Biosystems 7900
QPCR system (Applied Biosystems, Foster City, CA, USA) with a 20 µl
RT-qPCR reaction mixture, comprising forward primer (0.6 µl),
reverse primer (0.6 µl), cDNA (2 µl), ROX Reference Dye II (0.4
µl), SYBR Premix Ex Taq (10 µl), and doubly distilled
H2O (6.6 µl). The relative expression of miRNA-196b was
calculated using the 2−ΔΔCq method (21), with U6 RNA as the internal reference
compound.
 | Table II.Primers for performing reverse
transcription-quantitative polymerase chain reaction on miR-196b
and U6. |
Table II.
Primers for performing reverse
transcription-quantitative polymerase chain reaction on miR-196b
and U6.
| Gene | Primer
sequence |
|---|
| miR-196b |
|
|
Forward |
5′-TAGGTACCACTTTATCCCGTTCACCA-3′ |
|
Reverse |
5′-ATCTCGAGGCAGGGAGAGAGGAATAA-3′ |
| U6 |
|
|
Forward |
5′-CTCGCTTCGGCAGCACA-3′ |
|
Reverse |
5′-AACGCTTCACGAATTTGCGT-3′ |
Receiver operating characteristic
(ROC) curve analysis
The relative levels of miR-196 expression in the
serum of patients and controls were recorded. ROC curves were
generated, with the horizontal axis as specificity and the vertical
axis as sensitivity, using GraphPad Prism 5 software (GraphPad
Software, San Diego, CA, USA). According to the drawn ROC curves,
the diagnosis cut-off points and their specificity and sensitivity
were analyzed and calculated. The diagnostic value of miR-196b in
the serum in CRC was evaluated, and presented by the area under the
curve (AUC) and the standard error.
Survival analysis
Patients in the CRC group were divided into two
subgroups on the basis of whether the expression of miR-196b was
high or low. Survival analysis using the Kaplan-Meier method was
performed, using the data from a 5-year follow-up.
Statistical analysis
All data were performed using SPSS 19.0 software
(IBM SPSS, Armonk, NY, USA). Values are expressed as the mean ±
standard deviation. Differences and/or correlations between groups
were analyzed and calculated using the Student's t-test and
Chi-square test. Survival curves were drawn using the Kaplan-Meier
method, and the long-rank test was employed for survival analysis.
Prognosis analysis was performed using multivariate cox
proportional hazards regression analysis, and P<0.05 was
considered to indicate a statistically significant value.
Results
Serum miR-196b is upregulated in the
CRC group compared with the Ad group and healthy individuals, and
is a potential diagnostic marker for CRC
Serum miR-196b was detected in the three groups of
participants, including healthy individuals (n=100), Ad (n=51) and
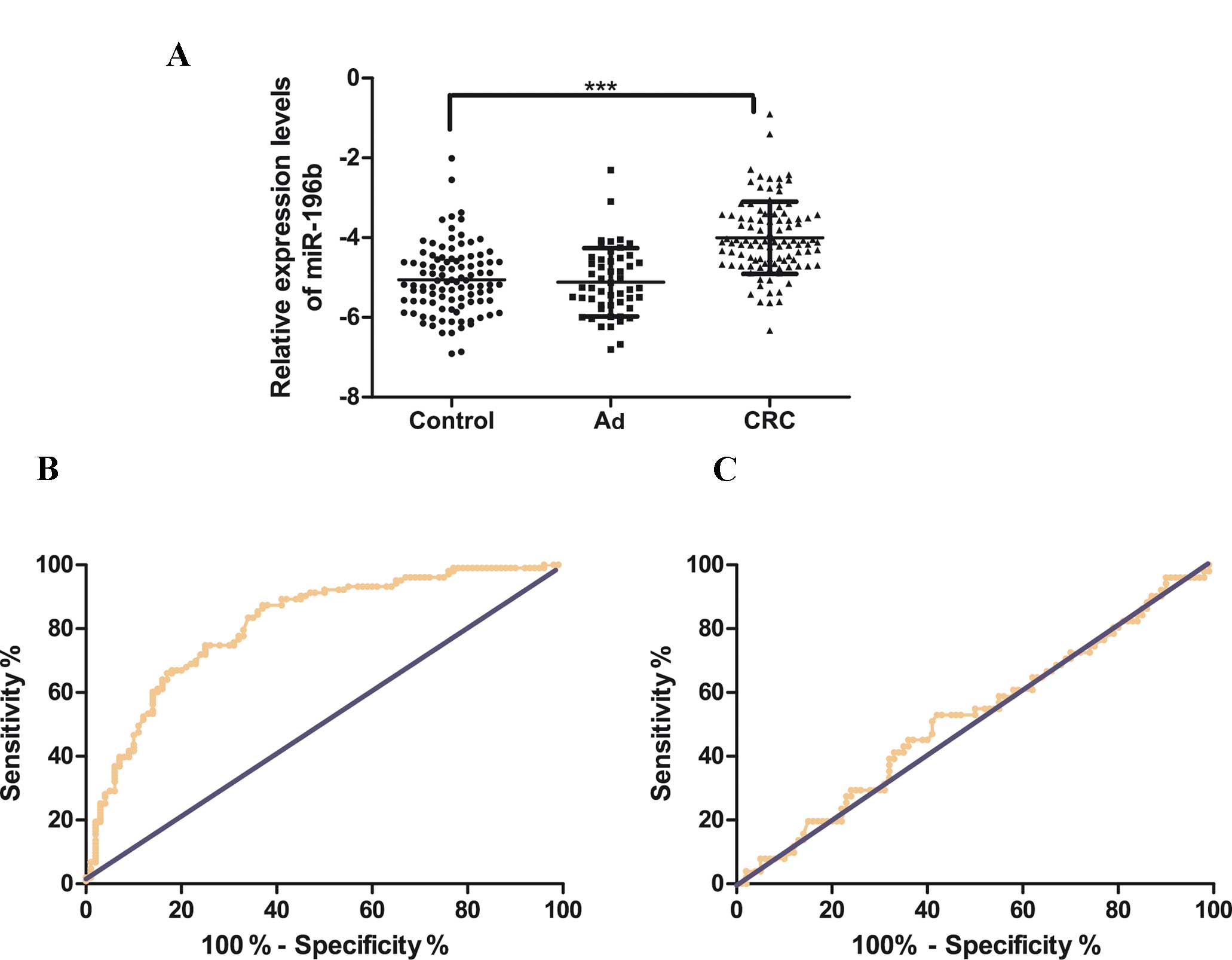
CRC (n=103). As shown in Fig. 1A,
compared with healthy controls, the serum level of miR-196b was
significantly upregulated in CRC (CRC vs. controls: −3.91±0.89 vs.
−5.10±0.85, P<0.001). However, the serum miR-196b levels were
slightly downregulated in Ad compared with the healthy controls,
although without statistical significance (Ad vs. controls:
−5.12±0.86 vs. −5.10±0.85, P=0.68). These results indicated that
the serum expression of miR-196b was significantly upregulated in
CRC. ROC curves were drawn to analyze and assess the diagnostic
power of serum miR-196b in CRC (Fig.
1B). The AUC was 0.8135 (95% confidence interval:
0.7546–0.8725), with a diagnostic threshold of −4.785, and
specificity and sensitivity were 87.38 and 63%, respectively.
However, as shown in Fig. 1C, serum
miR-196b had no diagnostic value in the Ad group.
Association between the
clinicopathological features and expression levels of serum
miR-196b in human CRC
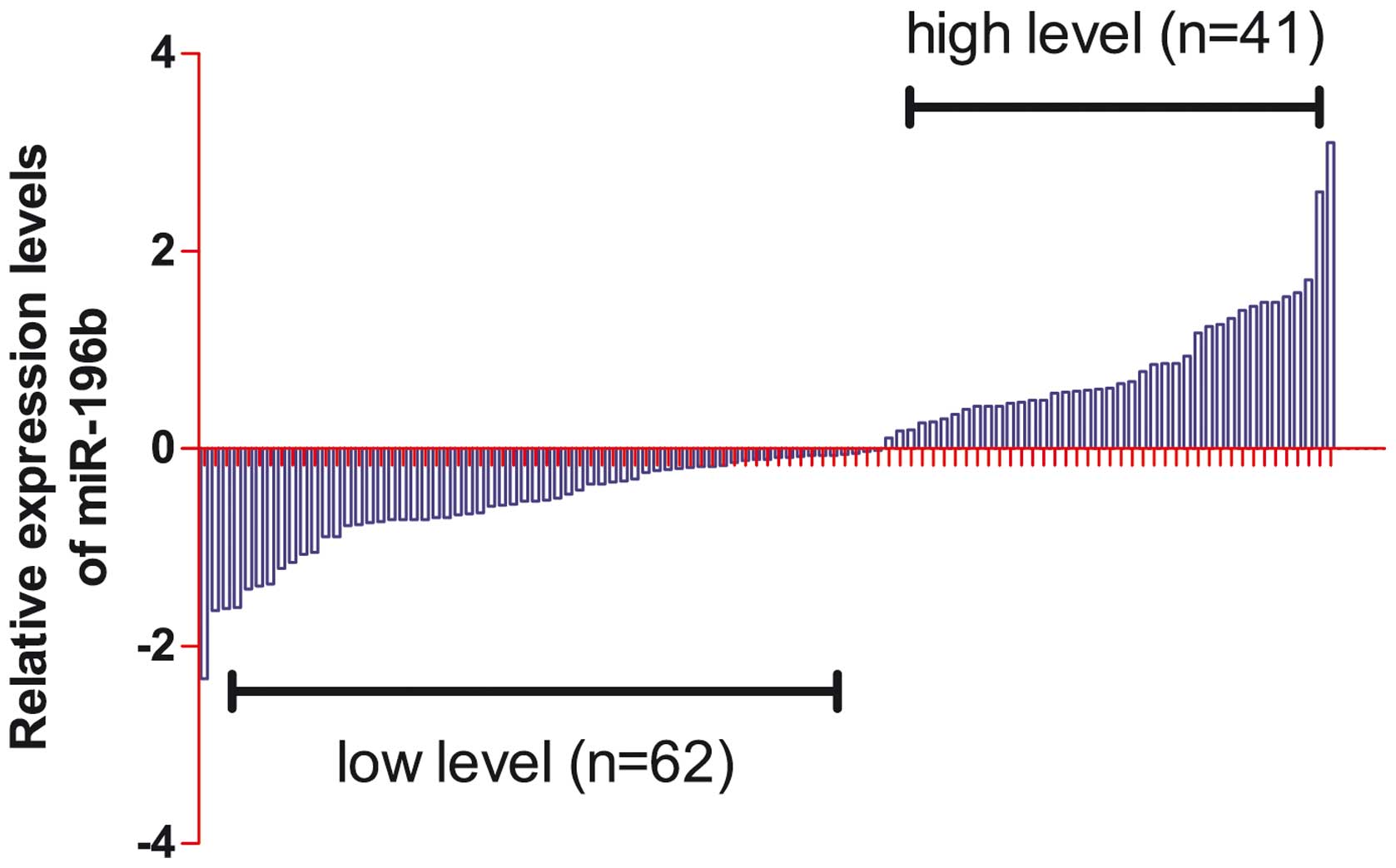
As shown in Fig. 2,
relative miR-196b levels detected by RT-qPCR in the serum of the
103 patients with CRC were divided into the two subgroups on the
basis of the expression levels of miR-196b: The high serum
expression of miR-196b group, and the low serum expression of
miR-196b group. The correlations between clinicopathological
features and serum miR-196b levels are shown in Table III. The results of the chi-square
test demonstrated that low expression of plasma miR-145 was
significantly associated with lymph node invasion, differentiation
and tumor-lymph nodes-metastasis (TNM) staging (all P<0.05),
whereas no significant associations were determined for age,
gender, history of alcohol consumption, tumor size, tumor invasion,
metastasis and site of the primary tumor (all P>0.05).
 | Table III.Associations between serum miR-196b
levels and clinicopathological parameters in patients with CRC. |
Table III.
Associations between serum miR-196b
levels and clinicopathological parameters in patients with CRC.
|
|
| Serum miR-196b
expression |
|
|---|
|
|
|
|
|
|---|
| Characteristic | Total no. | Low (n=62) | High (n=41) | P-value |
|---|
| Age |
|
|
|
|
|
<57 | 45 | 30 (44.6%) | 15 (41.4%) | 0.237 |
|
≥57 | 58 | 32 (55.4%) | 26 (48.6%) |
|
| Gender |
|
|
|
|
|
Male | 62 | 34 (57.1%) | 28 (63.8%) | 0.172 |
|
Female | 41 | 28 (42.9%) | 13 (36.2%) |
|
| History of alcohol
consumption |
|
|
|
|
|
Yes | 47 | 29 (67.9%) | 18 (60.3%) | 0.775 |
| No | 56 | 33 (32.1%) | 23 (39.7%) |
|
| Tumor size |
|
|
|
|
| <5
cm | 59 | 38 (26.8%) | 21 (48.3%) | 0.254 |
| ≥5
cm | 44 | 24 (73.2%) | 20 (51.7%) |
|
| Tumor invasion |
|
|
|
|
|
T1-T2 | 51 | 27 (52.9%) | 23 (47.1%) | 0.212 |
|
T3-T4 | 51 | 35 (68.6%) | 18 (31.4%) |
|
| Metastasis |
|
|
|
|
|
Yes | 33 | 22 (60.7%) | 11 (41.4%) | 0.357 |
| No | 70 | 40 (39.3%) | 30 (58.6%) |
|
| Lymph node
invasion |
|
|
|
|
|
Yes | 45 | 34 (67.9%) | 11 (56.9%) | 0.005 |
| No | 58 | 28 (32.1%) | 30 (43.1%) |
|
|
Differentiation |
|
|
|
|
|
Well | 21 | 15 (12.5%) | 6 (%) | 0.001 |
|
Moderate | 43 | 32 (26.8%) | 11 (43.1%) |
|
|
Poor | 39 | 15 (60.7%) | 24 (44.8%) |
|
| TNM stage |
|
|
|
|
|
I–II | 57 | 42 (19.0%) | 15 (41.4%) | 0.002 |
|
III–IV | 46 | 20 (81.0%) | 26 (58.6%) |
|
| Site of primary
tumor |
|
|
|
|
|
Right-sided | 40 | 25 | 15 | 0.875 |
|
Left-sided | 42 | 24 | 18 |
|
|
Rectum | 21 | 13 | 8 |
|
Prognostic effect of upregulation of
serum miR-196b in human CRC
Subsequently, the prognostic implications of an
increased expression of serum miR-196b in CRC was investigated
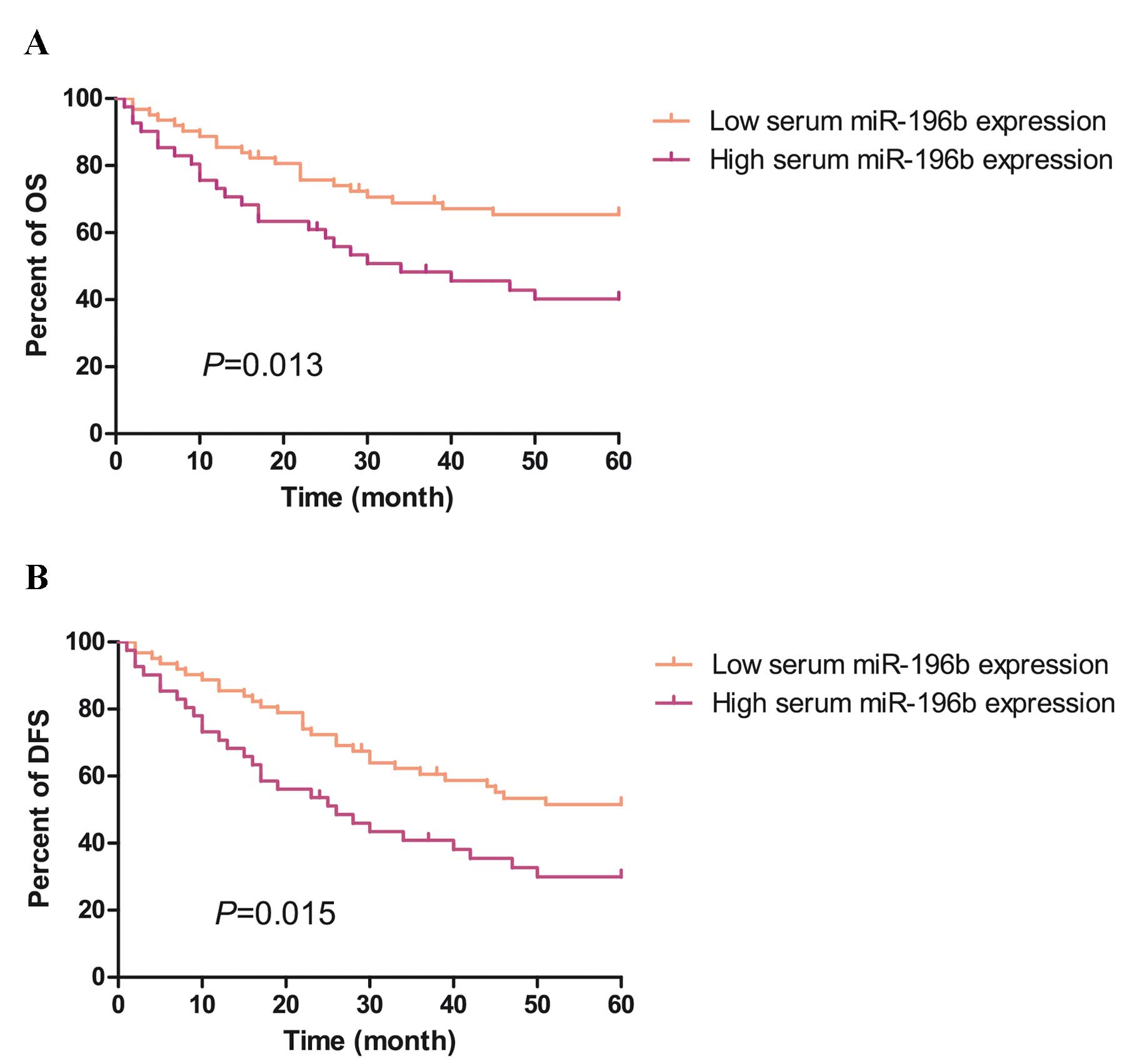
using the Kaplan-Meier method. As shown in Fig. 3A and B, survival analysis indicated
that overall survival (OS) and disease-free survival (DFS) rates of
patients with CRC with high serum miR-196b levels were
significantly lower than those of patients with low serum miR-196b
levels (OS: 40.2 vs. 65.4, P<0.05; DFS: 40.2 vs. 65.4,
P<0.05). Therefore, serum miR-196b was a prognostic indicator
for DFS and OS of patients with CRC.
Multivariate Cox's proportional hazard
regression analysis of the prognostic values of indicators in human
CRC
It remains unclear whether the independent
parameters of serum miR-196b expression in the prognosis of human
CRC are correlated with OS. In the present study, in order to
explore the associations between the independent predictors of
serum miR-196b expression in the prognosis of human CRC and OS, the
clinical characteristics, including serum miR-196b expression,
lymph node invasion, differentiation and TNM stage, which were
defined as statistically significant, were performed using
multivariate Cox's proportional hazard regression analysis.
According to the multivariate analysis, the serum miR-196b
expression, lymph node invasion and TNM stage were all identified
as having significant associations with OS. Therefore, the present
investigation has shown that independent predictors, including
serum miR-196b expression, lymph node invasion and TNM stage, are
potential risk factors for OS in human CRC (all P<0.05; Table IV).
 | Table IV.Multivariate Cox's proportional
hazards regression analysis of the prognostic values of indicators
on overall survival in human CRC. |
Table IV.
Multivariate Cox's proportional
hazards regression analysis of the prognostic values of indicators
on overall survival in human CRC.
|
|
|
|
| 95% CI for Exp
(B) |
|---|
|
|
|
|
|
|
|---|
| Characteristic | Unfavorable vs.
favorable | P-value | Exp (B) | Lower | Upper |
|---|
| Serum miR-196b
level | High vs. low | 0.045 | 2.8 | 1.023 | 7.664 |
| Lymph node
invasion | Yes vs. no | 0.013 | 0.256 | 0.088 | 0.749 |
| Differentiation
status | Poor vs. well,
moderate | 0.431 | 1.468 | 0.565 | 3.814 |
| TNM stage | III–IV vs.
I–II | 0.022 | 3.31 | 1.193 | 9.185 |
Discussion
At the present time, the mortality rate due to CRC
remains higher than that for a number of other common malignancies.
Recently, a burgeoning list of tumor biomarkers, including MYC,
HOTAIRM1 and colon cancer-associated transcript 1 (CCAT1), have
been reported to have a limited association with diagnosis and
prognosis in CRC (22–24). Therefore, it is of great interest to
identify further, more specific and sensitive biomarkers for the
diagnosis of CRC. In the present study, it was noted that, compared
with healthy individuals, the expression of serum miR-196b was
significantly upregulated in patients with CRC, and serum miR-196b
was identified to be a potential diagnostic marker for CRC.
Furthermore, Cox regression analysis revealed that serum miR-196b
expression, lymph node invasion, differentiation and the TNM stage
were potential risk factors for OS in human CRC.
Tissue miRNAs, as the biomarkers of diagnosis, are
most widely used in scientific research. Fadous-Khalifé et
al (25) demonstrated that,
compared with normal tissue, the expression of Kruppel-like factor
4 (KLF4) was markedly decreased in non-small cell lung cancer,
although it was overexpressed in small cell lung cancer. In
addition, Perini et al (26)
demonstrated that the overexpression of membrane epidermal growth
factor receptor is associated with decreased survival in pancreas
cancer tissue. However, patients with a number of different types
of cancer are unable to undergo surgery when they are diagnosed.
The diagnostic effectiveness of this method is unsatisfactory, due
to the limitation of there being a dependence on surgical
resection, and the invasive procedure for the collection of tissue
samples.
Given the great advances that have been made in
diagnostic technology, with the exception of that associated with
the tissues, it is possible that the expression of circulating
miRNAs, which can be detected in, and extracted from, a variety of
biological samples, including serum, plasma and urine, could be a
useful addition to the technologies available. The miR-196 family
of miRNAs, which is located at three paralogous loci in the
mammalian homeobox (HOX) clusters, is able to regulate a series of
genetic processes in the development of embryos (27). As a member of the miR-196 family, the
aberrant expression of miR-196b was determined to be associated
with a great number of cancer types, including lung, oral and
gastric cancers, and acute myeloid leukemia (28–31). Low
expression of miR-196b leads to the development of chronic myeloid
leukemia via upregulation of the expression of the BCR-ABL1 fusion
gene and HOXA9 (32). In addition, How
et al (33) revealed that the
low expression of miR-196b was significantly correlated with poorer
DFS rates for patients with cervical cancer, and that the recovery
of miR-196b expression resulted in reduced tumor angiogenesis and
tumor cell proliferation in vivo, and reduced cell growth,
clonogenicity, migration and invasion in vitro. Furthermore, Li
et al (34) demonstrated that
miR-196b expression was clearly associated with mixed lineage
leukemia-rearranged leukemia by directly targeting the HOXA9/MEIS1
oncogenes and the FAS tumor suppressor. In contrast, upregulation
of miR-196b expression was identified in glioblastoma, which was
associated with a poor prognosis via promoting cellular
proliferation (35). A similar result
in gastric cancer revealed that, via regulation of the
phosphoinositide 3-kinase/AKT/mammalian target of rapamycin
pathway, overexpression of miR-196b promotes the proliferation and
invasion of gastric cancer cells (36). Additionally, Wang et al
(37) demonstrated that miR-196b was
upregulated in colonic cancer tissues. Although the phenomenon of
the aberrant expression of miR-196b in CRC is partly understood,
little is known about the association between serum miR-196b
expression and the clinical relevance of such an aberration. In the
present study, based on three large cohorts, including CRC, Ad and
healthy individuals, we have demonstrated that serum miR-196b was
significantly upregulated in the CRC group compared with the Ad and
the healthy individuals groups. It was also demonstrated that high
levels of miR-196b in CRC sera were associated with positive lymph
node invasion, the TNM stage and poor differentiation. Furthermore,
miR-196b-high status was clearly correlated with shorter OS and DFS
rates compared with those with miR-196b-low status. Finally, the
Cox regression analysis indicated that high serum miR-196b
expression, lymph node invasion and the TNM stage were risk factors
for OS in human CRC.
The present study did have several limitations.
First, this study could be repeated with the analysis and
evaluation of a larger number of patients, which may help to
improve the accuracy of the data and credibility of the study.
Secondly, there may have been selection bias in operation when the
inclusion criteria were formulated.
In conclusion, the present study has revealed that
the serum expression of miR-196b was significantly higher in
patients with CRC compared with that in Ad patients or healthy
individuals. In addition, the results of our study have indicated
that the overexpression of serum miR-196b was significantly
correlated with lymph node invasion, the TNM stage, poor
differentiation and poor prognosis. Therefore, serum miR-196b may
have an application as a diagnostic tool and prognostic marker for
CRC.
Acknowledgements
Τhe present study was supported by the following
support fund: The Shanghai Municipal Commission of Health and
Family Planning Fund (grant no. 20154Y0207).
References
|
1
|
Wei Q, Wang X, Gao J, Li J, Li J, Qi C, Li
Y, Li Z and Shen L: Clinicopathologic and molecular features of
colorectal adenocarcinoma with signet-ring cell component. PLoS
One. 11:e01566592016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Strum WB: Colorectal Adenomas. N Engl J
Med. 374:1065–1075. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fantini S, Salsi V, Vitobello A, Rijli FM
and Zappavigna V: MicroRNA-196b is transcribed from an autonomous
promoter and is directly regulated by Cdx2 and by posterior Hox
proteins during embryogenesis. Biochim Biophys Acta.
1849:1066–1080. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kojima S, Goto Y and Naya Y: The roles of
microRNAs in the progression of castration-resistant prostate
cancer. J Hum Genet. Jun 9–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Melo SA and Esteller M: Dysregulation of
microRNAs in cancer: Playing with fire. FEBS Lett. 585:2087–2099.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng K, Li H, Huang H and Qiu M:
MicroRNAs and glial cell development. Neuroscientist. 18:114–118.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lizé M, Klimke A and Dobbelstein M:
MicroRNA-449 in cell fate determination. Cell Cycle. 10:2874–2882.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cao DD, Li L and Chan WY: MicroRNAs: Key
Regulators in the Central Nervous System and Their Implication in
Neurological Diseases. Int J Mol Sci. 17:E8422016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rea D, Del Vecchio V, Palma G, Barbieri A,
Falco M, Luciano A, De Biase D, Perdonà S, Facchini G and Arra C:
Mouse Models in Prostate Cancer Translational Research: From
Xenograft to PDX. Biomed Res Int. 2016:97507952016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Landi D, Moreno V, Guino E, Vodicka P,
Pardini B, Naccarati A, Canzian F, Barale R, Gemignani F and Landi
S: Polymorphisms affecting micro-RNA regulation and associated with
the risk of dietary-related cancers: A review from the literature
and new evidence for a functional role of rs17281995 (CD86) and
rs1051690 (INSR), previously associated with colorectal cancer.
Mutat Res. 717:109–115. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Skrzypski M, Dziadziuszko R and Jassem J:
MicroRNA in lung cancer diagnostics and treatment. Mutat Res.
717:25–31. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Acunzo M and Croce CM: Downregulation of
miR-15a and miR-16-1 at 13q14 in Chronic Lymphocytic Leukemia. Clin
Chem. 62:655–656. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang E, Liu R and Chu Y: miRNA-15a/16: as
tumor suppressors and more. Future Oncol. 11:2351–2363. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu XL, Cheng B, Li PY, Huang HJ, Zhao Q,
Dan ZL, Tian DA and Zhang P: MicroRNA-143 suppresses gastric cancer
cell growth and induces apoptosis by targeting COX-2. World J
Gastroenterol. 19:7758–7765. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marí-Alexandre J, Sánchez-Izquierdo D,
Gilabert-Estellés J, Barceló-Molina M, Braza-Boïls A and Sandoval
J: miRNAs Regulation and Its Role as Biomarkers in Endometriosis.
Int J Mol Sci. 17:E932016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Willeit P, Skroblin P, Kiechl S,
Fernandez-Hernando C and Mayr M: Liver microRNAs: potential
mediators and biomarkers for metabolic and cardiovascular disease?
Eur Heart J. Apr 20–2016.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mansfield JH, Harfe BD, Nissen R, Obenauer
J, Srineel J, Chaudhuri A, Farzan-Kashani R, Zuker M, Pasquinelli
AE, Ruvkun G, et al: MicroRNA-responsive ‘sensor’ transgenes
uncover Hox-like and other developmentally regulated patterns of
vertebrate microRNA expression. Nat Genet. 36:1079–1083. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Guan Y, Mizoguchi M, Yoshimoto K, Hata N,
Shono T, Suzuki SO, Araki Y, Kuga D, Nakamizo A, Amano T, et al:
MiRNA-196 is upregulated in glioblastoma but not in anaplastic
astrocytoma and has prognostic significance. Clin Cancer Res.
16:4289–4297. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan Y, Meng M, Zhang G, Han H and Zhou Q:
Oncogenic microRNAs in the genesis of leukemia and lymphoma. Curr
Pharm Des. 20:5260–5267. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ge J, Chen Z, Li R, Lu T and Xiao G:
Upregulation of microRNA-196a and microRNA-196b cooperatively
correlate with aggressive progression and unfavorable prognosis in
patients with colorectal cancer. Cancer Cell Int. 14:1282014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee KS, Kwak Y, Nam KH, Kim DW, Kang SB,
Choe G, Kim WH and Lee HS: c-MYC Copy-Number Gain Is an Independent
Prognostic Factor in Patients with Colorectal Cancer. PLoS One.
10:e01397272015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wan L, Kong J, Tang J, Wu Y, Xu E, Lai M
and Zhang H: HOTAIRM1 as a potential biomarker for diagnosis of
colorectal cancer functions the role in the tumour suppressor. J
Cell Mol Med. 20:2036–2044. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xin Y, Li Z, Shen J, Chan MT and Wu WK:
CCAT1: A pivotal oncogenic long non-coding RNA in human cancers.
Cell Prolif. 49:255–260. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fadous-Khalifé MC, Aloulou N, Jalbout M,
Hadchity J, Aftimos G, Paris F and Hadchity E: Krüppel-like factor
4: A new potential biomarker of lung cancer. Mol Clin Oncol.
5:35–40. 2016.PubMed/NCBI
|
|
26
|
Perini MV, Montagnini AL, Coudry R,
Patzina R, Penteado S, Abdo EE, Diniz A, Jukemura J and da Cunha
JE: Prognostic significance of epidermal growth factor receptor
overexpression in pancreas cancer and nodal metastasis. ANZ J Surg.
85:174–178. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yu H, Lindsay J, Feng ZP, Frankenberg S,
Hu Y, Carone D, Shaw G, Pask AJ, O'Neill R, Papenfuss AT, et al:
Evolution of coding and non-coding genes in HOX clusters of a
marsupial. BMC Genomics. 13:2512012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tellez CS, Juri DE, Do K, Picchi MA, Wang
T, Liu G, Spira A and Belinsky SA: miR-196b Is Epigenetically
Silenced during the Premalignant Stage of Lung Carcinogenesis.
Cancer Res. 76:4741–4751. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hou YY, You JJ, Yang CM, Pan HW, Chen HC,
Lee JH, Lin YS, Liou HH, Liu PF, Chi CC, et al: Aberrant DNA
hypomethylation of miR-196b contributes to migration and invasion
of oral cancer. Oncol Lett. 11:4013–4021. 2016.PubMed/NCBI
|
|
30
|
Li CY, Liang GY, Yao WZ, Sui J, Shen X,
Zhang YQ, Peng H, Hong WW, Ye YC, Zhang ZY, et al: Identification
and functional characterization of microRNAs reveal a potential
role in gastric cancer progression. Clin Transl Oncol. May
12–2016.(Epub ahead of print).
|
|
31
|
Díaz-Beyá M, Brunet S, Nomdedéu J, Tejero
R, Díaz T, Pratcorona M, Tormo M, Ribera JM, Escoda L, Duarte R, et
al: Cooperative AML group CETLAM (Grupo Cooperativo Para el Estudio
y Tratamiento de las Leucemias Agudas y Mielodisplasias): MicroRNA
expression at diagnosis adds relevant prognostic information to
molecular categorization in patients with intermediate-risk
cytogenetic acute myeloid leukemia. Leukemia. 28:804–812. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Y, Zheng W, Song Y, Ma W and Yin H:
Low expression of miR-196b enhances the expression of BCR-ABL1 and
HOXA9 oncogenes in chronic myeloid leukemogenesis. PLoS One.
8:e684422013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
How C, Hui AB, Alajez NM, Shi W, Boutros
PC, Clarke BA, Yan R, Pintilie M, Fyles A, Hedley DW, et al:
MicroRNA-196b regulates the homeobox B7-vascular endothelial growth
factor axis in cervical cancer. PLoS One. 8:e678462013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Z, Huang H, Chen P, He M, Li Y,
Arnovitz S, Jiang X, He C, Hyjek E, Zhang J, et al: miR-196b
directly targets both HOXA9/MEIS1 oncogenes and FAS tumour
suppressor in MLL-rearranged leukaemia. Nat Commun. 3:6882012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ma R, Yan W, Zhang G, Lv H, Liu Z, Fang F,
Zhang W, Zhang J, Tao T, You Y, et al: Upregulation of miR-196b
confers a poor prognosis in glioblastoma patients via inducing a
proliferative phenotype. PLoS One. 7:e380962012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Li NA, Wang W, Xu B and Gong H: miR-196b
regulates gastric cancer cell proliferation and invasion via
PI3K/AKT/mTOR signaling pathway. Oncol Lett. 11:1745–1749.
2016.PubMed/NCBI
|
|
37
|
Wang YX, Zhang XY, Zhang BF, Yang CQ, Chen
XM and Gao HJ: Initial study of microRNA expression profiles of
colonic cancer without lymph node metastasis. J Dig Dis. 11:50–54.
2010. View Article : Google Scholar : PubMed/NCBI
|