Introduction
Ulcerative colitis (UC) is a chronic inflammatory
disease of unknown etiology that occurs in the large bowel
(1). Previous study by our group has
identified alteration of microbiota and mucosa-associated
microbiota in patients with UC (2,3).
Probiotics are often employed as an adjuvant therapeutic strategy
(4,5).
Clinical studies involving probiotics as the sole subject include
those that have demonstrated the usefulness of
Bifidobacterium-fermented milk and highly concentrated
probiotic preparation (6–13). Fecal microbiota transplantation (FMT)
is performed with the aim of normalizing the gut microbiota.
Although single FMT has been reported to be insufficient (14), intensive FMT may be effective for
patients with mild to moderately active UC (15). Since the gut microbiota is generally
stable (16), bacteria that have been
ingested as probiotics do not remain in the gastrointestinal tract
but are excreted as transient flora (17). As such, for a probiotic therapy to be
successful, it should be preferable to continuously administer a
wide variety of bacterial strains in large quantities (11,15,18).
In the present study, a fermented vegetable beverage
containing Pediococcus pentosaceus (Pc. pentosaceus)
strain IDS885 (Yasai no Senshi®; Otsuka Foods Co., Ltd.,
Osaka, Japan) was evaluated. This beverage contains eight varieties
of vegetable (equivalent to 150 g in weight), which are fermented
with Pc. pentosaceus (a plant-derived lactic acid bacterium)
to eliminate smell and improve flavor. Per serving (100 g), this
fermented vegetable beverage contains 5×1010 Pc.
pentosaceus. Pc. pentosaceus is a lactic acid bacterium
that can be isolated in abundance from sourdough, rainbow trout,
fermented dairy products originated from India, traditional Asian
fermented foods including moromi (the solid fermenting mass of
swollen rice grains), mochi koji (rice cake malt) and gundruk (a
fermented leafy green vegetable popular in Nepal) (19). This bacterium is consumed widely as
part of human diet. However, to the best of our knowledge, Pc.
pentosaceus has not previously been tested in patients with
UC.
Herein this fermented vegetable beverage was
administered to patients with active UC for 8 weeks to evaluate its
effects on their gastrointestinal symptoms and environment. To
assess the intestinal environment, terminal restriction fragment
length polymorphism (T-RFLP) was performed for analysis of fecal
microbiota and organic acids.
Materials and methods
Study design
The present study was an open-label, randomized
controlled trial with approval of the Ethics Committee of Shiga
University of Medical Science, Otsu, Japan (26–205, UMIN000019753).
Enrolled patients had mildly to moderately active UC diagnosed
according to the Mayo Score/Disease Activity Index for Ulcerative
Colitis, with symptoms that had remained unchanged for at least 4
weeks between December 2015 and February 2017. Disease severity at
patient enrollment was judged according to the Mayo Score/Disease
Activity Index for Ulcerative Colitis, detailed previously
(20). If a patient had been
receiving a constant therapeutic regimen for 4 weeks prior to
enrollment, concomitant use of 5-aminosalicylate preparations,
prednisolone, immunomodulators, immunosuppressive agents and
anti-tumor necrosis factor (TNF)-α antibodies was permitted
throughout the trial period. Regarding anti-TNF-α antibodies, only
patients receiving infliximab every 8 weeks or adalimumab every 2
weeks were enrolled. Patients undergoing apheresis or patients
receiving antimicrobial agents were excluded. If patients
experienced exacerbation of the symptoms and required additional
treatment, they were withdrawn from the study. The primary endpoint
was improvement of the Rachmilewitz clinical activity index (CAI),
detailed previously (21), following
consumption of the fermented beverage.
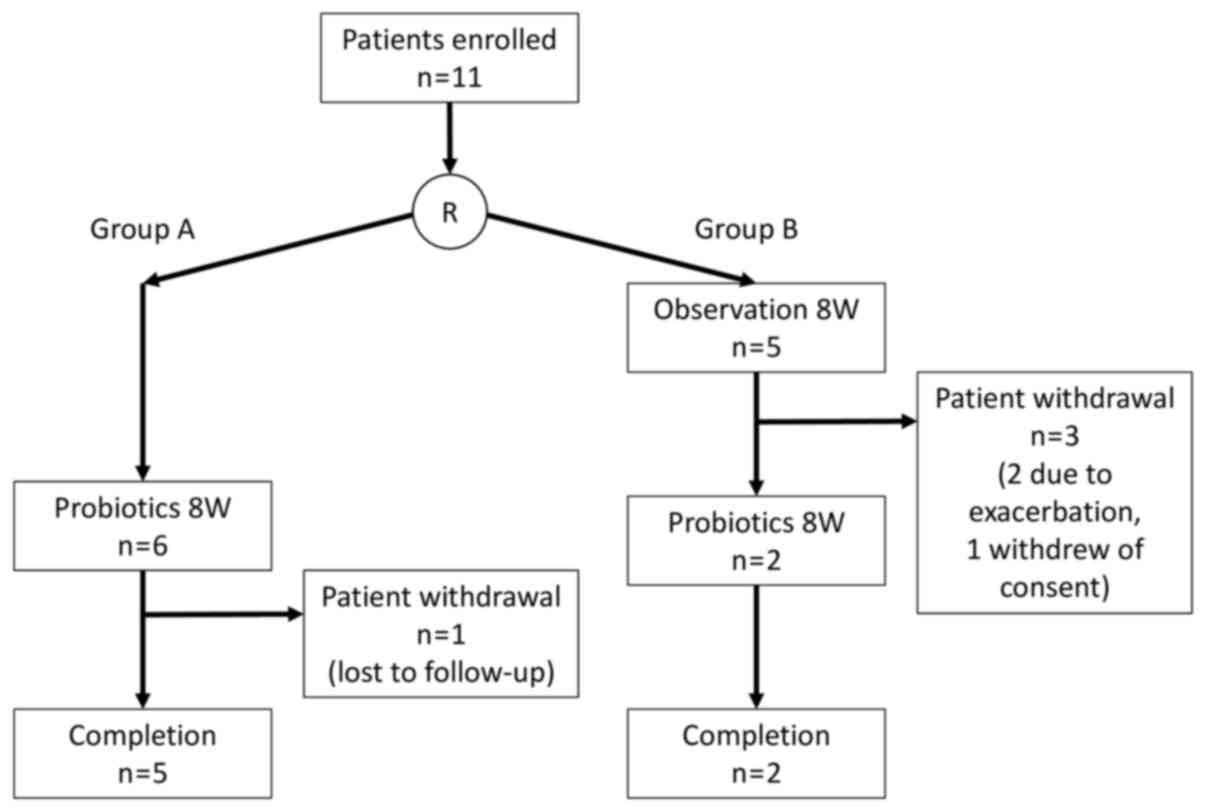
Consequently, 11 patients whose consent was obtained
were subjected to block randomization and divided into two groups:
Group A (n=6), in which the subjects consumed the fermented
vegetable beverage (Yasai no Senshi®; Otsuka Foods Co.,
Ltd.) for 8 weeks immediately following enrollment, and Group B
(n=5), in which the subjects were followed up for 8 weeks following
enrollment and then consumed the beverage over the ensuing 8 weeks
(Fig. 1). The component composition
of the fermented vegetable beverage is listed in Table I. The subjects were checked for
clinical symptoms, underwent sigmoidoscopy, and fecal samples were
subjected to T-RFLP and organic acid analysis immediately following
enrollment, and prior to and after the full course of
administration. The gastrointestinal symptom rating scale (GSRS)
described previously (22) was used
to evaluate the overall clinical symptoms. For the evaluation of UC
activity and endoscopic severity, the Rachmilewitz CAI and
uncreative colitis endoscopic index of severity were employed,
respectively. For the purposes of the current study, the subjects
whose Rachmilewitz CAI had decreased by ≥1 point were defined as
responders, whereas the subjects whose score had either been
unchanged or increased were defined as non-responders.
 | Table I.Nutritional composition of fermented
vegetable beverage (100 g). |
Table I.
Nutritional composition of fermented
vegetable beverage (100 g).
| Nutrient | Contents/100 g |
|---|
| Energy | 46.0 kcal |
| Protein | 0.9 g |
| Lipid | 0.3 g |
| Carbohydrate | 10.0 g |
| Na+ | 23.0 mg |
| K+ | 320.0 mg |
| Dietary fiber | 0.8 g (soluble 0.4
g, insoluble 0.4 g) |
| Carotenoid |
|
|
α-Carotene | 1,600.0 µg |
|
β-Carotene | 3,900.0 µg |
|
Lycopene | 2,000.0 µg |
|
Lutein | 1,130.0 µg |
|
Zeaxanthin | 130.0 µg |
|
Cryptoxanthin | 40.0 µg |
Polymerase chain reaction (PCR)
amplification and T-RFLP analysis
PCR and T-RFLP analyses were performed according to
the methods described previously (14,23). The
16S ribosomal RNA gene was amplified from human fecal DNA with use
of the fluorescently labeled 516F and 1510R primers (24). The PCR amplifications of DNA samples
(10 ng of each DNA) were performed according to a protocol
described by Nagashima et al (24).
The restriction enzyme was selected according to the
Nagashima et al study (24).
The purified PCR products (2 µl) were digested with 20 U of
BslI (Thermo Fisher Scientific, Inc., Waltham, MA, USA) at
37°C for 10 min. The length of the terminal restriction fragment
was determined with an ABI PRISM 3130xl genetic analyzer (Applied
Biosystems; Thermo Fisher Scientific, Inc.) in GeneMapper mode. The
fragment size was estimated with use of the local Southern method
taxonomic units as described by Nagashima et al (24). The major terminal restriction
fragments were identified by Human Fecal Microbiota T-RFLP
profiling (https://www.tecsrg.co.jp/t-rflp/t_rflp_hito_OTU.html).
Measurement of pH and organic
acids
Following sampling, the fecal pH of fecal samples
was immediately measured with a handy digital pH meter (KS701;
Shindengen Electric Manufacturing Co., Ltd., Tokyo, Japan). Fecal
samples were sterilized at 80°C for 15 min with Milli-Q water
(Merck KGaA, Darmstadt, Germany) and then homogenized. Following
centrifugation at 15,350 × g for 10 min at room temperature, the
supernatant fluid was passed through a 0.45-µm membrane filter and
assigned for measurement of 9 short chain fatty acids (succinic,
lactic, formic, acetic, propionic, iso-butyric, n-butyric,
iso-valeric and n-valeric) by high performance liquid
chromatography at Techno Suruga Laboratory Co., Ltd. (Shizuoka,
Japan).
Statistical analysis
The data were presented as median (interquartile
range). All statistical analyses were performed using Prism version
6.05 (GraphPad Software Inc., La Jolla, CA, USA). The χ2
test and Mann-Whitney's U test were used to compare the clinical
characteristics of patients between groups A and B. The Wilcoxon
signed-rank test was used to compare changes in measured parameters
prior to and following consumption of the fermented vegetable
beverage. P<0.05 was regarded to indicate statistical
significance.
Results
Study participants
The clinical characteristics of the 11 patients who
consented to participate in the study are listed in Table II. Group A patients (n=6) were
required to start consuming the fermented vegetable beverage
immediately following enrollment, and Group B patients (n=5)
started consuming the beverage when the follow-up period ended. In
Group A, 1 subject became unfollowable midway through due to
geographic relocation; however, exhibited unchanged clinical
symptoms at their final examination at 4 weeks after the start of
consumption. In group B, 2 of the 5 subjects required additional
treatment during the follow-up period and had to be withdrawn.
Another subject canceled their participation for personal reasons.
Consequently, a total of 7 subjects (5 in Group A and 2 in Group B)
completed the 8-week consumption regimen (Fig. 1). Due to the dropout rate, the total
cohort was treated as a single patient group for the following
analyses.
 | Table II.Clinical characteristics of patients
(n=11). |
Table II.
Clinical characteristics of patients
(n=11).
| Characteristic | Group A | Group B | P-value |
|---|
| Male:female | 3:3 | 2:3 | 1.000a |
| Age, median
(IQR) | 43 (36–48) | 58 (39–60) | 0.429b |
| Disease duration,
years, median (IQR) | 11.6
(4.7–12.1) | 10.0
(2.5–20.5) | 0.792b |
| Behavior (first
attack/relapse-remitting/chronic-persistent) | 1/3/2 | 0/3/2 | 0.632a |
| Location
(pancolitis/left-sided/proctitis) | 3/1/2 | 3/2/0 | 0.323a |
| Rachmilewitz CAI,
median (IQR) | 4
(3.0–4.8) | 3
(2.0–4.0) | 0.256b |
| Mayo score at
baseline, median (IQR) | 6
(4.3–6.0) | 5
(4.0–5.0) | 0.562b |
| UCEIS, median
(IQR) | 4
(3.0–4.8) | 3
(3.0–4.0) | 0.762b |
| Medication |
|
|
|
| 5-ASA
(time-dependent/pH-dependent) | 6/0 | 3/2 | 0.353a |
|
Azathioprine (yes/no) | 2/4 | 2/3 | 1.000a |
|
Probiotics (yes/no) | 2/4 | 2/3 | 1.000a |
Changes in clinical symptoms
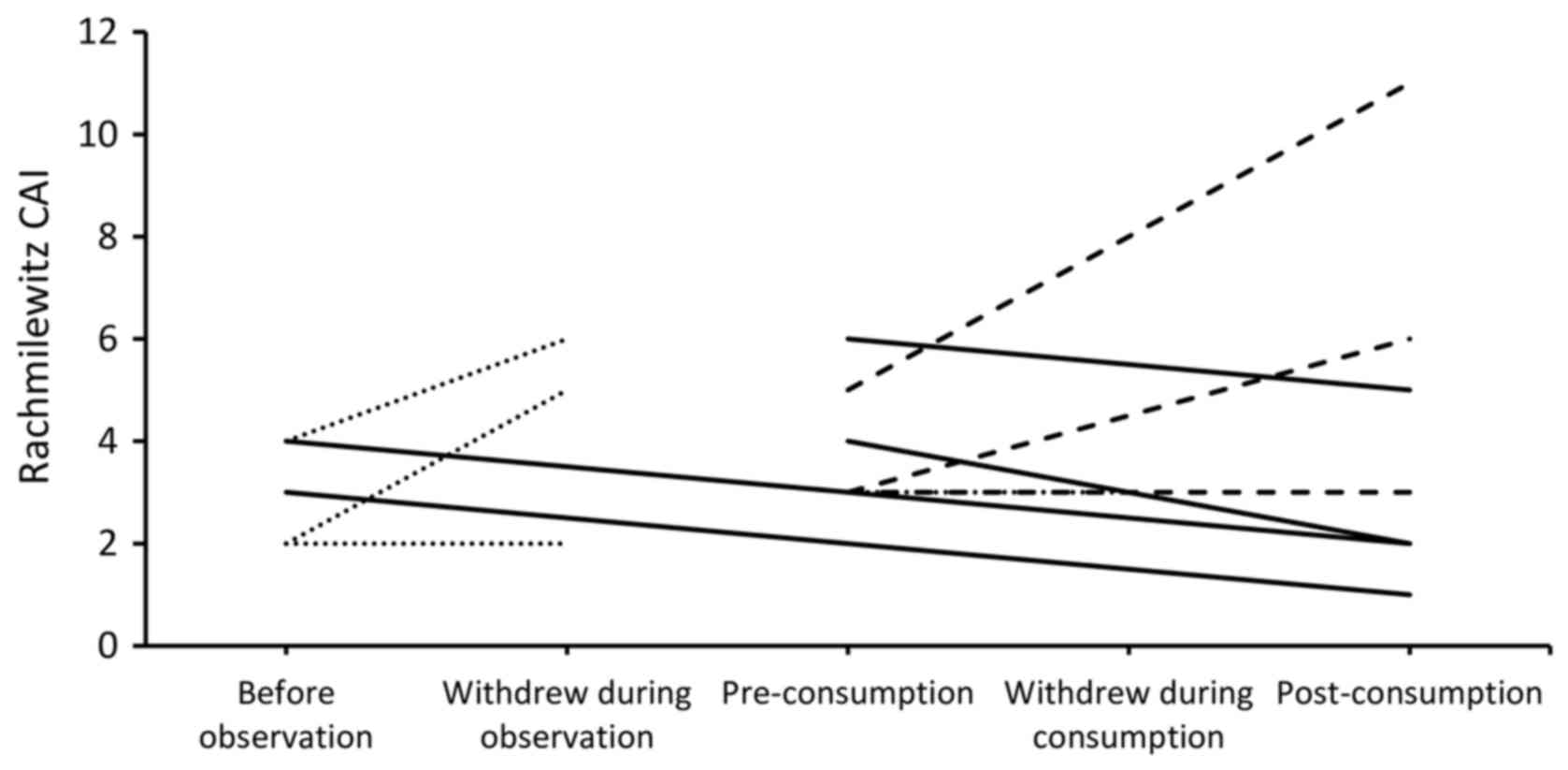
Fig. 2 presents the
Rachmilewitz CAI of all subjects enrolled in the study. No
significant changes were observed in the clinical activity and
endoscopic severity prior to and following consumption (Table III). Regarding the GSRS, there was
significant improvement in the total GSRS score (P=0.042) and for
Question 12 ‘Have you been bothered by loose stools during the past
week? [If your stools (motions) have been alternately hard and
loose, this question only refers to the extent you have been
bothered by the stools being loose.]’ (P=0.048; Table III). No significant changes were
observed in the T-RFLP occupancy, pH, fecal organic acids and
microbiota prior to and following the consumption period (Table III).
 | Table III.Changes in clinical parameters. |
Table III.
Changes in clinical parameters.
|
|
Pre-consumption |
Post-consumption | P-value |
|---|
| Rachmilewitz
CAI | 3 (3.0–4.5) | 3 (2.0–5.5) | 1.000 |
| UCEIS | 3 (3.0–4.0) | 4 (2.5–4.0) | 1.000 |
| Mayo score | 5 (4.0–6.0) | 5 (3.5–5.5) | 1.000 |
| Bowel
movement | 1 (0.5–2.0) | 1 (0.5–1.5) | 1.000 |
| Rectal
bleeding | 1 (0.0–1.0) | 1 (0.0–1.0) | 0.773 |
|
Endoscopic score | 2 (2.0–2.0) | 2 (2.0–2.0) | 1.000 |
| General
appearance | 1 (1.0–1.0) | 1 (1.0–1.0) | 1.000 |
| GSRS total
score | 35 (29.8–40.3) | 24 (24.0–31.0) | 0.042 |
| Pain or
discomfort in your upper abdomen or pit of the stomach | 1 (1.0–2.0) | 1 (1.0–1.0) | 0.710 |
|
Heartburn | 1 (1.0–1.5) | 1 (1.0–2.0) | 1.000 |
| Acid
reflux | 1 (1.0–1.5) | 1 (1.0–2.0) | 1.000 |
| Hunger
pains | 1 (1.0–2.0) | 1 (1.0–2.0) | 1.000 |
|
Nausea | 1 (1.0–2.0) | 1 (1.0–1.0) | 0.174 |
|
Rumbling | 1 (1.0–2.0) | 1 (1.0–1.5) | 1.000 |
|
Bloated | 1 (1.0–3.0) | 1 (1.0–1.0) | 1.000 |
|
Burping | 1 (1.0–2.0) | 1 (1.0–1.0) | 1.000 |
| Passing
gas or flatus | 3 (1.5–4.0) | 2 (2.0–3.0) | 0.577 |
|
Constipation | 1 (1.0–3.0) | 1 (1.0–1.0) | 0.371 |
|
Diarrhea | 4 (3.5–5.0) | 3 (1.0–3.0) | 0.057 |
| Loose
stools | 4 (2.5–4.0) | 3 (1.5–3.0) | 0.048 |
| Hard
stools | 1 (1.0–2.5) | 1 (1.0–1.0) | 0.423 |
| Urgent
need to have a bowel movement | 4 (3.5–6.0) | 2 (1.5–3.5) | 0.100 |
|
Sensation of not completely
emptying the bowels | 3 (2.0–4.5) | 2 (1.0–2.5) | 0.269 |
| Fecal pH | 6.25
(5.81–7.21) | 6.45
(6.10–6.67) | 1.000 |
| Fecal organic
acids, % positive detection and median mg/g (IQR) |
|
|
|
|
Succinic acid | 57%, 0.20
(0.15–0.55) | 57%, 0.12
(0.11–0.51) | 0.438 |
| Lactic
acid | 57%, 0.88
(0.30–2.20) | 43%, 0.55
(0.36–0.73) | 0.375 |
| Acetic
acid | 100%, 1.76
(1.48–2.76) | 100%, 1.64
(0.92–3.06) | 0.813 |
|
Propionic acid | 100%, 0.52
(0.40–0.99) | 100%, 0.41
(0.31–1.33) | 0.813 |
|
iso-butyric acid | 0% | 14%, 0.12
(0.12–0.12) | 1.000 |
|
n-butyric acid | 100%, 0.34
(0.19–0.39) | 100%, 0.48
(0.21–0.65) | 0.578 |
|
iso-valeric acid | 14%, 0.10
(0.10–0.10) | 43%, 0.19
(0.15–0.19) | 0.265 |
| T-RFLP, occupancy,
% |
|
|
|
|
Bifidobacterium | 26.28
(10.56–31.48) | 16.11
(12.35–27.57) | 0.109 |
|
Lactobacillales | 8.85
(6.86–18.20) | 5.19
(4.28–15.14) | 0.578 |
|
Bacteroides | 29.39
(14.53–40.37) | 31.97
(23.00–41.80) | 1.000 |
|
Prevotella | 0.00
(0.00–0.00) | 0.00
(0.00–0.00) | 1.000 |
|
Clostridium cluster
IV | 2.70
(1.83–6.78) | 4.05
(2.92–8.52) | 0.529 |
|
Clostridium subcluster
XIVa | 9.77
(7.17–14.83) | 12.10
(10.66–19.08) | 0.813 |
|
Clostridium cluster
IX | 1.04
(0.27–3.32) | 1.96
(0.51–3.04) | 0.578 |
|
Clostridium cluster
XI | 0.32
(0.00–4.12) | 0.00
(0.00–3.53) | 0.201 |
|
Clostridium cluster
XVIII | 1.75
(1.12–3.76) | 1.32
(0.79–2.87) | 0.059 |
|
Others | 5.09
(3.24–7.54) | 6.52
(5.10–8.80) | 0.469 |
|
Shannon's diversity index | 1.53
(1.45–1.90) | 1.69
(1.60–1.90) | 0.813 |
Comparison between responders and
non-responders
As depicted in Fig. 2,
4 subjects exhibited improvement of their Rachmilewitz CAI
(responders), whereas 3 experienced either unchanged or exacerbated
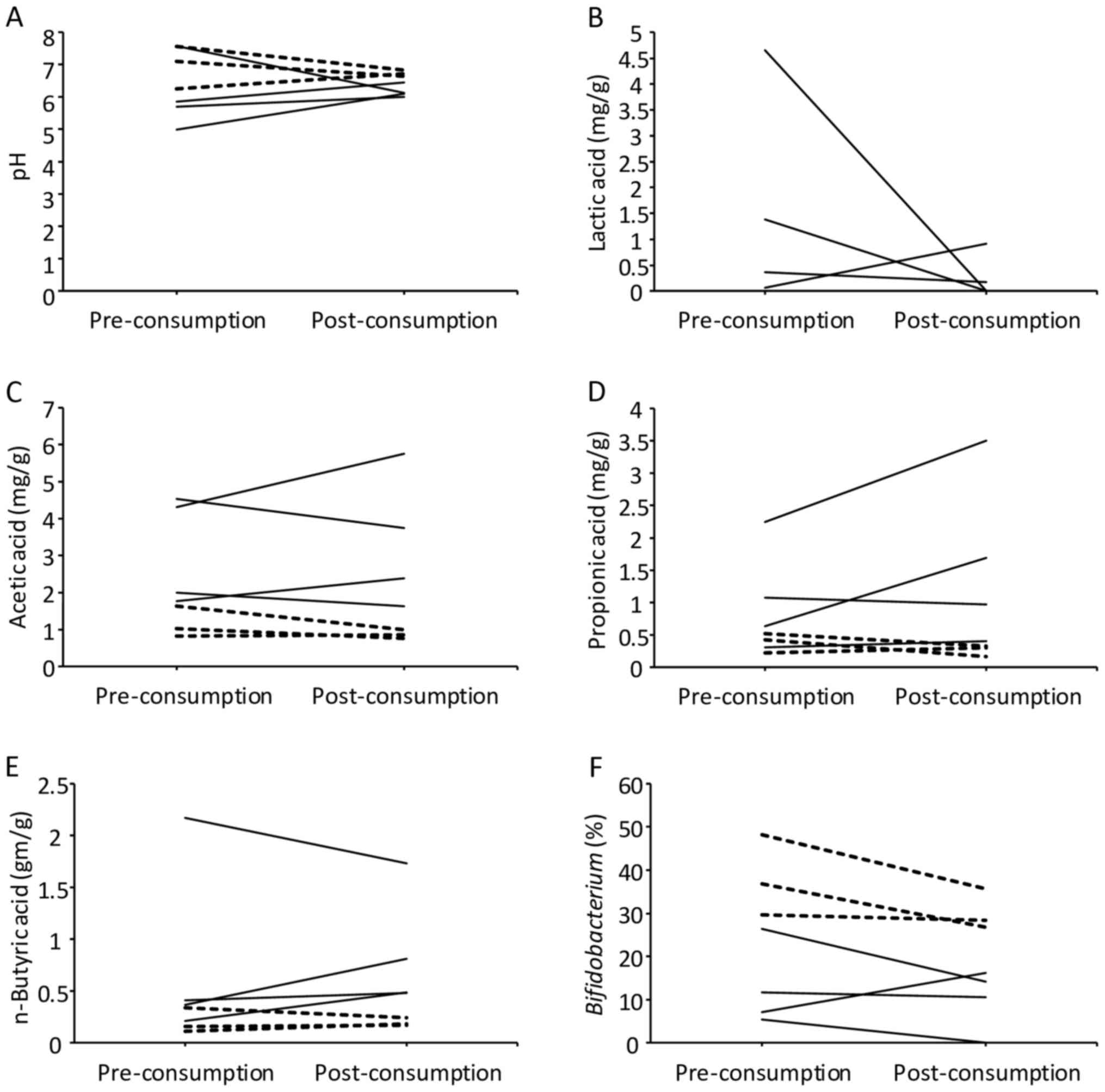
condition (non-responders). Fig. 3
presents the analytic results of fecal pH, organic acids and
microbiota. Although no statistical significance was present due to
the limited number of subjects, the responders appeared to start at
lower pH than the non-responders, and while they mostly exhibited
an increased pH following consumption, they still tended to exhibit
lower pH following consumption compared with the non-responders
(Fig. 3A). Regarding the measurement
of organic acids, the value of the limit of quantification was 0.05
mg/g for succinic acid, lactic acid, acetic acid and propionic
acid, and 0.10 mg/g for formic acid, iso-butyric acid, n-butyric
acid, iso-valeric acid and n-valeric acid. Formic acid and
n-valeric acid were not detected in all samples. While the levels
of lactic acid tended to decrease following consumption of the
fermented vegetable beverage among the responders, lactic acid was
not detected in the non-responders prior to consumption (Fig. 3B). The levels of acetic acid,
propionic acid and n-butyric acid tended to be lower prior to
consumption in the non-responders than in the responders (Fig. 3C-E). The levels of these organic acids
did not significantly change following consumption in responders
and non-responders (Table III). The
microbiota analysis revealed that the occupancy of
Bifidobacterium tended to be lower in the responders
compared with in the non-responders prior to and following
consumption (Fig. 3F).
Discussion
The present study observed that the 8-week
consumption of the fermented vegetable beverage by UC patients
marginally improved loose stool symptoms but did not affect the
disease activity of UC itself. The organic acid analysis revealed
that the levels of acetic acid, propionic acid and n-butyric acid
tended to be continuously higher in the responders than in the
non-responders.
Per serving (100 g), the fermented vegetable
beverage (Yasai no Senshi®) contains 8 varieties of
vegetable (equivalent to 150 g in weight) and approximately
5×1010 colony-forming units (CFU) of Pc.
pentosaceus. Pc. pentosaceus was classified as
Lactobacillales for the T-RFLP analysis. However, the
occupancy of Lactobacillales was not observed to increase
following consumption of the beverage. A previous study reported
that 106-107 CFU/g of Pc. pentosaceus
was detected in the feces of healthy subjects who had consumed
Yasai no Senshi® (19). As
the Pc. pentosaceus bacterial count was not proportionally
high within feces (19), the results
of the T-RFLP analysis may not have reflected their presence.
Regarding organic acid analysis, it has been
reported that the levels of acetic acid, propionic acid and butyric
acid are higher in healthy individuals compared with in UC patients
(25–28), whereas the levels of lactic acid are
reduced (26,29). In the present study, the responders
tended to continuously exhibit higher levels of acetic acid,
propionic acid and n-butyric acid; while their levels of lactic
acid tended to decrease following consumption of the fermented
vegetable beverage. While no significant differences were observed
in the index of UC activity between the responders and
non-responders, the organic acid analysis revealed that the
responders tended to exhibit higher levels of acetic acid,
propionic acid and n-butyric acid prior to consumption. This result
suggests that the responders probably had an intestinal environment
similar to healthy individuals compared with the non-responders
even prior to consuming the fermented vegetable beverage.
The gut microbiota analysis revealed that the
occupancy of Bifidobacterium tended to be lower in the
responders, particularly following consumption. The levels of
lactic acid also tended to decrease following consumption. The low
occupancy of Bifidobacterium in the responders is consistent
with the results of an FMT study targeting UC patients (14). Another previous study indicated that
increased levels of lactic acid coincided with exacerbated UC
symptoms (29). The source of this
lactic acid is considered to be Bifidobacterium (30). Furthermore, another study reported a
higher occupancy of Bifidobacterium in UC patients compared
with in healthy individuals (3).
Given these findings, it is possible that the consumption of the
fermented vegetable beverage improved the intestinal
environment.
Following consumption of the fermented vegetable
beverage, the present subjects experienced improvement in loose
stool symptoms. In addition to the effects of Pc.
pentosaceus, the symbiotic effects of soluble dietary fiber may
have contributed to this improvement.
The present study has limitations due to its
single-center nature. Therefore, the number of patients enrolled in
this study was limited. Although cases were randomly assigned to
two groups, the dropout rate only allowed analysis of a single
group; a total of 3 out of 11 patients dropped out, though the
effect on the validity of the statistical analysis is unknown.
Furthermore, when comparing between the responders and
non-responders, statistical significance could not be detected due
to the limited size of each group.
In conclusion, the fermented vegetable beverage
appeared to ameliorate loose stool symptoms, although the activity
of UC did not improve. Acquisition of further clinical case data is
expected to confirm the present findings.
Acknowledgements
Not applicable.
Funding
The present study was contracted research between
Shiga University of Medical Science (Otsu, Japan) and Otsuka Foods
Co., Ltd. (Osaka, Japan). Otsuka Foods supplied the fermented
vegetable beverage (Yasai no Senshi®), and expenses for
endoscopic examination and fecal microbiota and organic acid
analyses.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Study concept and design: SB and AA; acquisition of
patient data: SB, KT, HI and AN; statistical analysis: SB; drafting
of the manuscript: SB and KT; data interpretation and revision of
the manuscript: SB, KT, HI, AN, MK, OI, MSu, MSa and AA.
Ethics approval and consent to
participate
The present study was an open-label, randomized
controlled trial with approval of the Ethics Committee of Shiga
University of Medical Science, Otsu, Japan (26–205, UMIN000019753).
All participants provided written consent to participate in this
study.
Consent for publication
The consent form signed by participants included
agreement on the publishing of relevant data following
anonymization of personal information.
Competing interests
The present study was conducted in collaboration
with Otsuka Foods Co., Ltd. (Osaka, Japan), the manufacturer and
distributer of the fermented vegetable beverage (Yasai no
Senshi®). All authors declare that they have no other
competing interests.
Glossary
Abbreviations
Abbreviations:
|
UC
|
ulcerative colitis
|
|
CAI
|
clinical activity index
|
|
GSRS
|
gastrointestinal symptom rating
scale
|
|
T-RFLP
|
terminal restriction fragment length
polymorphism
|
|
FMT
|
fecal microbiota transplantation
|
|
TNF
|
tumor necrosis factor
|
|
Pc. pentosaceus
|
Pediococcus pentosaceus
|
|
PCR
|
polymerase chain reaction
|
|
CFU
|
colony-forming units
|
References
|
1
|
Chan HC and Ng SC: Emerging biologics in
inflammatory bowel disease. J Gastroenterol. 52:141–150. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Andoh A, Sakata S, Koizumi Y, Mitsuyama K,
Fujiyama Y and Benno Y: Terminal restriction fragment length
polymorphism analysis of the diversity of fecal microbiota in
patients with ulcerative colitis. Inflamm Bowel Dis. 13:955–962.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Nishino K, Nishida A, Inoue R, Kawada Y,
Ohno M, Sakai S, Inatomi O, Bamba S, Sugimoto M, Kawahara M, et al:
Analysis of endoscopic brush samples identified mucosa-associated
dysbiosis in inflammatory bowel disease. J Gastroenterol.
53:95–106. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Guandalini S: Update on the role of
probiotics in the therapy of pediatric inflammatory bowel disease.
Expert Rev Clin Immunol. 6:47–54. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abdin AA and Saeid EM: An experimental
study on ulcerative colitis as a potential target for probiotic
therapy by Lactobacillus acidophilus with or without
‘olsalazine’. J Crohn's Colitis. 2:296–303. 2008. View Article : Google Scholar
|
|
6
|
Ishikawa H, Akedo I, Umesaki Y, Tanaka R,
Imaoka A and Otani T: Randomized controlled trial of the effect of
bifidobacteria-fermented milk on ulcerative colitis. J Am Coll
Nutr. 22:56–63. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kato K, Mizuno S, Umesaki Y, Ishii Y,
Sugitani M, Imaoka A, Otsuka M, Hasunuma O, Kurihara R, Iwasaki A
and Arakawa Y: Randomized placebo-controlled trial assessing the
effect of bifidobacteria-fermented milk on active ulcerative
colitis. Aliment Pharmacol Ther. 20:1133–1141. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tursi A, Brandimarte G, Giorgetti GM,
Forti G, Modeo ME and Gigliobianco A: Low-dose balsalazide plus a
high-potency probiotic preparation is more effective than
balsalazide alone or mesalazine in the treatment of acute
mild-to-moderate ulcerative colitis. Med Sci Monit. 10:PI126–PI131.
2004.PubMed/NCBI
|
|
9
|
Bibiloni R, Fedorak RN, Tannock GW, Madsen
KL, Gionchetti P, Campieri M, De Simone C and Sartor RB: VSL#3
probiotic-mixture induces remission in patients with active
ulcerative colitis. Am J Gastroenterol. 100:1539–1546. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Soo I, Madsen KL, Tejpar Q, Sydora BC,
Sherbaniuk R, Cinque B, Di Marzio L, Cifone MG, Desimone C and
Fedorak RN: VSL#3 probiotic upregulates intestinal mucosal alkaline
sphingomyelinase and reduces inflammation. Can J Gastroenterol.
22:237–242. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sood A, Midha V, Makharia GK, Ahuja V,
Singal D, Goswami P and Tandon RK: The probiotic preparation, VSL#3
induces remission in patients with mild-to-moderately active
ulcerative colitis. Clin Gastroenterol Hepatol. 7:1202–1209. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tursi A, Brandimarte G, Papa A, Giglio A,
Elisei W, Giorgetti GM, Forti G, Morini S, Hassan C, Pistoia MA, et
al: Treatment of relapsing mild-to-moderate ulcerative colitis with
the probiotic VSL#3 as adjunctive to a standard pharmaceutical
treatment: A double-blind, randomized, placebo-controlled study. Am
J Gastroenterol. 105:2218–2227. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shen J, Zuo ZX and Mao AP: Effect of
probiotics on inducing remission and maintaining therapy in
ulcerative colitis, Crohn's disease, and pouchitis: Meta-analysis
of randomized controlled trials. Inflamm Bowel Dis. 20:21–35. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nishida A, Imaeda H, Ohno M, Inatomi O,
Bamba S, Sugimoto M and Andoh A: Efficacy and safety of single
fecal microbiota transplantation for Japanese patients with mild to
moderately active ulcerative colitis. J Gastroenterol. 52:476–482.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Paramsothy S, Kamm MA, Kaakoush NO, Walsh
AJ, van den Bogaerde J, Samuel D, Leong RWL, Connor S, Ng W,
Paramsothy R, et al: Multidonor intensive faecal microbiota
transplantation for active ulcerative colitis: A randomised
placebo-controlled trial. Lancet. 389:1218–1228. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Faith JJ, Guruge JL, Charbonneau M,
Subramanian S, Seedorf H, Goodman AL, Clemente JC, Knight R, Heath
AC, Leibel RL, et al: The long-term stability of the human gut
microbiota. Science. 341:12374392013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li SS, Zhu A, Benes V, Costea PI, Hercog
R, Hildebrand F, Huerta-Cepas J, Nieuwdorp M, Salojärvi J, Voigt
AY, et al: Durable coexistence of donor and recipient strains after
fecal microbiota transplantation. Science. 352:586–589. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nishida A, Inoue R, Inatomi O, Bamba S,
Naito Y and Andoh A: Gut microbiota in the pathogenesis of
inflammatory bowel disease. Clin J Gastroenterol. 11:1–10. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kumemura M, Saito M, Okamatsu H, Domae T
and Isono T: Survival of Pediococcus pentosaceus species in
the gastrointestinal tract and the effect of cultured vegetable
drink on fecal microflora in human. J Intestinal Microbiol.
16:139–143. 2002.
|
|
20
|
Schroeder KW, Tremaine WJ and Ilstrup DM:
Coated oral 5-aminosalicylic acid therapy for mildly to moderately
active ulcerative colitis. A randomized study. N Engl J Med.
317:1625–1629. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rachmilewitz D: Coated mesalazine
(5-aminosalicylic acid) versus sulphasalazine in the treatment of
active ulcerative colitis: A randomised trial. BMJ. 298:82–86.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dimenäs E, Glise H, Hallerbäck B,
Hernqvist H, Svedlund J and Wiklund I: Quality of life in patients
with upper gastrointestinal symptoms. An improved evaluation of
treatment regimens? Scand J Gastroenterol. 28:681–687. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Andoh A, Imaeda H, Aomatsu T, Inatomi O,
Bamba S, Sasaki M, Saito Y, Tsujikawa T and Fujiyama Y: Comparison
of the fecal microbiota profiles between ulcerative colitis and
Crohn's disease using terminal restriction fragment length
polymorphism analysis. J Gastroenterol. 46:479–486. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagashima K, Hisada T, Sato M and
Mochizuki J: Application of new primer-enzyme combinations to
terminal restriction fragment length polymorphism profiling of
bacterial populations in human feces. Appl Environ Microbiol.
69:1251–1262. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nemoto H, Kataoka K, Ishikawa H, Ikata K,
Arimochi H, Iwasaki T, Ohnishi Y, Kuwahara T and Yasutomo K:
Reduced diversity and imbalance of fecal microbiota in patients
with ulcerative colitis. Dig Dis Sci. 57:2955–2964. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vernia P, Gnaedinger A, Hauck W and Breuer
RI: Organic anions and the diarrhea of inflammatory bowel disease.
Dig Dis Sci. 33:1353–1358. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Takaishi H, Matsuki T, Nakazawa A, Takada
T, Kado S, Asahara T, Kamada N, Sakuraba A, Yajima T, Higuchi H, et
al: Imbalance in intestinal microflora constitution could be
involved in the pathogenesis of inflammatory bowel disease. Int J
Med Microbiol. 298:463–472. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Machiels K, Joossens M, Sabino J, De
Preter V, Arijs I, Eeckhaut V, Ballet V, Claes K, Van Immerseel F,
Verbeke K, et al: A decrease of the butyrate-producing species
Roseburia hominis and Faecalibacterium prausnitzii
defines dysbiosis in patients with ulcerative colitis. Gut.
63:1275–1283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Vernia P, Caprilli R, Latella G, Barbetti
F, Magliocca FM and Cittadini M: Fecal lactate and ulcerative
colitis. Gastroenterology. 95:1564–1568. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Berlec A, Ravnikar M and Strukelj B:
Lactic acid bacteria as oral delivery systems for biomolecules.
Pharmazie. 67:891–898. 2012.PubMed/NCBI
|