Introduction
Certain types of food exert beneficial effects on
human health; however, the effects are not explained by the
nutritional content, such as macronutrients, vitamins and minerals
(1). These types of food, termed
functional foods, are food-derived products that enhance normal
physiological or cognitive functions, or prevent the abnormal
function that underlies disease (1).
These food types also contribute to the promotion of
self-medication, i.e., the use of over-the-counter medicines,
including herbal and traditional products, to treat self-recognized
illnesses or symptoms (1,2). Of particular interest is the biological
activity and safety of natural products, including food,
traditional herbs, kampo and their phytochemicals (3–13).
Rosehip is the fruit of rose plants within the genus
Rosa, in particular Rosa canina L., also termed dog rose.
Rosehip has a particularly high vitamin C content compared with
other fruits and vegetables (14,15), and
contains other vitamins, minerals, sugars, fatty acids and
flavonoids (14). Rosehip has
traditionally been administered for the treatment of colds,
infectious diseases and inflammatory diseases (16). In support of its traditional uses,
various studies have reported that rosehip exhibited bioactivity,
including antioxidant (17,18), anti-inflammatory (19–22),
hepatoprotective (23), anti-diabetic
(17) and anti-obesity (24) effects. Therefore, rosehip may be
considered a functional food that promotes health. Although rosehip
has traditionally been administered for treating uric acid (urate)
metabolism disorders (16), its
effects have not been characterized in detail.
In humans, urate is the end product of purine
metabolism and is delivered from hypoxanthine following double
enzyme catalysis by xanthine oxidase (XO) in the liver (25). Serum urate production is regulated by
the endogenous (de novo purine synthesis and tissue catabolism
under normal circumstances) and exogenous (diet including animal
protein) precursor proteins delivered to the liver; whereas its
excretion is controlled by the kidneys through renal plasma flow,
glomerular filtration and proximal tubular exchange (26,27). The
imbalance of its production and excretion induces hyperuricemia,
which also develops into gout and kidney stones, and accelerates
the progression of renal and cardiovascular diseases (28,29).
Potassium oxonate (PO)-treated mice generally serve as a model of
urate overproduction (hyperuricemia), as its intraperitoneal
injection induces the overproduction of urate in mice (30–33). Indeed,
a previous study using PO-treated ABCG2-knockout mice reported that
a decrease of extra-renal urate excretion was one of the most
common causes of hyperuricemia (33).
Numerous studies have demonstrated that the
simultaneous administration of a food or beverage that inhibits
drug-metabolizing enzymes, such as cytochrome P450 (CYP), and a
drug that is metabolized by said enzyme alters blood
concentrations, although occasionally with adverse effects
(34). A phenomenon similar to this
food-drug interaction can be generated between food and drugs.
Indeed, it has previously been demonstrated that beverages and
food, such as beer, red wine, black and herbal tea, garlic, spices,
mace, nutmeg, fruit and fruit juice, tomato juice, and licorice
root inhibited enzyme-mediated drug metabolism (9,10,35–39).
Although rosehip has been used as a food and as a traditional
medicine (16), to the best of our
knowledge, there is no evidence of an interaction between rosehip
and CYP3A4-metabolizing drugs.
In the present study, the effects of hot water,
ethanol and ethyl acetate extracts of rosehip on XO activity were
investigated in an in vitro assay. In addition, the effect
of rosehip hot water extract on urate metabolism was evaluated
according to the level of serum urate in hyperuricemia model mice.
Furthermore, whether rosehip hot water extract inhibits CYP3A4
activity in vitro was investigated.
Materials and methods
Materials
Unless otherwise stated, the various reagents and
the Urate C-test Wako kit were purchased from Wako Pure Chemical
Industries, Ltd. (Osaka, Japan). Rosehip was obtained from the Tree
of Life Co. (Tokyo, Japan). Xanthine oxidase (from buttermilk),
nicotinamide adenine dinucleotide phosphate oxidized form
(β-NADP+), glucose-6-phosphate (G-6-P), and G-6-P
dehydrogenase (G-6-PDH) were purchased from Oriental Yeast, Ltd.
(Tokyo, Japan). Dimethyl sulfoxide (DMSO) and
11α-hydroxyprogesterone were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Human CYP3A4R Bactosomes (recombinant
CYP3A4) were purchased from Cypex Ltd. (Scotland, UK). Mightysil
RP-18 GP 250-4.6 (5 µm) [Octadecylsilyl (ODS) column] was purchased
from Kanto Chemical, Co., Inc. (Tokyo, Japan).
Preparation of rosehip hot water
extract
The extract was prepared from triturated powder (1
g) with 20 ml MilliQ-water at room temperature and decocted at
100°C for 30 min. The extract was cooled, filtered and evaporated
using a freeze dryer, after which the dried sample (yield, 65.2%)
was weighed and dissolved at a concentration of 50 mg/ml in
MilliQ-water.
Preparation of rosehip ethanol or
ethyl acetate extracts
The extracts were prepared from triturated powder (1
g) with 20 ml ethanol or ethyl acetate and agitated at room
temperature for 2 h. The extracts, with ethanol or ethyl acetate,
were subsequently filtered and evaporated, after which the dried
samples were weighed (yields, 5.66 and 0.85%, respectively) and
prepared at a concentration of 50 or 100 mg/ml in DMSO,
respectively.
Measurement of XO activity in
vitro
The measurement of XO activity was performed in
accordance with previously published methods with modifications
(40). Briefly, 151.5 µl Tris-HCl
buffer (pH 7.5; 100 mM), 7.5 µl XO (0.4 U/m) in 50 mM Tris-HCl
buffer (pH 7.5), and 9 µl rosehip extracts [concentrations, 10, 20,
50 and 100 mg/ml (final concentrations, 500, 1,000, 2,500 and 5,000
µg/ml, respectively) in the ethyl acetate extract, and 0.5, 1, 5,
10, 50 mg/ml (final concentrations, 25, 50, 250, 500 and 2,500
µg/ml, respectively) in the hot water and ethanol extracts] were
mixed in 1.5-ml tubes. These tubes were pre-incubated in a
heat-block at 37°C for 5 min. Subsequently, 12 µl xanthine (80 µM)
in 25 mM NaOH was added and incubated at 37°C for 30 min. The tubes
were incubated at 100°C for 1 min to terminate the reaction. To
measure the quantities of urate production, the Urate C-test Wako
kit was used according to the manufacturer's protocol. The relative
XO activity was expressed as a ratio of the absorbance of each
rosehip extract group to that of the corresponding vehicle control
group (MilliQ-water or DMSO). The half maximal inhibitory
concentration (IC50) values were calculated from the XO
activity curves.
Animals
All experiments and the care and handling of the
animals were approved by Josai University (Sakado, Japan)
Institutional Animal Care and Use Committee. Thirty male ddY mice
(age, 5 weeks), obtained from Sankyo Labo Service Corporation, Inc.
(Tokyo, Japan) were used. The mice were housed in six cages with
five mice per cage. They were exposed to a 12-h light/dark cycle
and maintained at a constant temperature of 22±2°C and humidity of
55±10%. The mice were allowed 1 week to adapt to the laboratory
environment prior to the experiments and fed laboratory pellet chow
(CE-2; CLEA Japan Inc., Tokyo, Japan) and water ad libitum. All
mice were euthanized by the intraperitoneal injection of
pentobarbital sodium following completion of the experiments.
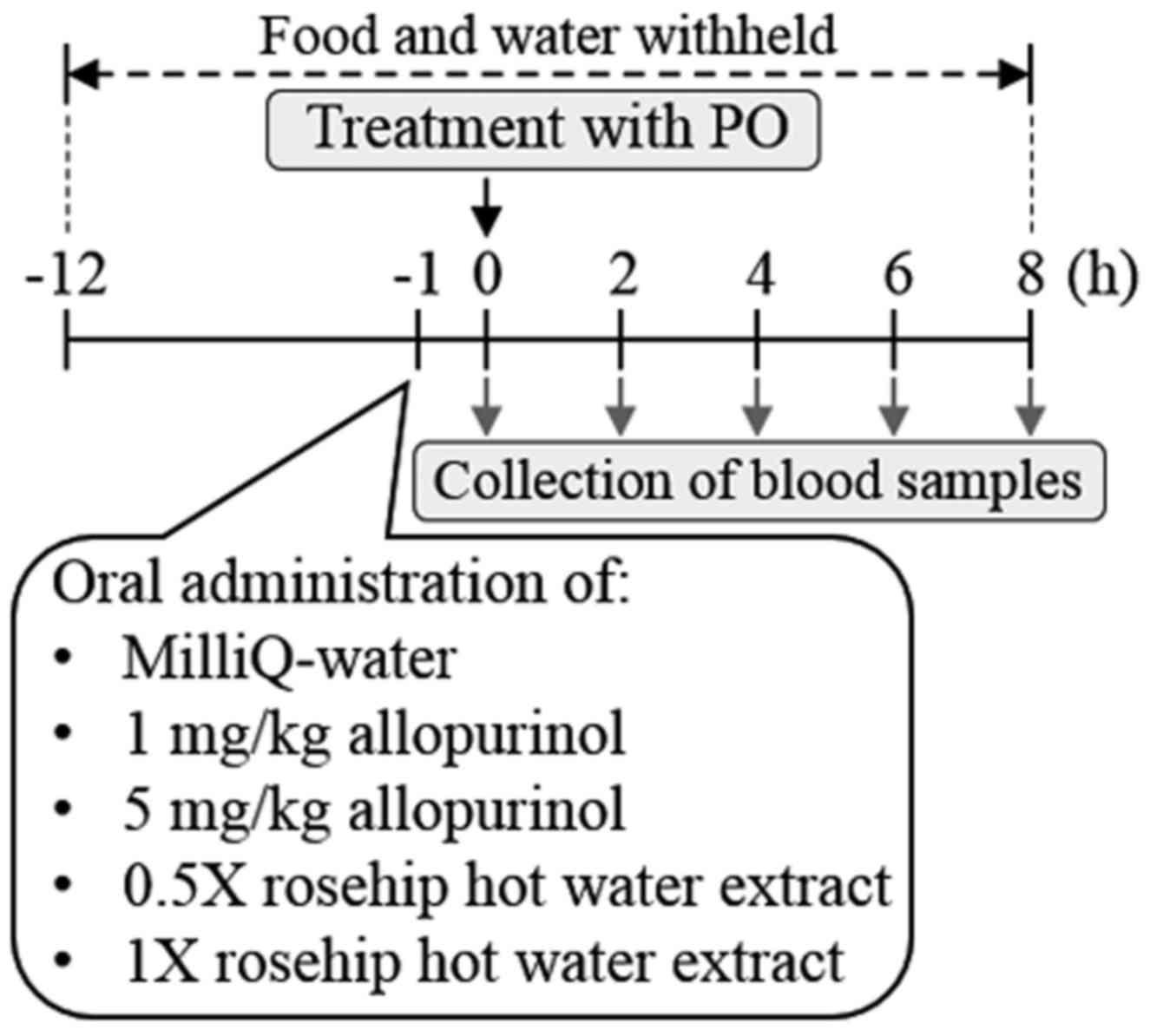
Treatment of hyperuricemia model mice with rosehip
hot water extracts and allopurinol PO, a uricase inhibitor, was
used to establish the hyperuricemia model mice, as described
previously (30–33). Briefly, pellet chow and water supplies
to the ddY mice (age, 6 weeks; body weight, 31.0±0.37 g) were
halted the night before the experiment. The mice were randomly
divided into five groups (n=7 in the control group; n=5 in the 5
mg/ml allopurinol group; and n=6 in the 1 mg/ml allopurinol, 0.5X
or 1X rosehip hot water extract groups. The mice were treated with
PO in 0.5% CMC-Na (280 mg/kg, i.p.) 1 h before oral administration
of 5 ml/kg MilliQ-water (as the control group), 1 or 5 mg/kg
allopurinol, or 5 ml/kg of 0.5X or 1X rosehip hot water extract
(~82.5 and ~165 mg/kg, respectively). A diagram of the timeline of
the experiment is presented in Fig.
1.
Measurement of serum urate
Blood samples (0.1 ml) were collected into 0.6-ml
tubes sequentially at 2, 4, 6 and 8 h via a small incision in the
tail vein using a razor blade. The blood samples were incubated for
1 h at room temperature and centrifuged at 800 × g at 4°C for 15
min. The supernatant (~20 µl) from each blood sample was collected
as serum samples and stored at −20°C until use. The quantity of
serum urate in 3.3 µl of each serum sample was measured using the
Urate C-test Wako kit, according to the manufacturer's
protocol.
Measurement of CYP3A4 activity in
vitro
Measurement of CYP3A4 activity was performed
according to a previously described method (10). Briefly, 125 µl NADPH regenerating
system [2.6 mM β-NADP+, 6.6 mM G-6-P and 6.6 mM
MgCl2 in 400 mM potassium phosphate buffer (pH 7.4)],
12.5 µl G-6-PDH (4 U/ml) in 100 mM Tris-HCl buffer (pH 7.4), 2.5 µl
recombinant CYP3A4 (1.0 nmol/ml), 3.75 µl rosehip hot water
extracts [concentrations, 0.8, 7.4, 22.2, 44.4 and 66.7 mg/ml
(final concentrations, 12, 111, 333, 666 and 1,000 µg/ml,
respectively)], and 105 µl MilliQ-water were mixed and preincubated
at 37°C for 10 min. The reaction was initiated by the addition of
1.25 µl testosterone (60 mM). The reaction was terminated by the
addition of 500 µl 11α-hydroxyprogesterone (10 µM) to the ethyl
acetate after 15 min. Following centrifugation (15,000 × g for 5
min), 400 µl supernatant was collected, dried and suspended in 200
µl methanol. Analyses of the metabolite, 6β-hydroxytestosterone,
were performed using a high-performance liquid chromatography
(HPLC) system (PU-2089, UV-2075 and AS-2057; JASCO Corp., Tokyo,
Japan) equipped with an ODS column. The mobile phase consisted of
70% (v/v) methanol and the metabolites were separated at a flow
rate of 1.0 ml/min. Quantification of the metabolite was performed
by comparing the HPLC peak area at 254 nm to that of
11α-hydroxyprogesterone, the internal standard. The retention times
for 6β-hydroxytestosterone, 11α-hydroxyprogesterone and
testosterone were ~4.3, ~6.2 and ~8.6 min, respectively. The
relative CYP3A4 activity was expressed as the ratio of the HPLC
peak area of each rosehip extract group to that of the
corresponding vehicle (MilliQ-water) control group.
Statistical analysis
Statistical analysis was performed using the
software BellCurve for Excel Ver. 2.1 (Social Survey Research
Information Co., Ltd., Tokyo, Japan). After applying a rejection
test, data were analyzed using Student's t-test and P<0.05 was
considered to indicate a statistically significant difference. Data
are reported as means ± standard deviation in vitro and as
means ± standard error of the mean in vivo.
Results
Dose-dependent effects of rosehip
extracts on XO activities
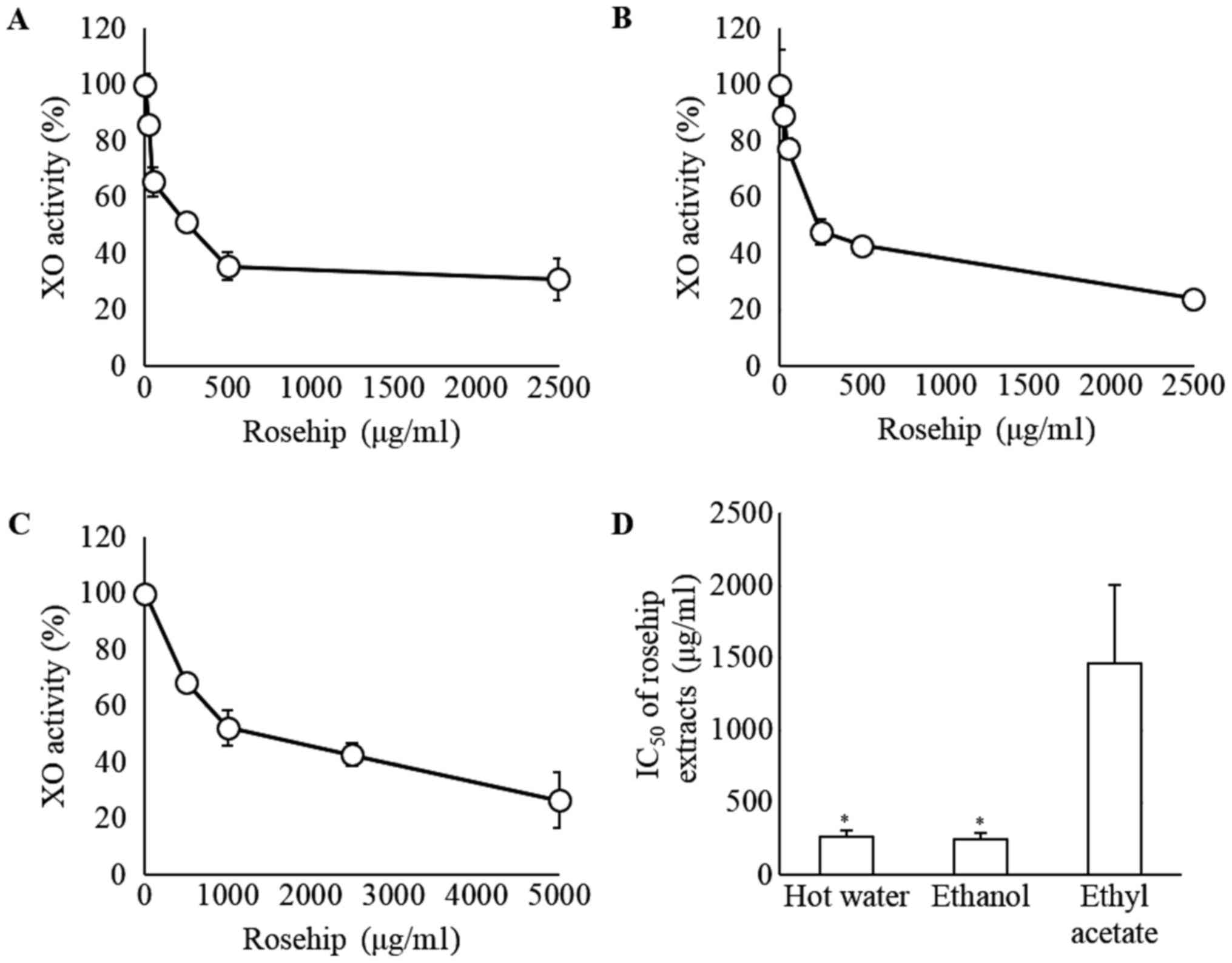
To investigate the XO inhibitory effects of rosehip
extracts, the XO activity was assessed in vitro. Significant
decreases in XO activity were observed in a dose-dependent manner
for the hot water, ethanol and ethyl acetate extracts (Fig. 2A-C, respectively). As shown in Fig. 2D, the IC50 values were
259.6±50.6, 242.5±46.2 and 1,462.8±544.2 µg/ml, respectively. These
values differed significantly between the ethyl acetate extract,
and the hot water or ethanol extract groups (P<0.05; Fig. 2D).
Effects of rosehip hot water extract
on serum urate levels in hyperuricemic mice
All mice were healthy prior to the experiment and
adverse events were not detected during the experiment itself.
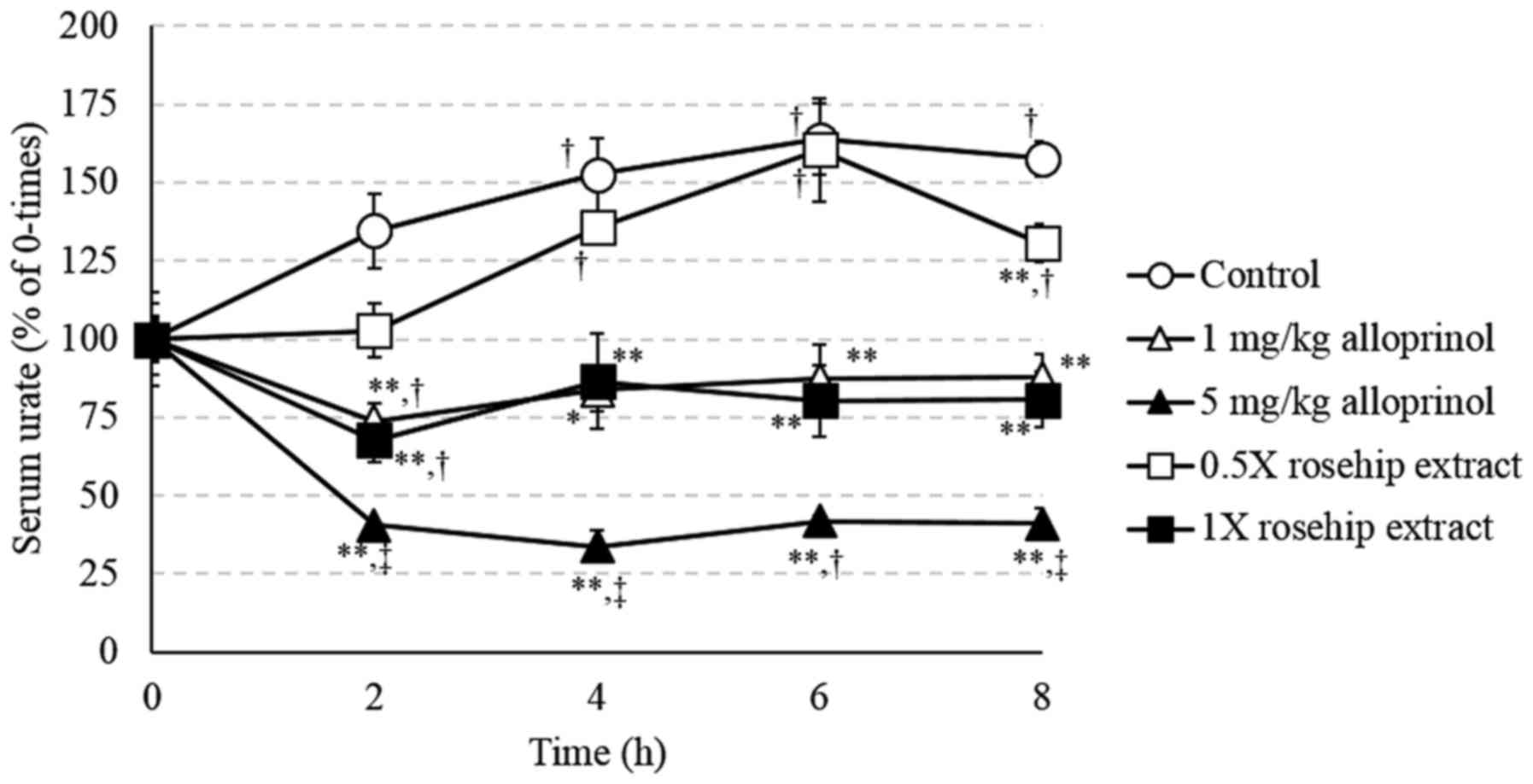
Treatment with PO, a uricase inhibitor, caused hyperuricemia in the
mice, as indicated by a significant increase in serum urate levels
at 4, 6, and 8 h (P<0.05; Fig. 3).
The levels of serum urate upon administration of 5 mg/kg
allopurinol (the 5 mg/kg allopurinol group) were significantly
lower when compared with time 0 (P<0.05 at 6 h; P<0.01 at the
other time-points) and compared with the levels in the control
groups at each time-point (P<0.01). The levels of serum urate
upon administration of 1 mg/kg allopurinol (the 1 mg/kg allopurinol
group) and 1X rosehip hot water extract (1X rosehip extract group)
were significantly lower at 2 h than at time 0 (P<0.05),
however, did not change at 4, 6 and 8 h. Furthermore, these levels
were significantly lower than the levels in the control group
(P<0.01), although there was no difference in these levels at
each point between the 1 mg/kg allopurinol group and the 1X rosehip
extract group (P>0.05). The levels of serum urate upon
administration of 0.5X rosehip hot water extract (the 0.5X rosehip
extract group) were significantly higher at 4, 6 and 8 h than at
time 0 (P<0.05). Furthermore, these levels were significantly
lower when compared with the levels in the control group at 8 h
(P<0.01).
Effects of rosehip hot water extract
on CYP3A4 activities
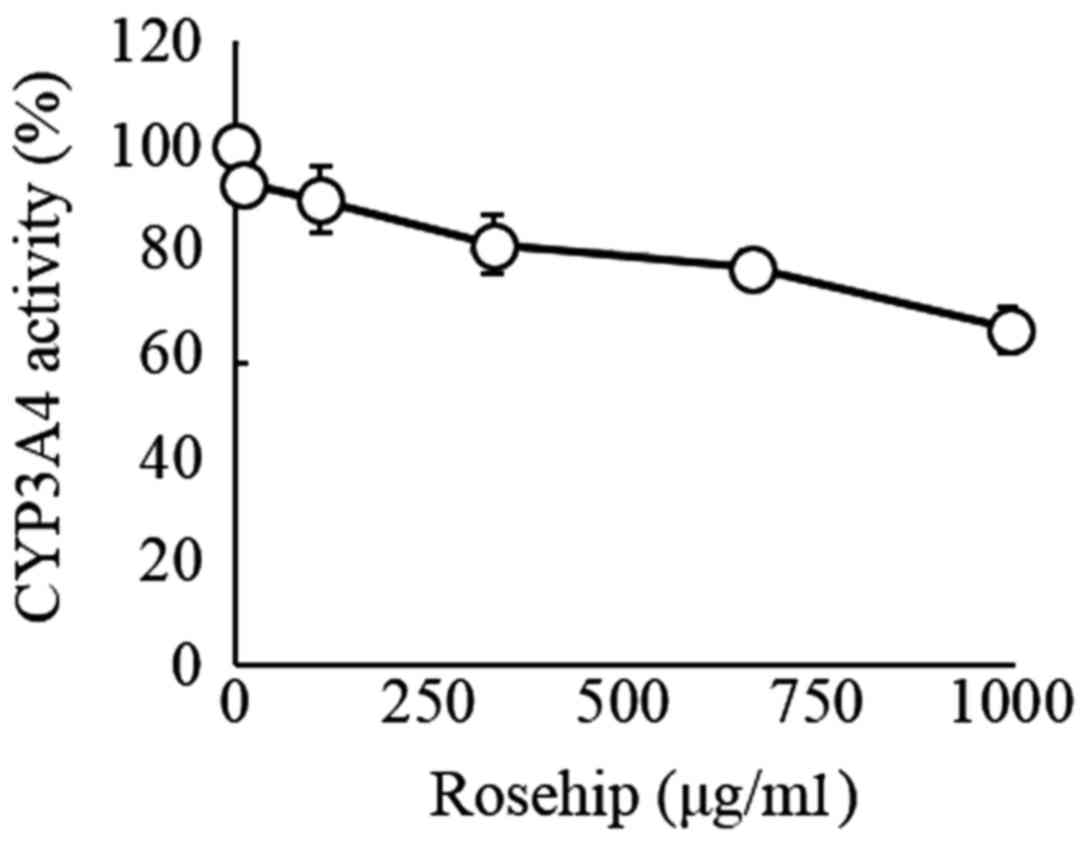
The addition of rosehip hot water extract tended to
decrease the CYP3A4 activity in a dose-dependent manner, although
this decrease was not significant (Fig.
4). Furthermore, at the highest examined concentration (1
mg/ml), rosehip hot water extract inhibited only 40% of CYP3A4
activity (IC50 value, >1 mg/ml).
Discussion
In the present study, it was demonstrated that each
of the rosehip extracts obtained using hot water, ethanol, and
ethyl acetate inhibited XO activity. The different methods of
extracting rosehip also generated different strengths of inhibitory
effect, namely, the hot water and ethanol extracts exhibited
markedly stronger dose-dependent inhibition of XO activity when
compared with the ethyl acetate extract. Numerous studies have
suggested that the particular polarity of an extraction solvent
affects the biological activity of the extract (20,41,42); for example, the free radical scavenging
activities associated with extracts of Galla chinensis, a
traditional Chinese herb, were in the following order: Ethyl
acetate (weak polarity) >ether polarity), because an antioxidant
phytochemical, tannin, in this extract is soluble in non-polar or
weak polar solvents (41). Certain
studies have reported that polyphenols, flavonoids and saponins are
potent XO inhibitors (43–46), and rosehip contains numerous phenolic
phytochemicals (47). Taking these
previous results and the observations of the current study into
account, the difference in the inhibitory effect of rosehip
extracts on XO activity may be associated with the polarity of the
extraction solvent, and rosehip containing phenolic phytochemicals,
the principal substances, are more likely to induce an inhibitory
effect. Identification of the XO-inhibiting constituents in rosehip
is currently underway.
The end product of purine metabolism varies between
species. In the majority of mammals, urate is converted to
allantoin, a more soluble product that is easily excreted in the
urine, by the enzyme uricase (also termed urate oxidase). However,
uricase was lost in hominoids during primate evolution (25). To mimic the purine metabolism of humans
who lack uricase, mice were exposed to PO, a uricase inhibitor
(30–33). In the current study, the oral
administration of hot water extract was selected in hyperuricemic
mice, as rosehip has typically been used for herbal tea and this
extract had a high yield when compared with extracts from other
extraction methods. In addition, allopurinol, an XO inhibitor, was
administered to serve as a positive control. The administration of
1 and 5 mg/kg allopurinol in hyperuricemic mice significantly and
dose-dependently decreased the level of serum urate at each
time-point, indicating that each dose of allopurinol effectively
inhibited XO activity in the hyperuricemic mice. Furthermore, the
administration of 1X rosehip extract reduced the increased serum
urate level to the same extent as the administration of 1 mg/kg
allopurinol. This is identical to the effect observed in the 1
mg/kg allopurinol group, suggesting that the inhibitory effect of
1X rosehip hot water extract on XO activity virtually mimicked the
effect of 1 mg/kg allopurinol.
CYP3A4 is considered to be the most important
drug-metabolizing enzyme, as it metabolizes >50% of all clinical
drugs (48). It is necessary to
consider herb-drug interactions in order to use herbs safely
(9,10,35–39). The present study attempted to determine
whether rosehip hot water extract inhibited CYP3A4 activity. In the
current study, although 12–1,000 µg/ml rosehip hot water extract
tended to exhibit dose-dependent inhibition of CYP3A4 activity, it
had a very weak effect. Therefore, the risk of interaction between
rosehip hot water extract and CYP3A4 substrate drugs appears to be
low. Furthermore, other studies reported that ethyl acetate,
n-butanol and ethanol extracts from rosehip did not induce toxicity
in an acute toxicity test in mice (20), and favorable results were obtained from
clinical trials in osteoarthritis (49–51). Thus,
the safety of rosehip has been confirmed by traditional experiences
of its use, as well as by safety testing.
In conclusion, the current study has demonstrated
for the first time, to the best of our knowledge, that hot water,
ethanol and ethyl acetate extracts of rosehip inhibited XO activity
in vitro and that this inhibitory effect was greater for the
hot water and ethanol extracts. In addition, the oral
administration of rosehip hot water extract decreased the levels of
serum urate in hyperuricemic mice, as a result of the inhibition of
XO activity. Notably, rosehip hot water extract exerted little
effect on CYP3A4 activity. Collectively, these results indicate
that rosehip hot water extract is a promising candidate as a
functional food for individuals with a high urate level and as a
therapeutic reagent of hyperuricemic patients.
Acknowledgements
The present study was supported by the Japan Medical
Herb Association (grant no. FY 2016 for KS). This manuscript was
edited for English language, grammar, punctuation and spelling by
Enago (Mumbai, India).
Glossary
Abbreviations
Abbreviations:
|
CYP
|
cytochrome P450
|
|
XO
|
xanthine oxidase
|
|
β-NADP+
|
nicotinamide adenine dinucleotide
phosphate oxidized form
|
|
G-6-P
|
glucose-6-phosphate
|
|
G-6-PDH
|
glucose-6-phosphate dehydrogenase
|
|
DMSO
|
dimethyl sulfoxide
|
|
ODS
|
octadecylsilyl
|
|
Tris
|
2-amino-2-hydroxymethyl-1,3-propanediol
|
|
PO
|
potassium oxonate
|
References
|
1
|
Galland L: Functional Foods: Health
Effects and Clinical Applications. Elsevier Ltd.; pp. 366–371.
2013
|
|
2
|
World Health Organization (WHO): The role
of the pharmacist in self-care and self-medication. WHO; Geneva:
pp. 151998
|
|
3
|
Kikuchi H, Yuan B, Yuhara E, Imai M,
Furutani R, Fukushima S, Hazama S, Hirobe C, Ohyama K, Takagi N, et
al: Involvement of histone H3 phosphorylation via the activation of
p38 MAPK pathway and intracellular redox status in cytotoxicity of
HL-60 cells induced by Vitex agnus-castus fruit extract. Int J
Oncol. 45:843–852. 2014.PubMed/NCBI
|
|
4
|
Kikuchi H, Yuan B, Yuhara E, Takagi N and
Toyoda H: Involvement of histone H3 phosphorylation through p38
MAPK pathway activation in casticin-induced cytocidal effects
against the human promyelocytic cell line HL-60. Int J Oncol.
43:2046–2056. 2013.PubMed/NCBI
|
|
5
|
Kikuchi H, Yuan B, Nishimura Y, Imai M,
Furutani R, Kamoi S, Seno M, Fukushima S, Hazama S, Hirobe C, et
al: Cytotoxicity of Vitex agnus-castus fruit extract and its major
component, casticin, correlates with differentiation status in
leukemia cell lines. Int J Oncol. 43:1976–1984. 2013.PubMed/NCBI
|
|
6
|
Imai M, Kikuchi H, Yuan B, et al: Enhanced
growth inhibitory effect of 5-fluorouracil in combination with
Vitex agnus-castus fruits extract against a human colon
adenocarcinoma cell line, COLO 201. J Chin Clin Med. 6:14–19.
2011.
|
|
7
|
Imai M, Yuan B, Kikuchi H, Saito M, Ohyama
K, Hirobe C, Oshima T, Hosoya T, Morita H and Toyoda H: Growth
inhibition of a human colon carcinoma cell, COLO 201, by a natural
product, Vitex agnus-castus fruits extract, in vivo and in vitro.
Adv Biol Chem. 2:20–28. 2012. View Article : Google Scholar
|
|
8
|
Yuan B, Imai M, Kikuchi H, et al:
Cytocidal Effects of Polyphenolic Compounds, Alone or in
Combination with, Anticancer Drugs Against Cancer Cells: Potential
Future Application of the Combinatory TherapyApoptosis and
Medicine. Ntuli T: InTech; Croatia: pp. 155–174. 2012
|
|
9
|
Ohno H, Miyoshi S, Araho D, et al:
Efficient utilization of licorice root by alkaline extractionVivo.
28. Brooklyn; pp. 785–794. 2014
|
|
10
|
Sunaga K, Ohkawa K, Nakamura K, Ohkubo A,
Harada S and Tsuda T: Mechanism-based inhibition of recombinant
human cytochrome P450 3A4 by tomato juice extract. Biol Pharm Bull.
35:329–334. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Imai M, Kikuchi H, Denda T, Ohyama K,
Hirobe C and Toyoda H: Cytotoxic effects of flavonoids against a
human colon cancer derived cell line, COLO 201: A potential natural
anti-cancer substance. Cancer Lett. 276:74–80. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sugaya E, Jin W, Sugaya A, Sunaga K and
Tsuda T: Inhibitory effects of peony root extract on the large
conductance calcium-activated potassium current essential in
production of bursting activity. J Herb Pharmacother. 6:65–77.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sunaga K, Sugaya E, Kajiwara K, Tsuda T,
Sugaya A and Kimura M: Molecular mechanism of preventive effect of
peony root extract on neuron damage. J Herb Pharmacother. 4:9–20.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Demir F and Özcan M: Chemical and
technological properties of rose (Rosa canina L.) fruits grown wild
in Turkey. J Food Eng. 47:333–336. 2001. View Article : Google Scholar
|
|
15
|
Ziegler SJ, Meier B and Sticher O: Fast
and Selective Assay of l-Ascorbic Acid in Rose Hips by RP-HPLC
Coupled with Electrochemical and/or Spectrophotometric Detection.
Planta Med. 52:383–387. 1986. View Article : Google Scholar
|
|
16
|
Chrubasik C, Duke RK and Chrubasik S: The
evidence for clinical efficacy of rose hip and seed: A systematic
review. Phytother Res. 20:1–3. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Orhan N, Aslan M, Hosbas S and Orhan
Deliorman D: Antidiabetic Effect and Antioxidant Potential of Rosa
canina Fruits. Pharmacogn Mag. 5:309–315. 2009. View Article : Google Scholar
|
|
18
|
Gao X, Björk L, Trajkovski V and Uggla M:
Evaluation of antioxidant activities of rosehip ethanol extracts in
different test systems. J Sci Food Agric. 80:2021–2027. 2000.
View Article : Google Scholar
|
|
19
|
Christensen LP: Galactolipids as potential
health promoting compounds in vegetable foods. Recent Pat Food Nutr
Agric. 1:50–58. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Orhan Deliorman D, Hartevioğlu A, Küpeli E
and Yesilada E: In vivo anti-inflammatory and antinociceptive
activity of the crude extract and fractions from Rosa canina
L. fruits. J Ethnopharmacol. 112:394–400. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jäger AK, Petersen KN, Thomasen G and
Christensen SB: Isolation of linoleic and α-linolenic acids as
COX-1 and −2 inhibitors in rose hip. Phytother Res. 22:982–984.
2008. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jäger AK, Eldeen IMS and van Staden J:
COX-1 and −2 activity of rose hip. Phytother Res. 21:1251–1252.
2007. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sadeghi H, Hosseinzadeh S, Touri
Akbartabar M, Ghavamzadeh M, Barmak Jafari M, Sayahi M and Sadeghi
H: Hepatoprotective effect of Rosa canina fruit extract against
carbon tetrachloride induced hepatotoxicity in rat. Avicenna J
Phytomed. 6:181–188. 2016.PubMed/NCBI
|
|
24
|
Ninomiya K, Matsuda H, Kubo M, Morikawa T,
Nishida N and Yoshikawa M: Potent anti-obese principle from Rosa
canina: Structural requirements and mode of action of
trans-tiliroside. Bioorg Med Chem Lett. 17:3059–3064. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu XW, Muzny DM, Lee CC and Caskey CT: Two
independent mutational events in the loss of urate oxidase during
hominoid evolution. J Mol Evol. 34:78–84. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lombardi R, Pisano G and Fargion S: Role
of serum uric acid and ferritin in the development and progression
of NAFLD. Int J Mol Sci. 17:5482016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shekarriz B and Stoller ML: Uric acid
nephrolithiasis: Current concepts and controversies. J Urol.
168:1307–1314. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Edwards NL: The role of hyperuricemia in
vascular disorders. Curr Opin Rheumatol. 21:132–137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Feig DI, Kang DH and Johnson RJ: Uric acid
and cardiovascular risk. N Engl J Med. 359:1811–1821. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen MTT, Awale S, Tezuka Y, Shi L,
Zaidi SF, Ueda JY, Tran QL, Murakami Y, Matsumoto K and Kadota S:
Hypouricemic effects of acacetin and 4,5-o-dicaffeoylquinic acid
methyl ester on serum uric acid levels in potassium
oxonate-pretreated rats. Biol Pharm Bull. 28:2231–2234. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhao X, Zhu JX, Mo SF, Pan Y and Kong LD:
Effects of cassia oil on serum and hepatic uric acid levels in
oxonate-induced mice and xanthine dehydrogenase and xanthine
oxidase activities in mouse liver. J Ethnopharmacol. 103:357–365.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Fridovich I: The Competitive Inhibition of
Uricase by Oxonate and by Related Derivatives of s-Triazines. J
Biol Chem. 240:2491–2494. 1965.PubMed/NCBI
|
|
33
|
Ichida K, Matsuo H, Takada T, Nakayama A,
Murakami K, Shimizu T, Yamanashi Y, Kasuga H, Nakashima H, Nakamura
T, et al: Decreased extra-renal urate excretion is a common cause
of hyperuricemia. Nat Commun. 3:7642012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Michalets EL: Update: Clinically
significant cytochrome P-450 drug interactions. Pharmacotherapy.
18:84–112. 1998.PubMed/NCBI
|
|
35
|
Foster BC, Vandenhoek S, Hana J, Krantis
A, Akhtar MH, Bryan M, Budzinski JW, Ramputh A and Arnason JT: In
vitro inhibition of human cytochrome P450-mediated metabolism of
marker substrates by natural products. Phytomedicine. 10:334–342.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Fujita T, Kawase A, Niwa T, Tomohiro N,
Masuda M, Matsuda H and Iwaki M: Comparative evaluation of 12
immature citrus fruit extracts for the inhibition of cytochrome
P450 isoform activities. Biol Pharm Bull. 31:925–930. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sand PG, Dreiseitel A, Stang M, Schreier
P, Oehme A, Locher S and Hajak G: Cytochrome P450 2C19 inhibitory
activity of common berry constituents. Phytother Res. 24:304–307.
2010.PubMed/NCBI
|
|
38
|
Kimura Y, Ito H and Hatano T: Effects of
mace and nutmeg on human cytochrome P450 3A4 and 2C9 activity. Biol
Pharm Bull. 33:1977–1982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim H, Yoon YJ, Shon JH, Cha IJ, Shin JG
and Liu KH: Inhibitory effects of fruit juices on CYP3A activity.
Drug Metab Dispos. 34:521–523. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishibuchi S, Morimoto H, Oe T, Ikebe T,
Inoue H, Fukunari A, Kamezawa M, Yamada I and Naka Y: Synthesis and
structure-activity relationships of 1-phenylpyrazoles as xanthine
oxidase inhibitors. Bioorg Med Chem Lett. 11:879–882. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian F, Li B, Ji B, Yang J, Zhang G, Chen
Y and Luo Y: Antioxidant and antimicrobial activities of
consecutive extracts from Galla chinensis: The polarity affects the
bioactivities. Food Chem. 113:173–179. 2009. View Article : Google Scholar
|
|
42
|
Miraj S: Phytochemical composition and in
vitro pharmacological activity of rose hip (Rosa canina L.).
Pharmachem. 8:117–122. 2016.
|
|
43
|
Owen PL and Johns T: Xanthine oxidase
inhibitory activity of northeastern North American plant remedies
used for gout. J Ethnopharmacol. 64:149–160. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Umamaheswari M, AsokKumar K, Somasundaram
A, Sivashanmugam T, Subhadradevi V and Ravi TK: Xanthine oxidase
inhibitory activity of some Indian medical plants. J
Ethnopharmacol. 109:547–551. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Nana FW, Hilou A, Millogo JF and Nacoulma
OG: Phytochemical composition, antioxidant and xanthine oxidase
inhibitory activities of Amaranthus cruentus L. and
Amaranthus hybridus L. Extracts. Pharmaceuticals (Basel).
5:613–628. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ozyürek M, Bektaşoğlu B, Güçlü K and Apak
R: Measurement of xanthine oxidase inhibition activity of phenolics
and flavonoids with a modified cupric reducing antioxidant capacity
(CUPRAC) method. Anal Chim Acta. 636:42–50. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kılıçgün H and Altıner D: Correlation
between antioxidant effect mechanisms and polyphenol content of
Rosa canina. Pharmacogn Mag. 6:238–241. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Guengerich FP: Role of cytochrome P450
enzymes in drug-drug interactions. Adv Pharmacol. 43:7–35. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Christensen R, Bartels EM, Altman RD,
Astrup A and Bliddal H: Does the hip powder of Rosa canina
(rosehip) reduce pain in osteoarthritis patients? - a meta-analysis
of randomized controlled trials. Osteoarthritis Cartilage.
16:965–972. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Rossnagel K, Roll S and Willich SN: The
clinical effectiveness of rosehip powder in patients with
osteoarthritis. A systematic review. MMW Fortschr Med. 149:51–56.
2007.(In German). PubMed/NCBI
|
|
51
|
Cohen M: Rosehip - an evidence based
herbal medicine for inflammation and arthritis. Aust Fam Physician.
41:495–498. 2012.PubMed/NCBI
|