Introduction
Recurrent spontaneous abortion (RSA), one of the
most common complications of pregnancy, refers to the occurrence of
at least two consecutive unexplained pregnancy losses before the
20th week of gestation (1). It is
reported that approximately 2% of women experience RSA (2). Although the pathogenic mechanism of RSA
remains to be fully elucidated, increasing data proposes that RSA
may occur as a result of certain fetal and maternal factors,
including genetic factors, endocrine and metabolic disorders, and
autoimmune abnormalities (3,4). However, the definitive cause of RSA is
undetermined in approximately 50% of cases (5). Genetic variations have been suggested as
an influential factor for RSA and as of 2012, approximately 100
candidate genes had been inspected (6).
The human vascular endothelial growth factor
(VEGF) gene (OMIM: 192240) is mapped to chromosome 6
(6p12-p21) and consists of 8 exons separated by 7 introns, the
alternative splicing of which produces a family of proteins
(7). VEGF, also known as VEGFA, is a
key regulator of physiological vasculogenesis and angiogenesis
during pregnancy (8). It has been
proposed that altered expression of the VEGF gene may serve
a role in the pathogenesis of RSA (9–13).
Numerous studies have investigated the VEGF genetic polymorphisms
and RSA risk in diverse ethnic groups (14–19); these
led to inconsistent results, indicating the varying degree of
association between VEGF polymorphism and RSA risk among
different ethnicities.
Polymorphisms in the promoter, introns, exons and
untranslated regions (3′-and 5′-UTRs) of a gene may affect the
manufacture or function of the corresponding protein. The
VEGF gene is highly polymorphic (20) and functional polymorphisms of the
VEGF gene modulate VEGF protein expression (13,21,22). A
functional 18-bp insertion/deletion (ins/del) polymorphism, located
at position −2549 in the promoter region of VEGF (21) affects gene expression, whereby the del
allele leads to a 1.95-fold increase in transcriptional activity
compared to the ins allele (22).
Due to the important roles of VEGF during pregnancy,
the dysregulated expression of the VEGF gene in RSA, and the
potential divergence in genetic risk among various populations, the
current study was designed to investigate the impact of the 18-bp
ins/del polymorphism (rs35569394) in VEGF on RSA risk in a
southeast Iranian population.
Patients and methods
Patients
A total of 186 subjects including 93 RSA cases and
93 controls were enrolled in the present case-control study. This
cohort was used in previous studies by our group on gene
polymorphisms and RSA risk, as detailed elsewhere (23,24). The
participants were selected between January 2015 and February 2016
from individuals attending the obstetrics and gynecology clinic at
the Ali ibn Abi Talib Hospital affiliated to Zahedan University of
Medical Sciences (Zahedan, Iran). RSA was defined as two or more
consecutive pregnancy losses before 20 weeks of gestation. All of
the patients were without anatomical, microbial, viral, hormonal or
genetic disease. The control group consisted of healthy fertile
women without any history of miscarriage. The local research Ethics
Committee of Zahedan University of Medical Sciences approved the
project and informed consent was obtained from all participants.
The salting-out method was used for genomic DNA extraction from
peripheral blood samples as described previously (25).
Genotyping
Genotyping of VEGF variants was performed
using a polymerase chain reaction (PCR) method as described
previously (26). The forward and
reverse primers used for detection of polymorphism were
5′-AAGATCTGGGTGGATAATCAGACT-3′ and 5′-AACTCTCCACATCTTCCCTAAGTG-3′,
respectively. These primers were produced by Macrogen, Inc. (Seoul,
Korea). PCR was performed using commercially available Prime Taq
Premix (Genet Bio, Inc., Daejeon, Korea) according to the
manufacturer's protocol. Briefly, into 0.20-ml PCR reaction tubes,
1 µl genomic DNA (~100 ng/µl), 1 µl of each primer (10 µM), 10 µl
2X Prime Taq Premix and 7 µl ddH2O were added. The PCR cycling
conditions were 5 min at 95°C, followed by 30 cycles of 30 sec at
95°C, 30 sec at 61°C and 30 sec at 72°C, with a final step at 72°C
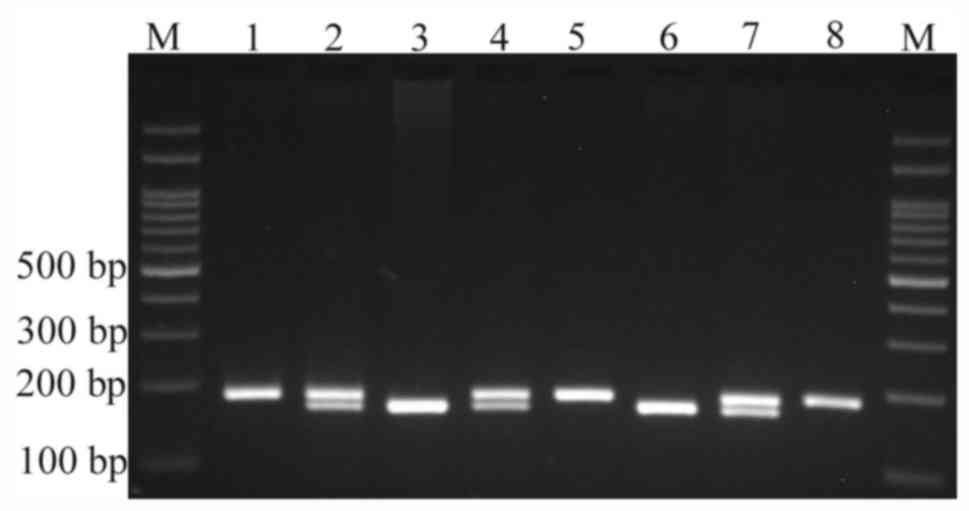
for 5 min. The PCR products were resolved on 2.5% agarose gel
electrophoresis containing 0.5 µg/ml ethidium bromide and
visualized with an ultraviolet transilluminator (Fig. 1). The product sizes for the ins and
del alleles were taken to be 188 and 170 bp, respectively (26).
Statistical analysis
Statistical analysis was performed using SPSS 20.0
software (IBM Corp., Armonk, NY, USA). Categorical data
(represented by the mean ± standard deviation) and continuous data
(represented by frequency) were analyzed by χ2 test and
independent sample t-test, respectively. The potential associations
between VEGF variants and RSA risk were evaluated by
computing the odds ratio (OR) and 95% confidence intervals (95% CI)
from unconditional logistic regression analysis. P<0.05 was
considered to indicate statistical significance.
Results and Discussion
As reported previously (23,24), the
RSA group consisted of 93 women with a mean age of 28.88±4.98 years
and the control group consisted of 93 unrelated healthy women with
a mean age of 30.01±4.77 years. There was no significant difference
between the groups regarding age (P=0.116).
With regard to the current experiment, the genotype
and allele frequencies of the VEGF 18-bp ins/del
polymorphism in the cases and controls are presented in Table I. The results indicated that the 18-bp
ins/del polymorphism significantly increased the risk of RSA under
codominant (ins/ins vs. del/del; OR=2.85, 95% CI=1.31–6.22,
P=0.019), dominant (del/ins+ins/ins vs. del/del; OR=2.19, 95%
CI=1.20–4.01, P=0.015) and allelic (ins vs. del; OR=1.90, 95%
CI=1.25–2.88, P=0.003) inheritance models. There were no
significant differences in the rates of RSA between the case and
control groups under the recessive and overdominant inheritance
models.
 | Table I.Genetic and allele frequencies of
VEGF 18-bp ins/del polymorphism in recurrent spontaneous
abortion cases and controls. |
Table I.
Genetic and allele frequencies of
VEGF 18-bp ins/del polymorphism in recurrent spontaneous
abortion cases and controls.
| VEGF 18-bp
ins/del polymorphism | Cases, n (%) | Controls, n (%) | OR (95% CI) | P-value |
|---|
| Codominant |
|
|
|
|
|
del/del | 27 (29.0) | 44 (47.3) | 1.00 | – |
|
del/ins | 38 (40.9) | 33 (35.5) | 1.88 (0.94–3.66) | 0.092 |
|
ins/ins | 28 (30.1) | 16 (17.2) | 2.85 (1.31–6.22) | 0.019 |
| Dominant |
|
|
|
|
|
del/del | 27 (29.0) | 44 (47.3) | 1.00 |
|
|
del/ins+ins/ins | 66 (71.0) | 49 (52.7) | 2.19
(1.20–4.01) | 0.015 |
| Recessive |
|
|
|
|
|
del/del+del/ins | 65 (69.9) | 77 (82.8) | 1.00 |
|
|
ins/ins | 28 (30.1) | 16 (17.2) | 2.07
(0.99–3.98) | 0.057 |
| Overdominant |
|
|
|
|
|
del/del+ins/ins | 55 (59.1) | 60 (64.5) | 1.00 | – |
|
del/ins | 38 (40.9) | 33 (35.5) | 1.26
(0.69–2.27) | 0.546 |
| Allele |
|
|
|
|
|
del | 92 (49.5) | 121 (65.1) | 1.00 | – |
|
ins | 94 (50.5) | 65 (34.9) | 1.90
(1.25–2.88) | 0.003 |
RSA is a multifactorial disorder caused by various
genetic and non-genetic factors. A number of studies have
demonstrated an association between genetic variants and the risk
of RSA (27–30). VEGF is among the established
regulators of angiogenesis during pregnancy and has been associated
with RSA (31,32). Increasing data indicates that
polymorphisms of the VEGF gene, including −1154 G/A
(rs1570360), −2578 A/C (rs699947), +936 C/T (rs3025039) and −2549
ins/del (rs35569394) are associated with VEGF expression level
(33–36).
In the present study, the possible association
between the 18-bp ins/del variant in the promoter region of
VEGF and the risk of RSA was inspected in a sample of the
southeast Iranian population. The results demonstrated that the
VEGF 18-bp ins/del variant significantly increased the risk
of RSA under codominant, dominant and allelic inheritance models.
Previous studies have evaluated the impact of VEGF
polymorphisms on the risk of RSA (16,37).
Saboori et al (37) examined
the possible association between +936 C/T, −1154 G/A, VEGF intron 5
C/T (rs3025010) and +5092 A/C (rs2146323) polymorphisms of the
VEGF gene and the risk of RSA. They identified a significant
association between the −1154 G/A and VEGF intron 5 C/T variants
and the risk of RSA. Pereza et al (16) reported that the VEGF 18-bp
ins/del polymorphism in men may be associated with RSA.
Furthermore, Vagnini et al (38) observed an association between the
VEGF −1154 G/A variant and recurrent implantation failure
(RIF). A meta-analysis performed by Xu et al (19) revealed that −1154 G/A, +936 C/T, −634
G/C (rs2010963) and −583 T/C (rs3025020) polymorphisms in the
VEGF gene were associated with increased RSA risk. In
particular, the −1154 G/A variant was significantly associated with
the risk of RSA among non-Asian populations, while the +936 C/T
variant was significantly associated with RSA risk among Asian
populations (19). In addition, the
findings of Almawi et al (32)
indicated an association of VEGF −460 T/C (rs833061), +398
G/A (rs833068), −583 T/C variants with the risk of RSA. On the
contrary, Samli et al (39)
demonstrated that the −2578 C/A (rs699947), −460 T/C and +936 C/T
polymorphisms of the VEGF gene were not associated with the
risk of RSA; while the −1154 G/A variant was related to the risk
(39).
More recently, Shim et al (40) investigated the association between
VEGF promoter polymorphisms −2578 C/A, −1154 G/A, −634 C/G
and +936 C/T and RIF. Their findings revealed that the −2578 AA
genotype was associated with an increased prevalence of RIF (≥4
implantation failures) compared with the CC genotype, whereas the
VEGF −634 CG+GG genotype was associated with an increased
incidence of total RIF and ≥4 RIFs compared with the CC
genotype.
There are certain limitations to the current study.
First, a relatively small sample size was used; second, only one
polymorphism of VEGF was evaluated, and thus other
polymorphisms of this gene should be assessed in equivalent
populations; and third, the serum levels of VEGF were not
determined to evaluate the association between the genotypes and
serum levels of VEGF, which warrants further study.
In conclusion, the present findings support an
association between VEGF 18-bp ins/del polymorphism and
increased risk of RSA. Further association studies on larger sample
sizes and different ethnicities are now required to verify the
current findings.
Acknowledgements
The authors would like to thank the patients and
control individuals who willingly participated in the study.
Funding
The present study was supported by Zahedan
University of Medical Sciences, Zahedan, Iran (grant no. 7188).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or available from the
corresponding author on reasonable request.
Authors' contributions
MH designed the study, analyzed and interpreted data
and drafted the manuscript. HD, FB, FT and MT performed experiments
and data analysis and gave final approval of the manuscript. MM and
GB collected, analyzed and interpreted data, and gave final
approval of the manuscript.
Ethics approval and consent to
participate
The local research Ethics Committee of Zahedan
University of Medical Sciences (Zahedan, Iran) approved the project
and informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Practice Committee of American Society for
Reproductive Medicine, . Definitions of infertility and recurrent
pregnancy loss: A committee opinion. Fertil Steril. 99:632013.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ford HB and Schust DJ: Recurrent pregnancy
loss: Etiology, diagnosis, and therapy. Rev Obstet Gynecol.
2:76–83. 2009.PubMed/NCBI
|
|
3
|
Brown S: Miscarriage and its associations.
Semin Reprod Med. 26:391–400. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Toth B, Jeschke U, Rogenhofer N, Scholz C,
Würfel W, Thaler CJ and Makrigiannakis A: Recurrent miscarriage:
Current concepts in diagnosis and treatment. J Reprod Immunol.
85:25–32. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
McNamee K, Dawood F and Farquharson R:
Recurrent miscarriage and thrombophilia: An update. Curr Opin
Obstet Gynecol. 24:229–234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rull K, Nagirnaja L and Laan M: Genetics
of recurrent miscarriage: Challenges, current knowledge, future
directions. Front Genet. 3:342012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vincenti V, Cassano C, Rocchi M and
Persico G: Assignment of the vascular endothelial growth factor
gene to human chromosome 6p21.3. Circulation. 93:1493–1495. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Burton GJ, Charnock-Jones DS and Jauniaux
E: Regulation of vascular growth and function in the human
placenta. Reproduction. 138:895–902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amirchaghmaghi E, Rezaei A, Moini A,
Roghaei MA, Hafezi M and Aflatoonian R: Gene expression analysis of
VEGF and its receptors and assessment of its serum level in
unexplained recurrent spontaneous abortion. Cell J. 16:538–545.
2015.PubMed/NCBI
|
|
10
|
Bagheri A, Kumar P, Kamath A and Rao P:
Association of angiogenic cytokines (VEGF-A and VEGF-C) and
clinical characteristic in women with unexplained recurrent
miscarriage. Bratisl Lek Listy. 118:258–264. 2017.PubMed/NCBI
|
|
11
|
Pang LH, Li MJ, Li MQ, Yang DM and Shi L:
Vascular endothelial growth factor (VEGF) and the VEGF soluble
receptor-1 (sFlt-1) in chorionic villus tissue from Chinese women
with early recurrent spontaneous abortion. J Int Med Res.
39:830–837. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pang L, Wei Z, Li O, Huang R, Qin J, Chen
H, Fan X and Chen ZJ: An increase in vascular endothelial growth
factor (VEGF) and VEGF soluble receptor-1 (sFlt-1) are associated
with early recurrent spontaneous abortion. PLoS One. 8:e757592013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Al-Khateeb GM, Mustafa FE, Sater MS and
Almawi WY: Effect of the functional VEGFA-583C/T variant on
vascular endothelial growth factor levels and the risk of recurrent
spontaneous miscarriage. Fertil Steril. 95:2471–2473. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ghasemi N, Dehghani Firouzabadi R and
Ahmadi S: Association of −460C/T and +405 G/C polymorphisms of
vascular endothelial growth factor gene and susceptibility to
ovarian hyperstimulation syndrome. Int J Reprod Biomed (Yazd).
15:87–92. 2017.PubMed/NCBI
|
|
15
|
Aggarwal S, Parveen F, Faridi RM, Phadke
S, Borkar M and Agrawal S: Vascular endothelial growth factor gene
polymorphisms in north Indian patients with recurrent miscarriages.
Reprod Biomed Online. 22:59–64. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pereza N, Ostojić S, Smirčić A, Hodžić A,
Kapović M and Peterlin B: The −2549 insertion/deletion polymorphism
in the promoter region of the VEGFA gene in couples with idiopathic
recurrent spontaneous abortion. J Assist Reprod Genet.
32:1789–1794. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Su MT, Lin SH and Chen YC: Genetic
association studies of angiogenesis- and vasoconstriction-related
genes in women with recurrent pregnancy loss: A systematic review
and meta-analysis. Hum Reprod Update. 17:803–812. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang B, Dai B, Zhang X and Wang Z:
Vascular endothelial growth factor and recurrent spontaneous
abortion: A meta-analysis. Gene. 507:1–8. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Xu X, Du C, Li H, Du J, Yan X, Peng L, Li
G and Chen ZJ: Association of VEGF genetic polymorphisms with
recurrent spontaneous abortion risk: A systematic review and
meta-analysis. PLoS One. 10:e01236962015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Rogers MS and D'Amato RJ: The effect of
genetic diversity on angiogenesis. Exp Cell Res. 312:561–574. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Brogan IJ, Khan N, Isaac K, Hutchinson JA,
Pravica V and Hutchinson IV: Novel polymorphisms in the promoter
and 5′ UTR regions of the human vascular endothelial growth factor
gene. Hum Immunol. 60:1245–1249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang B, Cross DF, Ollerenshaw M, Millward
BA and Demaine AG: Polymorphisms of the vascular endothelial growth
factor and susceptibility to diabetic microvascular complications
in patients with type 1 diabetes mellitus. J Diabetes
Complications. 17:1–6. 2003. View Article : Google Scholar
|
|
23
|
Hashemi M, Mokhtari M, Khazaeian S, Bahari
G, Rezaei M, Nakhaee A and Taheri M: Evaluation of HLA-G 14-bp
ins/del and +3142G>C polymorphisms with susceptibility to
recurrent spontaneous abortion. Taiwan J Obstet Gynecol.
56:276–280. 2017. View Article : Google Scholar
|
|
24
|
Hashemi M and Mokhtari M: Evaluation of
trancobalamin II rs1801198 and transcobalamin II receptor rs2336573
gene polymorphisms in recurrent spontaneous abortion. J Obstet
Gynaecol. 2017.
|
|
25
|
Hashemi M, Hanafi Bojd H, Eskandari Nasab
E, Bahari A, Hashemzehi NA, Shafieipour S, Narouie B, Taheri M and
Ghavami S: Association of adiponectin rs1501299 and rs266729 gene
polymorphisms with nonalcoholic fatty liver disease. Hepat Mon.
13:e95272013. View Article : Google Scholar
|
|
26
|
Rezaei M, Hashemi M, Sanaei S, Mashhadi MA
and Taheri M: Association between vascular endothelial growth
factor gene polymorphisms with breast cancer risk in an Iranian
population. Breast Cancer (Auckl). 10:85–91. 2016.
|
|
27
|
Barišić A, Pereza N, Hodžić A, Ostojić S
and Peterlin B: A single nucleotide polymorphism of DNA
methyltransferase 3B gene is a risk factor for recurrent
spontaneous abortion. Am J Reprod Immunol. 78:e127652017.
View Article : Google Scholar
|
|
28
|
Zhang Y, Wu YY, Qiao FY and Zeng WJ:
Association between p53 polymorphism at codon 72 and recurrent
spontaneous abortion. J Huazhong Univ Sci Technolog Med Sci.
36:402–405. 2016. View Article : Google Scholar
|
|
29
|
Wang G and Sun J: Interactive effects of
Snps located within CD28/B7pathway and environment on
susceptibility to recurrent spontaneous abortion. Cell Physiol
Biochem. 43:2185–2199. 2017. View Article : Google Scholar
|
|
30
|
Rah H, Chung KW, Ko KH, Kim ES, Kim JO,
Sakong JH, Kim JH, Lee WS and Kim NK: miR-27a and miR-449b
polymorphisms associated with a risk of idiopathic recurrent
pregnancy loss. PLoS One. 12:e01771602017. View Article : Google Scholar
|
|
31
|
Yalcintepe SA, Silan F, Hacivelioglu SO,
Uludag A, Cosar E and Ozdemir O: Fetal Vegf genotype is more
important for abortion risk than mother genotype. Int J Mol Cell
Med. 3:88–94. 2014.
|
|
32
|
Almawi WY, Saldanha FL, Mahmood NA,
Al-Zaman I, Sater MS and Mustafa FE: Relationship between VEGFA
polymorphisms and serum VEGF protein levels and recurrent
spontaneous miscarriage. Hum Reprod. 28:2628–2635. 2013. View Article : Google Scholar
|
|
33
|
Watson CJ, Webb NJ, Bottomley MJ and
Brenchley PE: Identification of polymorphisms within the vascular
endothelial growth factor (VEGF) gene: Correlation with variation
in VEGF protein production. Cytokine. 12:1232–1235. 2000.
View Article : Google Scholar
|
|
34
|
Awata T, Inoue K, Kurihara S, Ohkubo T,
Watanabe M, Inukai K, Inoue I and Katayama S: A common polymorphism
in the 5′-untranslated region of the VEGF gene is associated with
diabetic retinopathy in type 2 diabetes. Diabetes. 51:1635–1639.
2002. View Article : Google Scholar
|
|
35
|
Mohammadi M, Bazrafshani MR, Day PJ and
Ollier WE: Vascular endothelial growth factor production is
regulated by gene polymorphisms. Iran J Immunol. 6:119–129.
2009.
|
|
36
|
Papazoglou D, Galazios G, Papatheodorou K,
Liberis V, Papanas N, Maltezos E and Maroulis GB: Vascular
endothelial growth factor gene polymorphisms and idiopathic
recurrent pregnancy loss. Fertil Steril. 83:959–963. 2005.
View Article : Google Scholar
|
|
37
|
Saboori S, Noormohammadi Z and Zare-Karizi
S: Genetic variation in vascular endothelial growth factor gene and
its association with recurrent spontaneous abortion. Bratisl Lek
Listy. 117:80–86. 2016.
|
|
38
|
Vagnini LD, Nascimento AM, Canas MC, Renzi
A, Oliveira-Pelegrin GR, Petersen CG, Mauri AL, Oliveira JB,
Baruffi RL, Cavagna M and Franco JG Jr: The relationship between
vascular endothelial growth factor 1154G/A polymorphism and
recurrent implantation failure. Med Princ Pract. 24:533–537. 2015.
View Article : Google Scholar
|
|
39
|
Samli H, Demir BC, Ozgöz A, Atalay MA and
Uncu G: Vascular endothelial growth factor gene 1154 G/A, 2578 C/A,
460 C/T, 936 C/T polymorphisms and association with recurrent
pregnancy losses. Genet Mol Res. 11:4739–4745. 2012. View Article : Google Scholar
|
|
40
|
Shim SH, Kim JO, Jeon YJ, An HJ, Lee HA,
Kim JH, Ahn ΕΗ, Lee WS and Kim NK: Association between vascular
endothelial growth factor promoter polymorphisms and the risk of
recurrent implantation failure. Exp Ther Med. 15:2109–2119.
2018.
|