Introduction
Fractalkine (also termed C-X3-C motif chemokine
ligand 1; CX3CL1) is a unique chemokine of the CX3C subfamily. It
is induced by proinflammatory cytokines and identified as a
secreted chemokine that is an attractant for CX3CR1-expressing
mononuclear cells, and as a membrane-anchored protein that promotes
leukocyte adhesion (1).
Recently, fractalkine involvement in the tumor
microenvironment has been highlighted, indicating it as a potential
target of cancer immunotherapy (2). In
addition, fractalkine has been considered as an important marker
for cancer progression (3,4) including for colorectal cancer (CRC)
(5).
Fractalkine has also been involved in various
non-immune mechanisms associated with psychiatric disorders,
including inhibition of serotonergic neurotransmission by
enhancement of GABA activity at serotonergic neurons, inhibition of
glutamatergic activity in the hippocampal region, and regulation of
processes of neuroplasticity, such as long term potentiation
(6). These mechanisms are constantly
affected in the manifestation of depression and anxiety, which are
the most frequent comorbidities among CRC patients (7).
Mood disorders, such as depression and anxiety,
impair adherence to treatment regimens and affect the quality of
life of CRC patients. Although patient quality of life is often
normalized until the end of antitumor therapy, the frequent
psychological monitoring of patients at risk for depression and
anxiety is highly recommended (8).
Thus, it is becoming increasingly important to use chemokines as
risk biomarkers of psychological disorders, which could guide and
help clinical practice.
Evidence of a role of fractalkine in the
pathophysiology of depression and anxiety combined with the
presence of high levels of this chemokine in cancer patients,
including CRC patients (9), indicates
that it contributes to mood disorders in these individuals. In the
present study, the possible correlations between fractalkine and
psychological manifestations were analyzed in CRC patients at
various stages of antitumor therapy.
Materials and methods
Patient selection
A sample of 80 patients was selected between
October/2014 and May/2015 by convenience at the Clinical Hospital
of the Faculty of Medicine of Ribeirão Preto (Ribeirão Preto, SP,
Brazil). Eligible participants for the study were those diagnosed
with CRC, >18-years-old and male or female. These individuals
were approached in the period in which they were admitted for
surgical resection of the tumor, during the consultations for
chemotherapy and/or return for medical evaluation.
Socio-demographic data included age, sex, education and marital
status. The exclusion criteria were as follows: i) Individuals who
previously received or are receiving radiotherapy or chemotherapy;
ii) history of chronic inflammatory or autoimmune diseases, active
infectious diseases, kidney disease or diabetes; iii) use of
immunosuppressive drugs; iv) patients diagnosed with
schizoaffective disorder, bipolar disorder or panic disorder; and
v) individuals with cognitive impairment that prevents them from
understanding the study design and completing the Hospital Anxiety
and Depression Scale (HADS) questionnaire (10).
Study design
Patients enrolled in the present study were placed
in the following groups (n=20 per group): Pre-surgery group,
patients who were recently diagnosed (from 15 to 30 days) with CRC,
admitted for tumor resection at the Clinical Hospital of the
Faculty of Medicine of Ribeirão Preto; pre-chemotherapy group,
patients who underwent surgical resection and who had not commenced
adjuvant therapy; chemotherapy group, patients undergoing
chemotherapy for ~3 months (regardless of the regimen adopted for
adjuvant treatment); and post-chemotherapy group, patients who
completed the adjuvant chemotherapy regimen for ~6 months. Patients
in the pre- chemotherapy, chemotherapy, and post-chemotherapy
groups presented clinical stage III (local tumor in colon or rectum
>5 cm in diameter and/or has spread to regional lymph nodes)
according to staging system of the American Joint Committee on
Cancer/Union for International Cancer Control (11).
The control group included 20 healthy volunteers
free of any psychiatric or immune system disease. Blood samples
were collected from study participants with vacuum tubes
(Vacutainer; BD Biosciences, Franklin Lakes, NJ, USA). Depression
and anxiety were measured using the Brazilian Portuguese validated
version (12) of the HADS (10). The study was conducted in accordance
with The Code of Ethics of the World Medical Association
(Declaration of Helsinki) and was approved by the Ethics Committee
of College of Nursing, University of São Paulo (Ribeirão Preto, SP,
Brazil). All patients provided written informed consent.
Psychological assessment
HADS is a brief instrument for measuring
psychological distress in cancer patients. It consists of 14 items
and contains two subscales, anxiety and depression. Each item is
rated on a four-point scale, giving maximum scores of 21 for the
two subscales. Scores of 11–21 on each subscale are considered a
significant case of psychological morbidity, while scores of 8–10
represent ‘borderline’, and 0–7 are considered normal (10). For the combined anxiety and depression
score (the total HADS score), a cut-off score of 19 was used in
order to identify patients with severe affective psychopathology
(13).
Cytokine analysis
Venous blood samples (8 ml) were obtained after the
application of the questionnaires and were centrifuged at 1,000 × g
for 10 min at 4°C and the sera were stored at −80°C until assayed.
The concentrations of fractalkine were measured using cytometric
bead array (CBA) kits (BD Biosciences) according to the
manufacturer's instructions. CBA was performed using a BD™
FACSCanto flow cytometer. Quantitative analysis was performed using
FCAP Array™ v3.0 Software (BD Biosciences).
Statistical analysis
Data were analyzed using GraphPad Prism 5 software
(GraphPad Software, Inc., La Jolla, CA, USA). To analyze the
hypothesis of equal means between the groups, Kruskal-Wallis
one-way ANOVA followed by Dunn's multiple comparison post hoc test
were used. The results were expressed as means ± standard
deviation. Pearson's χ2 test was also performed to investigate
correlation among the evaluated parameters. Simple linear
regression was used to verify the correlation between variables.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Demographic characteristics
The characteristics of the study participants are
presented in Table I. Twenty healthy
volunteers formed the control group. The mean age was 47.8±9.0
years and eight (40%) were male. The majority were married (80%)
and had received a secondary education (65%). The pre-surgery group
consisted of patients with CRC who did not undergo surgical
resection. The mean age was 63±11.8 years, nine (45%) were male,
the majority were married (80%), and had completed primary
education (65%). Patients who underwent surgical resection and who
did not start adjuvant therapy formed the pre-chemotherapy group
(mean age, 56.8±7.2 years). Among the 20 subjects in the group,
nine (45%) were male, married, and had completed secondary
education. Patients undergoing chemotherapy for ~3 months formed
the chemotherapy group, with mean age of 56.4±8.6 years. Twelve
(60%) were married, 10 (50%) were male and had completed secondary
education. The post-chemotherapy group consisted of patients who
had completed an adjuvant chemotherapy regimen for ~6 months. The
mean age was 59.9±8.7 years, 10 (50%) were male and married and
nine (45%) had completed primary education. No significant
differences regarding age, sex, marital status or education level
were identified between the groups.
 | Table I.Demographic characteristics of study
participants. |
Table I.
Demographic characteristics of study
participants.
| Characteristics | Healthy volunteers
no. (%) | Pre-surgery, no.
(%) | Pre-chemotherapy, no.
(%) | Chemotherapy, no.
(%) | Post-chemotherapy,
no. (%) | P-value |
|---|
| Age (mean ± SD) | 47.8±9 | 63±11.8 | 56.8±7.2 | 56.4±8.6 | 59.9± 8.7 | nsa |
| Sex |
|
|
|
|
|
|
| Male | 8
(40) | 9
(45) | 9
(45) | 10 (50) | 10 (50) | nsb |
|
Female | 12 (60) | 11 (55) | 11 (55) | 10 (50) | 10 (50) |
|
| Marital status |
|
|
|
|
|
|
|
Single | 2
(10) | 1 (5) | 7
(35) | 7
(35) | 6
(30) | nsb |
|
Married | 16 (80) | 16 (80) | 9
(45) | 12 (60) | 10 (50) |
|
|
Divorced | 1 (5) | 1 (5) | – | – | – |
|
|
Widowed | 1 (5) | 2
(10) | 4
(20) | 1 (5) | 4
(20) |
|
| Education level |
|
|
|
|
|
|
|
Primary | 6
(30) | 13 (65) | 9
(45) | 10 (50) | 8
(40) | nsb |
|
Secondary | 13 (65) | 6
(30) | 6
(30) | 8
(40) | 9
(45) |
|
|
College/university | 1 (5) | 1 (5) | 5
(25) | 2
(10) | 3
(15) |
|
HADS score
Clinically significant levels of anxiety or
depression (indicated by respective HADS scores between 11 and 21)
were verified in CRC patients at different stages of antitumor
therapy (Table II). Each evaluated
group had at least 40% patients with a severe combination of
depression and anxiety (HADS total score, >19).
 | Table II.Depression and anxiety scores of study
participants. |
Table II.
Depression and anxiety scores of study
participants.
| Characteristics | Healthy volunteers
no. (%) | Pre-surgery, no.
(%) | Pre-chemotherapy, no.
(%) | Chemotherapy, no.
(%) | Post-chemotherapy,
no. (%) | P-value |
|---|
| Anxiety (HADS) |
| 0–7 | 17 (85) | 3
(15) | 4
(20) | 5
(25) | 6
(30) |
<0.001a |
| 8–10 | 3
(15) | 6
(30) | 4
(20) | 9
(45) | 9
(45) |
|
|
11–21 | 0 (0) | 11 (55) | 12 (60) | 6
(30) | 5
(25) |
|
| Mean ±
SD | 3.2±2.7 | 11±3.7 | 11±3.9 | 10±3.0 | 9.2±3.3 |
|
| Depression
(HADS) |
| 0–7 | 20
(100) | 3
(15) | 5
(25) | 3
(15) | 3
(15) |
<0.001a |
| 8–10 | 0 (0) | 7
(35) | 9
(45) | 4
(20) | 12 (60) |
|
|
11–21 | 0 (0) | 10 (50) | 6
(30) | 13 (65) | 5
(25) |
|
| Mean ±
SD | 2.5±2.3 | 11.0±3.4 | 9.9±3.7 | 12.0±3.2 | 9.3±2.9 |
|
| Depression and
anxiety (total HADS score) |
| ≤19 | 20
(100) | 7
(35) | 8
(40) | 8
(40) | 12 (60) |
<0.001a |
|
>19 | 0 (0) | 13 (65) | 12 (60) | 12 (60) | 8
(40) |
|
Serum levels of fractalkine
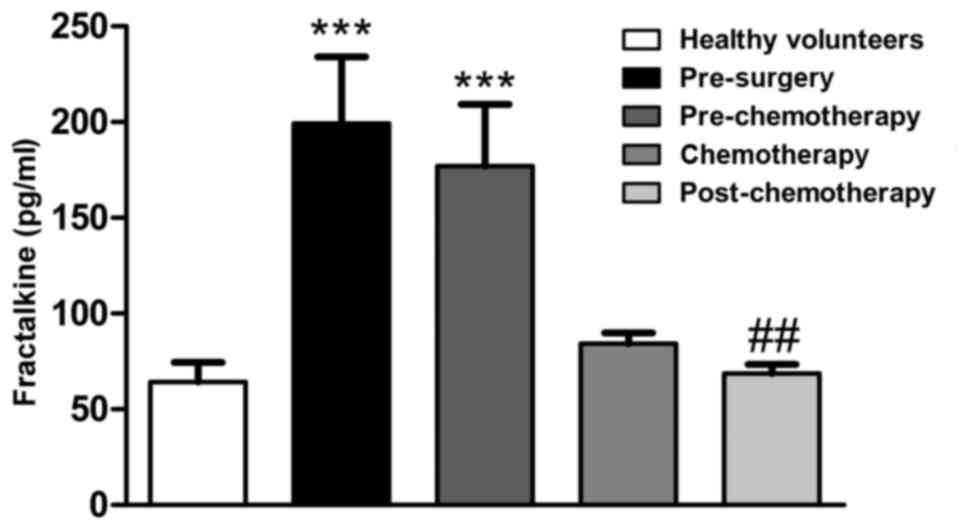
The fractalkine serum levels were observed to be
elevated in CRC patients at the pre-surgery (P<0.001) and
pre-chemotherapy (P<0.001) stages, but reduced following
chemotherapy (P<0.05) when compared with the healthy group
(Fig. 1).
Correlations between serum levels of
fractalkine and HADS scores
Fractalkine serum levels positively correlated
(P<0.05) with anxiety and/or depression (HADS scores) in CRC
patients at different stages of antitumor therapy (Table III). However, in the
post-chemotherapy group, no correlation was identified between
fractalkine levels and depression scores. The global correlation
between fractalkine levels and HADS scores, regardless of the
group, was also quantified, and the highest depression and anxiety
scores were associated with the highest fractalkine serum
levels.
 | Table III.Correlation between serum levels of
cytokines and HADS score in each group (n=20). |
Table III.
Correlation between serum levels of
cytokines and HADS score in each group (n=20).
| Group | Anxiety (HADS) | Depression
(HADS) | Depression and
anxiety (total HADS) |
|---|
| Healthy
volunteers | 0.16 | 0.18 | 0.16 |
| Pre-surgery |
0.45a |
0.35b |
0.26c |
|
Pre-chemotherapy |
0.32b |
0.40b |
0.42b |
| Chemotherapy |
0.40b |
0.24c |
0.38b |
|
Post-chemotherapy |
0.25c | 0.16 |
0.26c |
Discussion
In the current study, the possible correlation of
fractalkine with anxiety and depression symptoms was analyzed in
CRC patients. Elevated fractalkine serum levels were identified in
CRC patients, which varied between the different stages of
antitumor therapy, and positive correlations were observed between
fractalkine levels and depression and anxiety scores. Therefore, it
was hypothesized that fractalkine is involved in such mood
disorders that are observed in CRC patients.
Although various mechanisms act on the regulation of
fractalkine expression in different types of cells, a major
mechanism seems to involve the activation of the nuclear factor
(NF)-κB signaling pathway by tumor necrosis factor (TNF)-α that may
occur on tumor cells allowing the release of the chemokine in the
tumor microenvironment and its reach to the bloodstream (14). Indeed, the level of NF-κB expression
has been shown to be increased in many types of tumors, including
CRC (15) and elevated TNF-α levels
have been identified in CRC patients (16). Thus, increased expression levels of
fractalkine may be a consequence of antitumor immune responses,
which in turn triggers systemic effects that include those in the
central nervous system, which are emphasized in the present
study.
The role of fractalkine in the regulation of the
immune response in the brain has been extensively investigated.
This chemokine is involved in microglial activation by neurons and
the local expression of this mediator enables the modulation of
inflammatory activity in the central nervous system (17). However, fractalkine participates in the
normal physiology of neuronal cells with a relevant role in
neurodegenerative diseases and impaired recovery from sickness
behavior (18). Furthermore, studies
using animal models have described the effects of fractalkine on
brain physiology, which are associated with depression and anxiety
(6). Mice deficient in CX3CR1 cells
have been shown to exhibit resistance to stress-induced
depression-like behavior and to not respond to antidepressant
treatment (19). Therefore, high
levels of systemic fractalkine seems to be linked to the incidence
of depression and anxiety.
In the current study, pre-surgery CRC patients (who
had not begun chemotherapy) exhibited the highest levels of
depression and anxiety symptoms, which may be attributed to the
fact that these patients must deal with the fear, frustration and
uncertainty of successful treatment. These patients also presented
the highest serum levels of fractalkine and the highest level of
agreement in the correlation analysis. Such a correlation is not
arbitrary, and the current study hypothesized that the fractalkine
secreted in the tumor environment reaches the brain, via the
circulation, affecting areas responsible for anxious/depressive
behavior. However, patients in the final stages of treatment, who
underwent surgical resection of the tumor, exhibited depression and
anxiety symptoms, and fractalkine levels similar to those of
healthy volunteers, which indicates that the tumor and other cells
in the tumor microenvironment are a major source of serum
fractalkine in CRC patients.
Based on the current results, it is recommended that
all CRC patients be routinely screened for psychological distress,
particularly if they exhibit increased serum levels of fractalkine.
Routine dosage of this serum chemokine may serve as a biomarker for
the manifestation of depression and anxiety in cancer patients, for
diagnosis and monitoring the patient. Thus, an integral
understanding of the mechanisms linking fractalkine to mood
disorders will allow the design of interventions that lead to an
improved quality of life and overall survival of CRC patients.
Limitations of this study design include its
cross-sectional nature and the relatively small number of
colorectal patients that were included. Therefore, further studies
are required to confirm the current results. In conclusion, these
preliminary findings indicate that fractalkine may serve as a
useful clinical biomarker of depression and/or anxiety risk among
CRC patients undergoing antitumor therapy. Furthermore, as
fractalkine is potentially involved in the pathophysiology of these
comorbidities, it is proposed that fractalkine is included in the
routine screening of CRC patients to allow for psychological
intervention to oncological patients in high-risk situations.
Acknowledgements
The present study was supported by a grant for São
Paulo Research Foundation (FAPESP) (grant nos. 2011/17118-9 and
2013/01262-9). The authors would like to thank the staff of the
Service of Clinical Oncology of Clinical Hospital of the Faculty of
Medicine of Ribeirão Preto (Ribeirão Preto, SP, Brazil), for
assistance with participant recruitment, and all the participants
who generously gave their time to take part.
References
|
1
|
Marchesi F, Locatelli M, Solinas G, Erreni
M, Allavena P and Mantovani A: Role of CX3CR1/CX3CL1 axis in
primary and secondary involvement of the nervous system by cancer.
J Neuroimmunol. 224:39–44. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nelson PJ and Muenchmeier N:
Membrane-anchored chemokine fusion proteins: A novel class of
adjuvants for immunotherapy. OncoImmunology. 2:e266192013.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yao X, Qi L, Chen X, Du J, Zhang Z and Liu
S: Expression of CX3CR1 associates with cellular migration,
metastasis, and prognosis in human clear cell renal cell carcinoma.
Urol Oncol. 32:162–170. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wada A, Ito A, Iitsuka H, Tsuneyama K,
Miyazono T, Murakami J, Shibahara N, Sakurai H, Saiki I, Nakayama
T, et al: Role of chemokine CX3CL1 in progression of multiple
myeloma via CX3CR1 in bone microenvironments. Oncol Rep.
33:2935–2939. 2015.PubMed/NCBI
|
|
5
|
Agalliu I, Xue X, Cushman M, Cornell E,
Hsing AW, Kaplan RC, Anastos K, Rajpathak S and Ho GY:
Detectability and reproducibility of plasma levels of chemokines
and soluble receptors. Results Immunol. 3:79–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rogers JT, Morganti JM, Bachstetter AD,
Hudson CE, Peters MM, Grimmig BA, Weeber EJ, Bickford PC and Gemma
C: CX3CR1 deficiency leads to impairment of hippocampal cognitive
function and synaptic plasticity. J Neurosci. 31:16241–16250. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dunn J, Lynch B, Rinaldis M, Pakenham K,
McPherson L, Owen N, Leggett B, Newman B and Aitken J: Dimensions
of quality of life and psychosocial variables most salient to
colorectal cancer patients. Psychooncology. 15:20–30. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Russell L, Gough K, Drosdowsky A,
Schofield P, Aranda S, Butow PN, Westwood JA, Krishnasamy M, Young
JM, Phipps-Nelson J, et al: Psychological distress, quality of
life, symptoms and unmet needs of colorectal cancer survivors near
the end of treatment. J Cancer Surviv. 9:462–470. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Grivennikov SI and Karin M: Inflammatory
cytokines in cancer: Tumour necrosis factor and interleukin 6 take
the stage. Ann Rheum Dis. 70 Suppl 1:i104–i108. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zigmond AS and Snaith RP: The hospital
anxiety and depression scale. Acta Psychiatr Scand. 67:361–370.
1983. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
American Joint Committee on Cancer (AJCC),
. AJCC Cancer Staging Manual. Edge SB, Byrd DR, Compton CC, Fritz
AG, Greene FL and Trotti A: 7th. Springer; New York, NY: pp.
1432010
|
|
12
|
Botega NJ, Bio MR, Zomignani MA, Garcia C
Jr and Pereira WA: Mood disorders among inpatients in ambulatory
and validation of the anxiety and depression scale HAD. Rev Saude
Publica. 29:355–363. 1995.(In Portuguese). PubMed/NCBI
|
|
13
|
Razavi D, Delvaux N, Farvacques C and
Robaye E: Screening for adjustment disorders and major depressive
disorders in cancer in-patients. Br J Psychiatry. 156:79–83. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isozaki T, Kasama T, Takahashi R, Odai T,
Wakabayashi K, Kanemitsu H, Nohtomi K, Takeuchi HT, Matsukura S and
Tezuka M: Synergistic induction of CX3CL1 by TNF alpha and IFN
gamma in osteoblasts from rheumatoid arthritis: Involvement of
NF-kappa B and STAT-1 signaling pathways. J Inflamm Res. 1:19–28.
2008.PubMed/NCBI
|
|
15
|
Wang S, Liu Z, Wang L and Zhang X:
NF-kappaB signaling pathway, inflammation and colorectal cancer.
Cell Mol Immunol. 6:327–334. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Miranda D Oliveira, de Lima TA Soares,
Azevedo L Ribeiro, Feres O, da Rocha JJ Ribeiro and
Pereira-da-Silva G: Proinflammatory cytokines correlate with
depression and anxiety in colorectal cancer patients. BioMed Res
Int. 2014:7396502014.PubMed/NCBI
|
|
17
|
Zujovic V, Schussler N, Jourdain D,
Duverger D and Taupin V: In vivo neutralization of endogenous brain
fractalkine increases hippocampal TNFalpha and 8-isoprostane
production induced by intracerebroventricular injection of LPS. J
Neuroimmunol. 115:135–143. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Briones TL, Woods J and Wadowska M:
Retraction Note: Chronic neuroinflammation and cognitive impairment
following transient global cerebral ischemia: role of
fractalkine/CX3CR1 signaling. J Neuroinflammation. 12:2202015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hellwig S, Brioschi S, Dieni S, Frings L,
Masuch A, Blank T and Biber K: Altered microglia morphology and
higher resilience to stress-induced depression-like behavior in
CX3CR1-deficient mice. Brain Behav Immun. 55:126–137. 2016.
View Article : Google Scholar : PubMed/NCBI
|