Introduction
Malignant glioma may cause severe damage to human
health, and accounts for ~70% of primary malignant brain tumors,
with an annual incidence of ~5/100,000 in China (1). Also in China, the number of new cases
exceeds 14,000 each year (2). For
patients with grade IV glioblastoma according to World Health
Organization criteria (3), median
survival time is between 14 and 17 months and total annual
mortalities may reach 30,000 worldwide (4). Temozolomide (TMZ) is a novel type of
glioma therapeutic drug, which is able to penetrate the blood-brain
barrier to exert targeted therapeutic effects on brain tumor tissue
(5,6).
To date, studies have aimed to enhance the efficacy and reduce the
toxic side effects of TMZ by reconstructing its chemical structure
(7,8).
To build on these previous studies, the present study utilized the
molecular biological differences between tumor and normal cells to
design TMZ derivatives with enhanced selectivity and targeting
activities through rational computer-aided drug design (CADD).
Alkylglycerone phosphate synthase (AGPS) is
considered to serve as an oncogene through its involvement in lipid
metabolic processes associated with cancer pathogenicity (9). A previous study by our group
demonstrated through RNA interference technology that silencing of
AGPS expression in glioma and liver cancer cells inhibited the
proliferation and invasion of the tumor cells, and improved the
drug sensitivity of resistant cells (10). Thus, AGPS may be a therapeutic
candidate for glioma therapy.
CADD may overcome the disadvantages of traditional
drug development processes, including their long clinical trial
cycle period, low combinatorial variety and low efficiency, by
simulating the interaction between drugs and receptors of
biological macromolecules (11,12). CADD
has identified numerous drugs to date, and is becoming a core
technology in the field of novel drug development (13). In particular, it may aid to identify
more optimized TMZ derivatives with enhanced therapeutic effect in
glioma patients.
On the basis of previous study (14), the current study has taken AGPS as a
target, built a 3D structure-activity relationship model through
CADD technology, and conducted molecular docking of isothiocyanate
(ITC)-TMZ compounds. A total of 10 ITC-TMZ derivatives were
identified as novel compounds with adequate targeting of AGPS.
Additionally, predictions on the absorption, distribution,
metabolism and excretion (ADME) processes and toxicity of the
ITC-TMZ derivatives were established, along with anti-tumor
activity in vitro, in order to elucidate the
structure-activity relationship of the candidate compounds, and
thus further optimize the structure of the lead compounds.
Materials and methods
Protein structure database
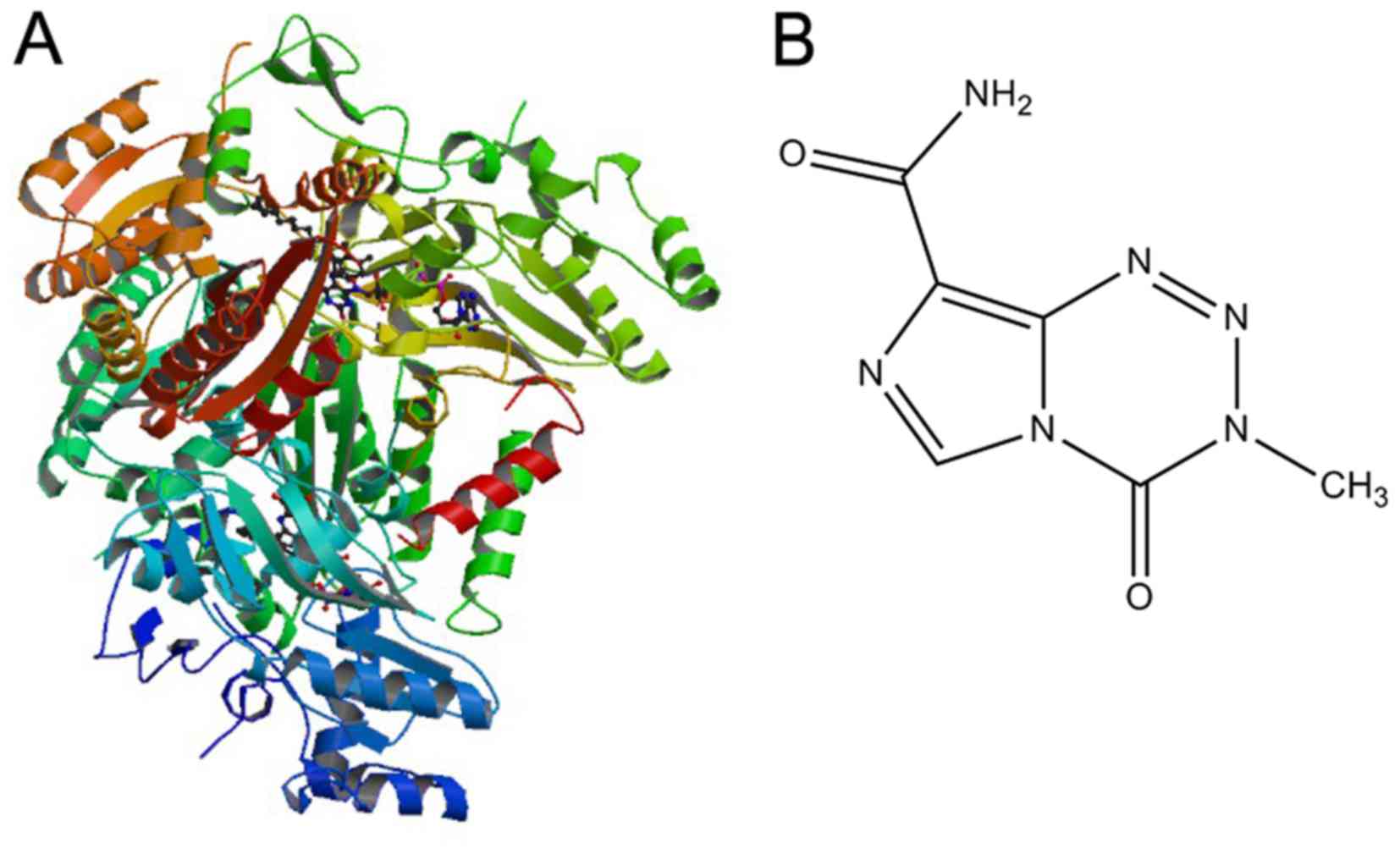
A 3D structure model of AGPS (Fig.1A) was downloaded from the Protein Data
Bank (www.rcsb.org; PDB ID: 2UUV) and the protein
modules were processed using Discovery Studio 3.5 software (BIOVIA,
San Diego, CA, USA) as follows: A single chain (the A chain) in the
tetramer was retained; missing atoms to residues were added; the
loop area was repaired; combinations and free water molecules were
removed; unsaturated atoms were hydrogenated; valence was repaired;
protein and original ligands were retained; and six basic
pharmacophore elements were selected, namely hydrogen bond
acceptor, hydrogen bond donor, hydrophobic, positive electricity
center, negative electricity center and aromatic center. This
obtained a final 3D AGPS structure for docking. The structure of
TMZ used is depicted in Fig. 1B
(15).
Virtual screening
The structures of TMZ derivatives were combined with
benzyl isothiocyanate (BITC) and the ITC-TMZ derivatives were drawn
using ChemBioOffice 2010 (PerkinElmer, Waltham, MA, USA), and each
structure was saved in mol2 format. The structures were imported
into Discovery Studio 3.5 for virtual screening. The CDOCKER
protocol in Discovery Studio 3.5 was used for molecular docking;
‘CDOCKER energy’ was used as a scoring index, with higher scores
indicating greater docking affinity.
The experiment screened 50
molecules
Firstly, it used the pharmacophore model generated
by the HipHop algorithm to perform fit value sorting of the
compounds based on the characteristics of the pharmacophore model,
with small molecular compounds with the highest scores retained
(16). The pharmacophore model
generated by the Counting Belief Propagation algorithm was then
used on reserved compounds to obtain preferable compounds that
conformed to the characteristics of the two pharmacophore types
(17). BITC was used as a control for
the virtual screening.
ADME and toxicity prediction
Protein structure processing was completed with the
QikProp module of Schrodinger Suite 2009 software (Schrodinger,
LLC, Cambridge, MA, USA). The downloaded AGPS target crystal
structure (PDB ID: 2UUV) was imported into the Schrodinger software
and processed as follows: Metal ions were removed; unsaturated
atoms were hydrogenated; amino acid residues at the N and C termini
were made neutral; and protein and original ligands were retained
to remove atom and water molecule impurities. The OPLS_2005
position was selected to optimize protein structure and utilize
sample water orientations as parameters to optimize hydrogen
bonding.
Ligand preparation utilized ChemBioDraw Ultra 11.0
in the ChemBioOffice 2010 to draw planar structures of the
micromolecules, which were imported into ChemBio3D Ultra 11.0 for
the generation of 3D structures, which were saved in mol2 format.
The mol2fileswere then imported into Schrodinger Suite 2009
software and processed with the LigPrep module, complete desalting,
add charge and produce ionization consistent with human body pH.
The OPLS_2005 position was also selected for the optimized
molecules to match that of the optimized acceptor. ADME parameters,
namely oil-water partition coefficient (logPo/w), polarization
surface area (PSA), water solubility (logS) and apparent Maden
Darby Canine Kidney cell permeability (PMDCK), and toxicity
factors, namely rodent carcinogenicity, mutagenicity, skin
irritancy, ocular irritation and aerobic biodegradability, were
subsequently simulated in the Schrodinger Suite 2009 software.
Cell lines and culture
The human glioma cell lines U87MG and U251 were
obtained from the Cell Bank of Type Culture Collection of Chinese
Academy of Sciences (Shanghai, China). Cells were cultured in
Dulbecco's modified Eagle's medium (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) with 10% fetal bovine serum (Thermo Fisher
Scientific, Inc.) at 37°C with 5% CO2. It is important to note that
U87MG has been identified to differ from the original cell line,
and is probably an authentic human glioblastoma cell line of
unknown origin according to DNA profiling (18).
MTS assay
A total of 5×103 cells/well were cultured in 96-well
plates at 37°C with 5% CO2 for 24 h. ITC-TMZ derivatives were
designed and synthesized via CADD (14) and added to the medium at final
concentrations of 0 (negative control), 1, 2, 5, 10, 20, 50 and 100
µM, respectively. Subsequently, the cells were cultured for 72 h at
37°C, after which the medium was replenished with 200 µl
plasma-free medium and 40 µl MTS, followed by incubation at 37°C
for another 4 h. The optical density (OD) value was measured using
an enzyme-labeled meter at 490 nm. Inhibition rate (%) was
calculated as follows: (1-OD value of the medicine-treated group/OD
value of the negative control group) × 100%. Half maximal
inhibitory concentrations (IC50s) were subsequently determined
using GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla,
CA, USA).
Results
Virtual screening and core
hopping
Visual screening of 50 small molecular compounds was
performed using Schrodinger Suite 2009. A total of 10 ITC-TMZ
derivatives and the compound BITC exhibited micromolecular
structures with improved combination with the target protein AGPS
compared with TMZ, determined by referring to the combination mode
of the micromolecules and surrounding amino acids, and evaluation
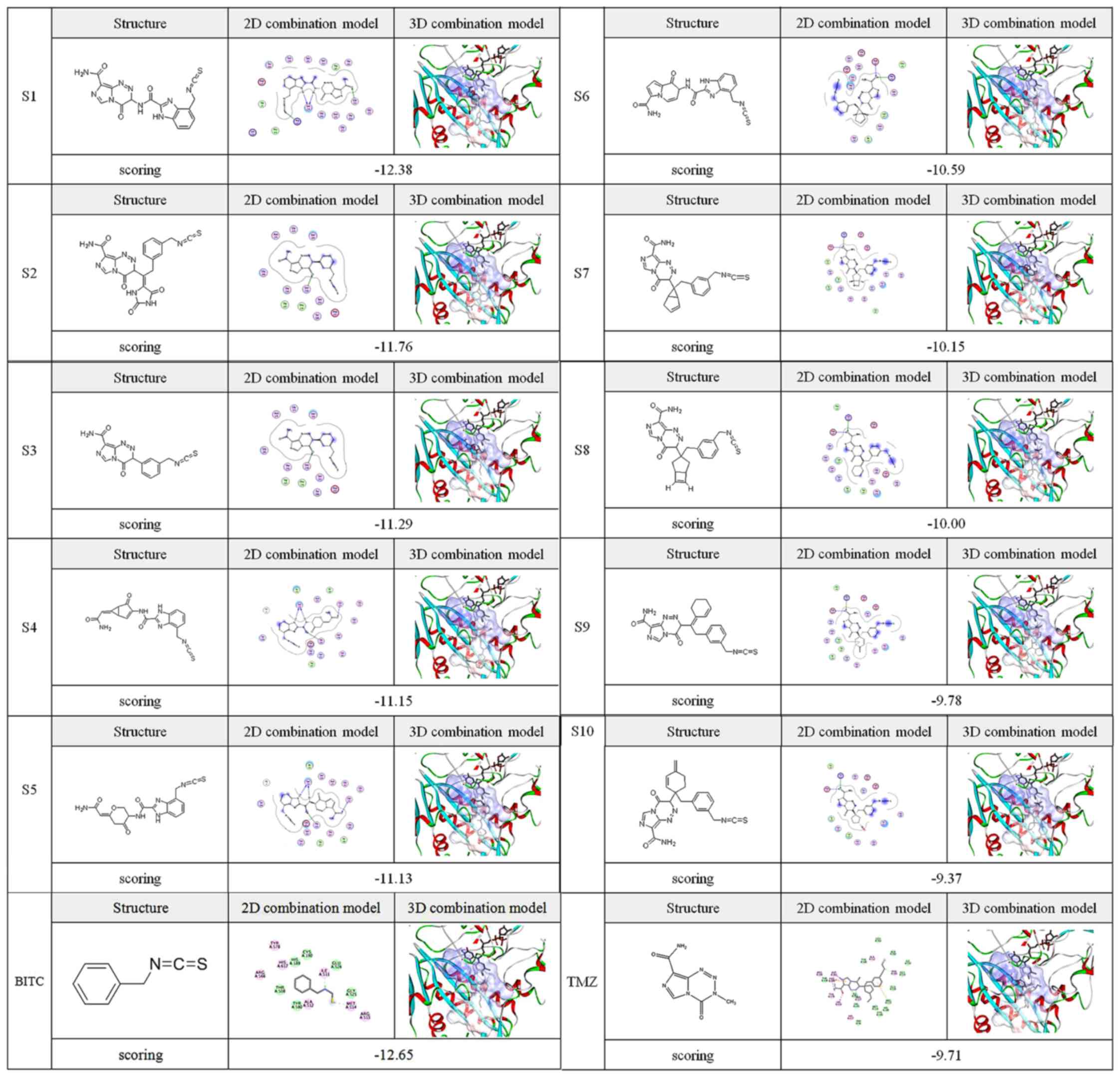
of scoring function from CDOCKER energy. The scoring of the docking
between these micromolecules [structures (S)1-10 and BITC] and the
AGPS active site is presented in Fig.
2. The 11 candidate compounds exhibited marked interactions
with the protein cavity, with key amino acid residues forming
hydrogen bonds and the hydrophobic effect occurring with nitrogen
and sulfur atoms and carbon-sulfur covalent bonds of the ITC-TMZ
derivative molecules. The other substituent residues in the
micromolecules formed certain hydrogen bonding interactions with
surrounding residues to an extent. As a result, as evident in the
pattern diagrams (Fig. 2), the main
ITC active group and small regions of substitute residues in the
TMZ derivatives with some impurity atoms were respectively
connected to both sides of the hydrophobic and rigid ring planes.
These structural data suggest improved interactions and combination
of the ITC-TMZ derivatives with the AGPS target compared with TMZ.
Indeed, the docking scores of S1-9 and BITC were increased compared
with that of TMZ, suggesting enhanced AGPS targeting.
ADME and toxicity predictions
ADME properties, including logPo/w, PSA, logS and
PMDCK, and toxicity properties, including rodent carcinogenicity,
mutagenicity, skin irritancy, ocular irritation and aerobic
biodegradability, aid to determine whether therapeutic candidates
are of clinical standard (16). The
present results on ADME (Table I) and
toxicity (Table II) parameters
indicated the 10 ITC-TMZ derivatives to have improved interaction
with AGPS. Firstly, the results suggested adequate ADME parameters
for BITC regarding physico-chemical properties. In turn, the ADME
parameters of S3, −6, −7, −8, −9 and −10 were optimized compared
with TMZ, particularly regarding logPo/w, logS and PMDCK,
suggesting the structure of BITC may improve TMZ physico-chemical
properties. Meanwhile, the general toxicity parameters of the above
compounds were not increased according to the predictions.
 | Table I.ADME predictions of isothiocyanate-TMZ
derivatives. |
Table I.
ADME predictions of isothiocyanate-TMZ
derivatives.
|
| ADME parameter |
|---|
|
|
|
|---|
| Candidate | PSAa | logPo/wb | logSc | PMDCKd |
|---|
| S1 | 204.93 |
0.596 |
−4.40485 |
13.345 |
| S2 | 205.35 |
1.243 |
−5.29240 |
10.342 |
| S3 | 147.15 |
2.015 |
−4.10003 |
111.705 |
| S4 | 162.39 |
0.869 |
−4.41749 |
46.410 |
| S5 | 171.62 |
0.896 |
−4.14306 |
50.200 |
| S6 | 167.32 |
1.560 |
−4.32601 |
70.105 |
| S7 | 147.15 |
2.536 |
−4.94407 |
183.872 |
| S8 | 147.15 | 2.99 |
−5.45929 |
481.063 |
| S9 | 147.15 |
3.623 |
−6.24712 |
121.998 |
| S10 | 147.15 |
3.293 |
−5.66361 |
875.571 |
| BITC |
44.45 |
3.321 | −2.5125 | 10,000.000 |
| TMZ |
79.92 | −1.810 | −1.1118 |
66.744 |
 | Table II.Toxicity prediction of
isothiocyanate-TMZ derivatives. |
Table II.
Toxicity prediction of
isothiocyanate-TMZ derivatives.
| Candidate | Mouse female NTP
prediction | Mouse male NTP
prediction | Rat female NTP
prediction | Rat male NTP
prediction | Ames test
prediction | Skin irritancy | Ocular irritancy | Aerobic
biodegradability prediction |
|---|
| S1 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S2 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S3 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S4 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S5 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S6 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S7 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S8 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S9 | NC | NC | NC | NC | NM | Mild | Moderate | ND |
| S10 | NC | NC | NC | NC | NM | Mild | Mild Severe | ND |
| TMZ | NC | NC | NC | NC | NM | None | Mild | ND |
| BITC | NC | NC | C | C | NM | Mild | Severe | ND |
Anti-tumor activity of ITC-TMZ
derivatives
MTS assay was performed to observe the effect of the
ITC-TMZ derivatives on the viability of human glioma U87MG and U251
cells in vitro. On determination of IC50 values, S1, −3, −8
and −10 were identified to exert greater inhibitory effect on
glioma cell viability compared with TMZ (Table III).
 | Table III.IC50 values of isothiocyanate-TMZ
derivatives in human glioma cell lines. |
Table III.
IC50 values of isothiocyanate-TMZ
derivatives in human glioma cell lines.
|
| IC50 (µM) |
|---|
|
|
|
|---|
| Candidate | U87MG | U251 |
|---|
| S1 |
21.7 |
28.3 |
| S2 |
35.8 |
47.4 |
| S3 |
24.5 |
31.2 |
| S4 | 115.2 | 168.5 |
| S5 |
76.7 |
62.5 |
| S6 |
98.6 |
87.1 |
| S7 |
87.4 |
88.5 |
| S8 |
41.8 |
32.2 |
| S9 | 157.6 |
91.5 |
| S10 |
45.5 |
35.8 |
| BITC |
15.2 |
18.7 |
| TMZ |
54.5 |
37.5 |
Discussion
In 1920, cancer was described as a metabolic disease
by Otto Warburg, due to his identification of the high glycolytic
rate of tumor cells (19). A
subsequent study demonstrated that the process of tumor development
is accompanied by abnormal lipid metabolism (20). Notably, inactivation of the key enzyme
for ether lipid synthesis, AGPS, has been reported to reduce ether
lipid levels in tumor cells and thus decrease cancer pathogenicity
(10). Additionally, its
overexpression may enhance the ether lipid levels of tumor cells,
increase the survival and motility of tumor cells, and promote the
growth and invasion of tumors (21).
Therefore, AGPS is considered to be a novel target of antitumor
drugs, with specific inhibitors expected to have marked advantages
over traditional chemotherapy methods. The characteristic elements
of an effective inhibitor include the following: A hydrogen bond
acceptor, a hydrogen bond donor, hydrophobicity, a positive
electricity center, a negative electricity center and an aromatic
center (22,23). The present study identified elevated
docking scores for S1-9 compared with TMZ, suggesting enhanced AGPS
targeting over TMZ.
ADME and toxicity are considered as important drug
properties (24). In previous years,
drug discovery principally focused on the discovery of active
compounds. However, prior to the development stage, this method
encountered problems regarding pharmacokinetics (PK), toxicity,
solubility and stability (25). A
previous study reported that drug failure in the developmental
stage typically occurred due to poor biopharmaceutical properties
(PK and bioavailability) (26). Due
to high development costs, this failure results in major economic
loss in the field of drug development. Therefore, it may be
concluded that drug ADME and toxicity are now key considerations in
the process of drug discovery, and may be used to guide the
selection and optimization of lead compounds.
The present study identified that while the ADME
parameters of TMZ were suitable, those of BITC were enhanced
regarding physico-chemical properties, thus implicating BITC as a
favorable modification for TMZ. Additionally, the physical and
chemical ADME parameters of S3, −6, −7, −8, −9 and −10 were
optimized compared with TMZ, particularly regarding logPo/w, logS
and PMDCK. The thiocyanate group may serve a key role in these
improvements. However, all of the derivatives exhibited similar
toxicity to TMZ, suggesting the thiocyanate group is unable to
decrease the toxicity of TMZ.
Nevertheless, the ADME parameters of S3, −6, −7, −8,
−9 and −10 indicated the suitability of these candidates as
potential clinical drugs, which thus require further study.
Additionally, the present study investigated the activities of
S1-10 in vitro following their synthesis, and identified
that the ITC-TMZ derivatives S1, −8 and −10 suppressed the
proliferation of U87MG and U251 cells to a greater extent than TMZ,
again indicating their potential as anti-cancer drugs. Due to the
established anti-tumor activity of TMZ, the thiocyanate group may
also be key to proliferation inhibition.
In conclusion, the present study identified the
ITC-TMZ derivatives S1, −8 and −10 to be potential anti-cancer
drugs. The results for S8 and −10 were in accordance with the
predictions by CADD, while S1 exhibited more optimal activity than
predicted from docking score. Thus, the thiocyanate group may be
involved in a complex mechanism influenced by differences in
simulated and in situ environments, compound stability and cell
membrane interference, among other factors, which warrants further
investigation. Additionally, although U87MG was implicated to be of
unknown origin, albeit an authentic human glioblastoma cell line,
the results indicated the anti-tumor activity of S1, −3, −8 and −10
against glioblastoma.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant nos. 31501159 and
81702637), the Tianjin Public Health Key Research Project (grant
no. 15KG108), the Tianjin Science and Technology Key Project on
Chronic Diseases Prevention and Treatment (grant no.
16ZXMJSY00020), the Natural Science Foundation of Tianjin (grant
no. 16JCQNJC12100) and the Special Program of Talent Development
for Excellent Youth Scholars in Tianjin, China (grant no.
TJTZJH-QNBJRC-2-9).
Availability of data and materials
All data and materials relevant to this study are
described in this published article or available from the
corresponding author on reasonable request.
Authors' contributions
BY was responsible for cell experiments, XL for
computer-aided drug design, LH for the MTS assay and YZ for the
study design.
Ethics approval and consent to
participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang H, Nie W, Zhang X, Zhang G, Li Z, Wu
H, Shi Q, Chen Y, Ding Z, Zhou X, et al: NEDD4-1 regulates
migration and invasion of glioma cells through CNrasGEF
ubiquitination in vitro. PLoS One. 8:e827892013. View Article : Google Scholar
|
|
2
|
Kahlert UD, Nikkhah G and Maciaczyk J:
Epithelial-to-mesenchymal(-like) transition as a relevant molecular
event in malignant gliomas. Cancer Lett. 331:131–138. 2013.
View Article : Google Scholar
|
|
3
|
Louis DN, Perry A, Reifenberger G, von
Deimling A, Figarella-Branger D, Cavenee WK, Ohgaki H, Wiestler OD,
Kleihues P and Ellison DW: The 2016 World Health Organization
Classification of Tumors of the Central Nervous System: A summary.
Acta Neuropathol. 131:803–820. 2016. View Article : Google Scholar
|
|
4
|
Zhu Y, Yang P, Wang Q, Hu J, Xue J, Li G,
Zhang G, Li X, Li W, Zhou C, et al: The effect of CXCR4 silencing
on epithelial-mesenchymal transition related genes in glioma U87
cells. Anat Rec (Hoboken). 296:1850–1856. 2013. View Article : Google Scholar
|
|
5
|
Gai XJ, Wei YM, Tao HM, An DZ, Sun JT and
Li BS: Comparison of long-term survival between temozolomide-based
chemoradiotherapy and radiotherapy alone for patients with
low-grade gliomas after surgical resection. Onco Targets Ther.
9:5117–5121. 2016. View Article : Google Scholar
|
|
6
|
Ashby LS, Smith KA and Stea B: Gliadel
wafer implantation combined with standard radiotherapy and
concurrent followed by adjuvant temozolomide for treatment of newly
diagnosed high-grade glioma: A systematic literature review. World
J Surg Oncol. 14:2252016. View Article : Google Scholar
|
|
7
|
Коbylinska LI, Klyuchivska OY, Grytsyna
II, Finiuk N, Panchuk RR, Starykovych MO, Lehka L, Lesyk RB,
Zіmenkovsky BS and Stoika RS: Differential pro-apoptotic effects of
synthetic 4-thiazolidinone derivative Les-3288, doxorubicin and
temozolomide in human glioma U251 cells. Croat Med J. 58:150–159.
2017. View Article : Google Scholar
|
|
8
|
Chen TC, Cho HY, Wang W, Wetzel SJ, Singh
A, Nguyen J, Hofman FM and Schönthal AH: Chemotherapeutic effect of
a novel temozolomide analog on nasopharyngeal carcinoma in vitro
and in vivo. J Biomed Sci. 22:712015. View Article : Google Scholar
|
|
9
|
Piano V, Benjamin DI, Valente S, Nenci S,
Marrocco B, Mai A, Aliverti A, Nomura DK and Mattevi A: Discovery
of Inhibitors for the Ether Lipid-Generating Enzyme AGPS as
Anti-Cancer Agents. ACS Chem Biol. 10:2589–2597. 2015. View Article : Google Scholar
|
|
10
|
Zhu Y, Liu XJ, Yang P, Zhao M, Lv LX,
Zhang GD, Wang Q and Zhang L: Alkylglyceronephosphate synthase
(AGPS) alters lipid signaling pathways and supports chemotherapy
resistance of glioma and hepatic carcinoma cell lines. Asian Pac J
Cancer Prev. 15:3219–3226. 2014. View Article : Google Scholar
|
|
11
|
Cai Z, Zhang G, Zhang X, Liu Y and Fu X:
Current insights into computer-aided immunotherapeutic design
strategies. Int J Immunopathol Pharmacol. 28:278–285. 2015.
View Article : Google Scholar
|
|
12
|
Scotti L and Scotti MT: Computer Aided
Drug Design Studies in the Discovery of Secondary Metabolites
Targeted Against Age-Related Neurodegenerative Diseases. Curr Top
Med Chem. 15:2239–2252. 2015. View Article : Google Scholar
|
|
13
|
Baig MH, Ahmad K, Rabbani G, Danishuddin
and Choi I: Danishuddin and Choi I: Computer aided drug design and
its application to the development of potential drugs for
neurodegenerative disorders. Curr Neuropharmacol. Oct 16–2017.(Epub
ahead of print). View Article : Google Scholar
|
|
14
|
Zhu Y, Liu A, Zhang X, Qi L, Zhang L, Xue
J, Liu Y and Yang P: The effect of benzyl isothiocyanate and its
computer-aided design derivants targeting alkylglycerone phosphate
synthase on the inhibition of human glioma U87MG cell line. Tumour
Biol. 36:3499–3509. 2015. View Article : Google Scholar
|
|
15
|
Hvizdos KM and Goa KL: Temozolomide. CNS
Drugs. 12:237–243. 1999. View Article : Google Scholar
|
|
16
|
Zhang J, Hao QQ, Liu X, Jing Z, Jia WQ,
Wang SQ, Xu WR, Cheng XC and Wang RL: Molecular docking, 3D-QSAR
and structural optimization on imidazo-pyridine derivatives dually
targeting AT1 and PPARg. Oncotarget. 8:25612–25627. 2017.
|
|
17
|
Ma Y, Jin YY, Wang YL, Wang RL, Lu XH,
Kong DX and Xu WR: The discovery of a novel and selective inhibitor
of PTP1B over TCPTP: 3D QSAR pharmacophore modeling, virtual
screening, synthesis, and biological evaluation. Chem Biol Drug
Des. 83:697–709. 2014. View Article : Google Scholar
|
|
18
|
Allen M, Bjerke M, Edlund H, Nelander S
and Westermark B: Origin of the U87MG glioma cell line: Good news
and bad news. Sci Transl Med. 8:354re32016. View Article : Google Scholar
|
|
19
|
Seyfried TN: Cancer as a mitochondrial
metabolic disease. Front Cell Dev Biol. 3:432015. View Article : Google Scholar
|
|
20
|
Liegel RP, Ronchetti A and Sidjanin DJ:
Alkylglycerone phosphate synthase (AGPS) deficient mice: Models for
rhizomelic chondrodysplasia punctate type 3 (RCDP3) malformation
syndrome. Mol Genet Metab Rep. 1:299–311. 2014. View Article : Google Scholar
|
|
21
|
Zhu Y, Li WM, Zhang L, Xue J, Zhao M and
Yang P: Inhibitory effect of isothiocyanate derivant targeting AGPS
by computer-aid drug design on proliferation of glioma and hepatic
carcinoma cells. Int J Clin Exp Pathol. 8:812–817. 2015.
|
|
22
|
Turku A, Borrel A, Leino TO, Karhu L,
Kukkonen JP and Xhaard H: A pharmacophore model to discover OX1 and
OX2 orexin receptor ligands. J Med Chem. 59:8263–8275. 2016.
View Article : Google Scholar
|
|
23
|
Medeiros Turra K, Pineda Rivelli D,
Berlanga de Moraes Barros S and Mesquita Pasqualoto KF:
Constructing and Validating 3D-pharmacophore Models to a Set of
MMP-9 Inhibitors for Designing Novel Anti-melanoma Agents. Mol
Inform. 35:238–252. 2016. View Article : Google Scholar
|
|
24
|
Battu MB, Chandra AM, Sriram D and
Yogeeswari P: Pharmacophore-based 3DQSAR and molecular docking
studies to identify new non-peptidic inhibitors of cathepsin S.
Curr Med Chem. 21:1910–1921. 2014. View Article : Google Scholar
|
|
25
|
Chakrabarti S and Michor F:
Pharmacokinetics and Drug Interactions Determine Optimum
Combination Strategies in Computational Models of Cancer Evolution.
Cancer Res. 77:3908–3921. 2017. View Article : Google Scholar
|
|
26
|
Valasani KR, Chaney MO, Day VW and Shidu
Yan S: Acetylcholinesterase inhibitors: Structure based design,
synthesis, pharmacophore modeling, and virtual screening. J Chem
Inf Model. 53:2033–2046. 2013. View Article : Google Scholar
|