Introduction
Brain tumors in animals are more commonly observed
in dogs rather than in cats, and are frequently detected in
middle-aged to geriatric dogs (1). The
incidence of canine brain tumors has been reported to be 4.5%
globally (2). The most common type of
canine brain tumors is meningioma, followed by glioma (including
astrocytoma and oligodendroglioma) (2–4). As canine
brain tumors are not detectable by routine blood analysis or X-ray
examination of the skull, they are best diagnosed using magnetic
resonance imaging (MRI) (5). However,
opportunities of MRI examination for canines are limited due to the
high cost of this technique and its limited availability in
veterinary facilities. The majority of brain tumors are not
suspected until after clinical signs appear, and it is difficult to
acquire MRI scans immediately in dogs with suspected brain tumors.
Furthermore, numerous canine brain tumors are diagnosed at an
advanced stage. Thus, development of a noninvasive diagnostic
biomarker with high sensitivity and specificity is required for the
early detection of canine brain tumors.
In human medicine, recent studies have reported that
the levels of plasma free amino acid (PFAA) profiles are altered in
certain diseases, such as in cancer (including pancreatic, breast
and lung cancers) and cardiovascular diseases, and that these
changes can predict future development of disease (6,7). Therefore,
it has been considered that PFAA profiles may be used as efficient
disease biomarkers. In veterinary medicine, it has also been
reported that PFAA levels are altered in dogs with malignant
mammary tumors, oral melanoma and lymphoma (8–10). In
addition, it has been reported that the levels of glutamic acid in
the CSF are increased in dogs with idiopathic epilepsy (11).
Various metabolites, such as amino acids and
choline, have been analyzed in human glioma patients by means of
proton magnetic resonance spectroscopy (MRS) or from tissue
specimens obtained during surgery or autopsy (12,13).
However, there have been few reports on PFAA analysis in
intracranial disorders (14). To the
best of our knowledge, the association between PFAA levels, canine
brain tumors and canine idiopathic epilepsy has not been
investigated thus far. Therefore, the present study investigated
the PFAA level profiles in dogs with brain tumors and compared the
data with healthy control dogs and dogs with idiopathic
epilepsy.
Materials and methods
Animals
A total of 12 dogs with brain tumors brought to the
Animal Medical Center of Tottori University and Japan Animal
Referral Medical Center from October 2014 to June 2015 were
evaluated in the current study. Diagnosis of brain tumors was based
on clinical signs, and intracranial MRI and/or pathological
examination. Generalized seizure, changes of character, depression,
circling and tetraparesis were common clinical signs. MRI was
performed using a 0.4T unit (AIRIS; Hitachi Medico, Tokyo, Japan)
and 1.5T unit (Echelon Vega; Hitachi Medico, Tokyo, Japan). In all
dogs, T1-, T2- and contrast-enhanced T1-weighted images were
obtained. The age at diagnosis ranged between 7 and 14 years, with
a mean age of 10.5 years. Six dogs were male and six dogs were
female. A total of 6 dogs were suspected with glioma, 4 dogs were
suspected or confirmed to suffer from meningioma, and 2 dogs had
metastatic brain tumor of the mammary gland. In addition, 8 dogs
diagnosed with idiopathic epilepsy were included, with an age at
diagnosis ranging between 3 and 10 years (mean age, 5.5 years). A
total of 16 healthy control dogs were also included in the study,
with an age ranging between 1 and 14 years (mean age, 8.1 years).
All information concerning these dogs is summarized in Table I. The study protocol was approved by
the Ethics Committee on Animal Trials of the Japan Animal Referral
Medical Center (Tokyo, Japan).
 | Table I.Characteristics of dogs included in
the present study. |
Table I.
Characteristics of dogs included in
the present study.
| Breed | Gender | Age (years) | Body weight (kg) | Diagnosis |
|---|
| French Bulldog | M | 7 | 9 | Brain tumor
(glioma) |
| French Bulldog | M | 11 | 10 | Brain tumor
(glioma) |
| Shiba Inu | F | 10 | 7 | Brain tumor
(glioma) |
| Golden Retriever | F | 10 | 26 | Brain tumor
(glioma) |
| Mongrel | M | 11 | 27 | Brain tumor
(glioma) |
| French Bulldog | M | 9 | 13 | Brain tumor
(glioma) |
| Shiba Inu | M | 9 | 15 | Brain tumor
(meningioma) |
| Shiba Inu | F | 10 | 10 | Brain tumor
(meningioma) |
| Miniature
Schnauzer | F | 12 | 6 | Brain tumor
(meningioma) |
| Shiba Inu | M | 14 | 11 | Brain tumor
(meningioma) |
| Yorkshire
Terrier | F | 10 | 2 | Brain metastases of
mammary gland tumor |
| Miniature
Dachshund | F | 13 | 4 | Brain metastases of
mammary gland tumor |
| Miniature
Dachshund | F | 3 | 3 | Idiopathic
epilepsy |
| Beagle | M | 3 | 18 | Idiopathic
epilepsy |
| Yorkshire
Terrier | M | 6 | 2 | Idiopathic
epilepsy |
| Chihuahua | F | 5 | 3 | Idiopathic
epilepsy |
| Toy Poodle | M | 6 | 3 | Idiopathic
epilepsy |
| Toy Poodle | F | 6 | 3 | Idiopathic
epilepsy |
| Mongrel | F | 5 | 3 | Idiopathic
epilepsy |
| Mongrel | F | 10 | 11 | Idiopathic
epilepsy |
| Mongrel | M | 14 | 11 | Control |
| Miniature
Dachshund | F | 6 | 4 | Control |
| Toy Poodle | F | 12 | 3 | Control |
| Chihuahua | F | 6 | 3 | Control |
| Miniature
Dachshund | F | 7 | 6 | Control |
| Mongrel | M | 12 | 14 | Control |
| Miniature
Dachshund | F | 12 | 6 | Control |
| Pug | F | 9 | 9 | Control |
| Maltese | F | 8 | 4 | Control |
| Pekingese | M | 2 | 3 | Control |
| Miniature
Schnauzer | F | 1 | 6 | Control |
| Miniature
Dachshund | F | 14 | 7 | Control |
| Mongrel | M | 1 | 3 | Control |
| Miniature
Dachshund | F | 10 | 8 | Control |
| Chihuahua | M | 10 | 4 | Control |
| Pekingese | M | 6 | 6 | Control |
Blood collection
The dogs were fasted for 8 h before blood
collection. Venous blood was collected in tubes containing heparin
and was immediately centrifuged at 1,700 × g for 15 min at 4°C.
Following the centrifugation, plasma was promptly removed and
frozen at −80°C until required for PFAA measurements. The plasma
was deproteinized in methanol [plasma:methanol (v/v), 1:9] for 20
min, the samples were then centrifuged at 15,000 × g for 10 min at
4°C, and the supernatant fluid was obtained.
PFAA measurements
Analysis of PFAA levels in the plasma of the dogs
was performed using a pre-column technique with liquid
chromatography according to previously reported methods (15). All reagents, including standard amino
acid solutions (type H), were purchased from Wako Pure Chemical
Industries Ltd. (Osaka, Japan). A liquid chromatography system with
automated pre-column derivatization functionality was used in the
analysis (Nexera X2; Shimadzu Corporation, Kyoto, Japan). A total
of 20 compounds were measured in the analysis, including the
following basic amino acids and associated molecules: Alanine
(Ala), arginine (Arg), asparagine (Asn), aspartic acid (Asp),
cysteine-cysteine (Cys-Cys), glutamic acid (Glu), glutamine (Gln),
glycine (Gly), histidine (His), isoleucine (Ile), leucine (Leu),
lysine (Lys), methionine (Met), phenylalanine (Phe), proline (Pro),
serine (Ser), threonine (Thr), tryptophan (Trp), tyrosine (Tyr) and
valine (Val). Plasma levels of the amino acids are expressed in
µmol/l.
Statistical analysis
Data are expressed as the mean ± standard deviation.
All figures were prepared by Excel 2013 (Microsoft Japan Co., Ltd.,
Tokyo, Japan). Differences between groups were evaluated using the
Steel-Dwass test (4-Step Excel Statistics version 3; OMS publishing
Inc., Tokyo, Japan). P<0.05 was considered to indicate a
statistically significant difference. The data are presented in
radar charts. The radar charts show the percentage of the mean PFAA
levels in dogs with brain tumors and idiopathic epilepsy, as
compared to the control groups (taken as 100%).
Immunohistochemical analysis
Immunohistochemical analysis for L-type amino acid
transporter 1 (LAT1) was performed in two cases (case 1: Shiba inu,
male, aged 14 years, 11 kg; case 2: Shiba inu, female, aged 10
years, 10 kg) of surgically resected meningioma. For immunochemical
analysis, rabbit anti-canine LAT1 polyclonal antibody was used,
donated by Dr Ochiai. This antibody was prepared with the synthetic
peptide antigen designed according to the C-terminus amino acid
sequence of canine LAT1 (16).
Immunohistochemistry was performed as described previously
(17,18). Briefly, following deparaffinization,
the sections were heated 5 times for 3 min in a microwave oven in
0.01 M citric acid (pH 6.0) and rinsed with Dulbecco's
phosphate-buffered saline (Wako Pure Chemical Industries, Ltd.).
Rabbit anti-canine LAT1 polyclonal antibody was incubated with the
sections after inactivation of endogenous peroxidase with methanol
containing 0.3% H2O2 at room temperature for
20 min. Immunostaining was performed using a commercial kit
(Histofine Simple Stain MAX PO; Nichirei, Tokyo, Japan). Then,
3,3′-diaminobenzidine (DAB) H2O2 solution was
applied to induce a color development reaction. Following the
reaction with DAB, the specimens were washed in deionized water
three to four times, followed by staining of the nuclei with
hematoxylin for observation.
Results
Association of PFAA levels with brain
tumors in dogs
The results of the PFAA analysis are presented in
Fig. 1, where the radar chart presents
the percentage of the mean PFAA levels and the PFAA levels of the
control group were considered to be 100%. Three amino acids (Ala,
Pro and Ile) were significantly increased in the plasma of brain
tumor dogs when compared with the control dogs (Ala, P=0.03; Pro,
P=0.009; Ile, P=0.009). The plasma levels of Ala, Pro and Ile in
dogs with brain tumors were increased by 1.6-, 1.5- and 1.6-fold as
compared with those in the controls, respectively (Fig. 1). In addition, the brain tumor dogs
presented higher Lys and lower Asp levels compared with healthy
dogs, although no significant difference was observed. By contrast,
the concentrations of the other amino acids (including Arg, Asn,
Cys-Cys, Glu, Gln, Gly, His, Leu, Met, Phe, Ser, Thr, Trp, Tyr and
Val) remained unchanged between brain tumor and control dogs.
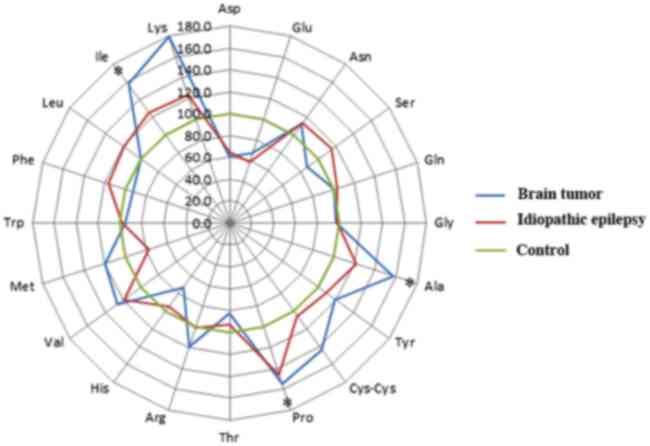 | Figure 1.Alterations in PFAA levels in dogs
with brain tumors and epilepsy. The radar chart presents the mean
PFAA levels, and the PFAA levels of the control group were
considered to be 100%. P-values were calculated using the
Steel-Dwass test. *P<0.05. PFAA, plasma free amino acid; Ala,
alanine; Arg, arginine; Asn, asparagine; Asp, aspartic acid;
Cys-cys, cysteine-cysteine; Glu, glutamic acid; Gln, glutamine;
Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys,
lysine; Met, methionine; Phe, phenylalanine; Pro, proline; Ser,
serine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val,
valine. |
The association of PFAA levels with
the type of tumor (glioma or meningioma)
As shown in Fig. 2,
significantly increased levels of Asn and Gln were observed in the
plasma of dogs with glioma when compared with those in dogs with
meningiomas (P<0.05). However, there were no statistically
significant changes in other amino acids between dogs with
meningioma and those with glioma.
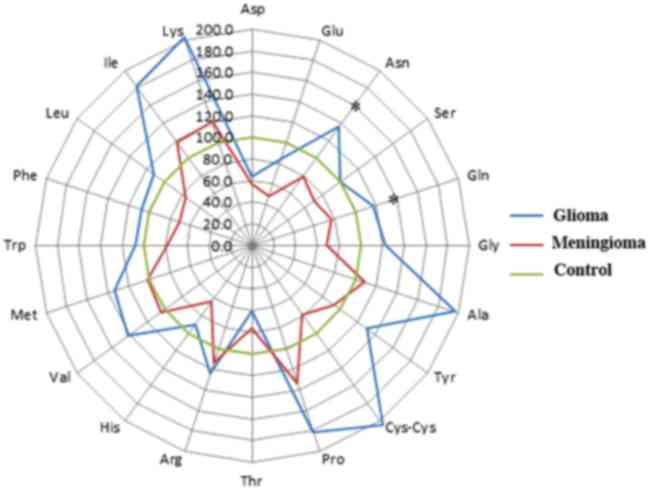 | Figure 2.Alterations in PFAA levels in dogs
with glioma and meningioma. The radar chart is presents the mean
PFAA levels, and the PFAA levels of the control group were
considered to be 100%. P-values were calculated using the
Steel-Dwass test. *P<0.05. PFAA, plasma free amino acid; Ala,
alanine; Arg, arginine; Asn, asparagine; Asp, aspartic acid;
Cys-cys, cysteine-cysteine; Glu, glutamic acid; Gln, glutamine;
Gly, glycine; His, histidine; Ile, isoleucine; Leu, leucine; Lys,
lysine; Met, methionine; Phe, phenylalanine; Pro, proline; Ser,
serine; Thr, threonine; Trp, tryptophan; Tyr, tyrosine; Val,
valine. |
PFAA levels association with
idiopathic epilepsy in dogs
As shown in Fig. 1, no
significant changes were observed in the levels of any amino acids
in dogs with idiopathic epilepsy, as compared with dogs with brain
tumors and healthy dogs. Although no significant difference was
observed, the levels of Asp obtained from the idiopathic epilepsy
dog plasma were lower than those of the healthy dogs.
Immunohistochemistry
Immunohistochemistry of LAT1 localization
demonstrated no signal in the tumor cells in case 1. While in case
2, the tumor cells exhibited a weakly positive reaction.
Discussion
In the present study, the plasma levels of the amino
acids Ala, Pro and Ile in dogs with brain tumor were increased by
1.6-, 1.5- and 1.6-fold, respectively, over the levels reported in
healthy dogs (Fig. 1). In addition,
the brain tumor dogs presented higher Lys and lower Asp levels
compared with healthy dogs, although no significant difference was
observed due to variations in the measurements. These results
suggest that the PFAA profile in the peripheral blood reflects
brain tumor metabolism.
It has been suggested that certain amino acids are
associated with human brain tumor metabolism (19). In cerebrospinal fluid (CSF) analysis of
human patients with brain tumors, there were significantly higher
levels of Pro, Met, taurine, Lys, Ser, Phe and Gln among patients
with malignant glioma compared with those in the controls (19). In another study, amino acid levels in
surgically removed glioma and peritumoral tissues were measured,
and significantly increased levels of Ile and Val were identified
within the peritumoral tissue compared with those in normal brain
tissue (13). In MRS studies of human
meningioma, increased alanine levels were observed in 91.3% of
cases (20). Although the mechanism
underlying these amino acid changes are not clear, they may be due
to downregulation of amino acid transporter expression, such as
L-type amino acid transporter 1 (LAT1), in tumor cells. In the
present study, immunohistochemical analysis for LAT1 was performed
in two samples of meningioma, and the results indicated no signal
in the samples, which were very weakly stained for LAT1 antibody.
This suggests that LAT1 may be downregulated in canine brain
tumors. Previously, Ochiai et al (16) prepared a synthetic peptide antigen,
which was designed according to the C-terminus amino acid sequence
of canine LAT1 to investigate LAT1 protein expression in various
types of tissue (16). This antibody
was used in the present study. Therefore, this antibody is
considered to be specific for canine LAT1.
When comparing human patients and dogs with brain
tumors, certain amino acids, including Ala, Pro and Ile, were
increased in the two species according to the present and previous
studies (13,19,20). By
contrast, specific amino acids, such as Met, taurine, Lys, Ser, Phe
and Gln were increased only in human patients (13,19). This
difference between levels in humans and dogs may be due to the
source of the sample. In human patients, CSF samples were used
(19), whereas plasma obtained from
venous blood samples in dogs was used in the present study. It is
possible that changes in amino acid levels associated with brain
diseases, including brain tumors, would be observed in the CSF as
well as in the plasma. To understand the correlation between canine
brain tumors and amino acid levels, the associations between plasma
and CSF levels of amino acids should be investigated. In addition,
the present study demonstrated that Asp levels were decreased in
dogs with idiopathic epilepsy, as well as in those with brain
tumors, suggesting that the decreased levels of this amino acid may
be associated with epileptic seizures, although no evidence is
available in the literature.
In veterinary medicine, it has been reported that
Thr, Pro and Ser plasma levels were decreased in dogs with
melanoma, the Phe and Glu serum levels were increased in dogs with
lymphoma, and the Met, Ser, Asn, Gln, Ala, taurine and citrulline
plasma levels were decreased in dogs with non-metastatic mammary
gland tumors, as compared with these levels in control dogs
(8–10).
These changes in PFAA levels differed from those observed in the
present study, suggesting that the changes noted in the present
study were specific to canine brain tumors.
Glu levels in the CSF increased in dogs with
idiopathic epilepsy (11). In the
present study, PFAA levels were also examined in dogs with
idiopathic epilepsy, as well as in healthy dogs and dogs with brain
tumors. However, no significant change was observed for any of the
amino acids in dogs with idiopathic epilepsy, as compared with the
healthy dogs. These findings may be explained based on the
functions of the blood-brain barrier (BBB). Amino acid levels may
have been altered in the CSF, but not in the peripheral blood of
dogs with idiopathic epilepsy, due to the presence of an intact
BBB. Generalized convulsive seizures temporarily increase the BBB
permeability; however, the loss of BBB integrity recovers within 24
h (21). In the present study, brain
MRI examination was performed in all cases. All dogs with brain
tumors showed enhancement in the tumor tissues when using a
contrast agent (gadoteridol), suggesting impairment of the BBB.
Therefore, the PFAA levels may be altered in dogs with brain tumors
(13). Screening of PFAA levels in
patients with epileptic seizures may, thus, facilitate the early
differential diagnosis of idiopathic epilepsy and symptomatic
epilepsy, particularly in the context of brain tumors as a cause of
seizures.
An MRS study in humans has suggested that the levels
of several metabolites, such as lactic acid and choline, are
elevated in malignant gliomas (12).
Another MRS study demonstrated no difference in glycine levels
between glioblastoma and meningioma patients, although these levels
were altered according to the grade of the glioma (22). By contrast, a study of CSF has
demonstrated no difference in the amino acid levels in the CSF with
the grade of brain tumor (14). In the
present study, there were significant differences in the plasma Asn
and Gln levels between dogs with meningioma and those with glioma.
In addition, in the case of glioma, the levels of Ala, Cys-Cys,
Pro, Ile and Lys were higher in brain tumor dogs compared with
those in healthy dogs, although there were no statistically
significant differences. This result suggests that amino acid
levels may be associated with tumor type and grade. Furthermore, it
is possible that amino acid levels may change prior to and
following treatment, however further investigations are
required.
In conclusion, the present study demonstrated that
the PFAA levels of dogs with brain tumors were different from those
of healthy dogs, with respect to certain amino acids. PFAA levels
can be determined by a simple test using blood samples, without the
need for special treatments, such as general anesthesia. In
addition, multiple types of cancer besides brain tumors can be
simultaneously tested for in a single sample, which provides a
useful screening test for the early detection of cancer. Future
investigations should be conducted in a larger sample size to
examine changes and prognoses, depending on tumor types, in order
to identify potential diagnostic biomarkers.
Acknowledgements
The authors would like to thank Dr Ogihara who
performed the immunohistochemical analysis.
References
|
1
|
Vandevelde M: Brain tumors in domestic
animals. Proceedings of a conference on brain tumors in man and
animals. Research Triangle Park, NC. 1984;
|
|
2
|
Song RB, Vite CH, Bradley CW and Cross JR:
Postmortem evaluation of 435 cases of intracranial neoplasia in
dogs and relationship of neoplasm with breed, age, and body weight.
J Vet Intern Med. 27:1143–1152. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heidner GL, Kornegay JN, Page RL, Dodge RK
and Thrall DE: Analysis of survival in a retrospective study of 86
dogs with brain tumors. J Vet Intern Med. 5:219–226. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Snyder JM, Shofer FS, Van Winkle TJ and
Massicotte C: Canine intracranial primary neoplasia: 173 cases
(1986–2003). J Vet Intern Med. 20:669–675. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
LeCouteur RA: Current concepts in the
diagnosis and treatment of brain tumours in dogs and cats. J Small
Anim Pract. 40:411–416. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Proenza AM, Oliver J, Palou A and Roca P:
Breast and lung cancer are associated with a decrease in blood cell
amino acid content. J Nutr Biochem. 14:133–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukutake N, Ueno M, Hiraoka N, Shimada K,
Shiraishi K, Saruki N, Ito T, Yamakado M, Ono N, Imaizumi A, et al:
A novel multivariate index for pancreatic cancer detection based on
the plasma free amino acid profile. PLoS One. 10:e01322232015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azuma K, Osaki T, Tsuka T, Imagawa T,
Minami S and Okamoto Y: Plasma free amino acid profiles of canine
mammary gland tumors. J Vet Sci. 13:433–436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tamai R, Furuya M, Hatoya S, Akiyoshi H,
Yamamoto R, Komori Y, Yokoi S, Tani K, Hirano Y, Komori M, et al:
Profiling of serum metabolites in canine lymphoma using gas
chromatography mass spectrometry. J Vet Med Sci. 76:1513–1518.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawabe M, Baba Y, Tamai R, Yamamoto R,
Komori M, Mori T and Takenaka S: Profiling of plasma metabolites in
canine oral melanoma using gas chromatography-mass spectrometry. J
Vet Med Sci. 77:1025–1028. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasegawa T, Sumita M, Horitani Y, Tamai R,
Tanaka K, Komori M and Takenaka S: Gas chromatography-mass
spectrometry-based metabolic profiling of cerebrospinal fluid from
epileptic dogs. J Vet Med Sci. 76:517–522. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yamasaki F, Kurisu K, Kajiwara Y, Watanabe
Y, Takayasu T, Akiyama Y, Saito T, Hanaya R and Sugiyama K:
Magnetic resonance spectroscopic detection of lactate is predictive
of a poor prognosis in patients with diffuse intrinsic pontine
glioma. Neuro-oncol. 13:791–801. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bianchi L, De Micheli E, Bricolo A,
Ballini C, Fattori M, Venturi C, Pedata F, Tipton KF and Corte L
Della: Extracellular levels of amino acids and choline in human
high grade gliomas: An intraoperative microdialysis study.
Neurochem Res. 29:325–334. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Piek J, Adelt T, Huse K and Bock WJ:
Cerebrospinal fluid and plasma aminograms in patients with primary
and secondary tumors of the CNS. Infusionsther Klin Ernahr.
14:73–77. 1987.(In German). PubMed/NCBI
|
|
15
|
Azuma K, Hirao Y, Hayakawa Y, Murahata Y,
Osaki T, Tsuka T, Imagawa T, Okamoto Y and Ito N: Application of
pre-column labeling liquid chromatography for canine plasma-free
amino acid analysis. Metabolites. 6:E32016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ochiai H, Morishita T, Onda K, Sugiyama H
and Maruo T: Canine Lat1: Molecular structure, distribution and its
expression in cancer samples. J Vet Med Sci. 74:917–922. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ogihara K, Onda K, Sato R, Naya Y and
Ochiai H: Evidence of LAT1 expression in canine caput epididymis. J
Vet Med Sci. 77:85–88. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shi SR, Key ME and Kalra KL: Antigen
retrieval in formalin-fixed, paraffin-embedded tissues: An
enhancement method for immunohistochemical staining based on
microwave oven heating of tissue sections. J Histochem Cytochem.
39:741–748. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Locasale JW, Melman T, Song S, Yang X,
Swanson KD, Cantley LC, Wong ET and Asara JM: Metabolomics of human
cerebrospinal fluid identifies signatures of malignant glioma. Mol
Cell Proteomics. 11:0146882012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demir MK, Iplikcioglu AC, Dincer A, Arslan
M and Sav A: Single voxel proton MR spectroscopy findings of
typical and atypical intracranial meningiomas. Eur J Radiol.
60:48–55. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Danjo S, Ishihara Y, Watanabe M, Nakamura
Y and Itoh K: Pentylentetrazole-induced loss of blood-brain barrier
integrity involves excess nitric oxide generation by neuronal
nitric oxide synthase. Brain Res. 1530:44–53. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Righi V, Andronesi OC, Mintzopoulos D,
Black PM and Tzika AA: High-resolution magic angle spinning
magnetic resonance spectroscopy detects glycine as a biomarker in
brain tumors. Int J Oncol. 36:301–306. 2010.PubMed/NCBI
|
















