Introduction
Coronary artery disease (CAD) comprises the largest
proportion of cardiovascular diseases (CVDs) and accounts for more
than one third of all mortalities worldwide (1). Risk factors include hypertension,
cigarette smoking, type 2 diabetes mellitus, increased cholesterol
concentration and obesity (2).
Atherosclerosis, the formation of plaque inside the arteries, is
the main cause of CAD (3). Several
pathological events contribute to atherosclerosis, including
endothelial dysfunction, extensive lipid deposition in the tunica
intima, exacerbated innate and adaptive immune responses, vascular
smooth muscle cell proliferation and remodeling of the
extracellular matrix (4).
Two major hypotheses have been proposed to describe
the origin of atherosclerosis: i) the thrombogenic theory, which
suggests that thickening of the intima layer of vessels is a result
of the organization of fibrin by fibroblasts, associated with
secondary lipid enrichment; and ii) the lipogenic theory, which
suggests that the deposition of lipid inside the arterial walls is
caused by an imbalance between the mechanisms responsible for lipid
accumulation and removal (5–7). To date, several lines of study have
indicated a role for oxidative stress in atherosclerosis and CVDs
(8–11). Oxidative stress is the result of
enhanced production of reactive oxygen species (ROS), which are the
key molecules in the signaling pathways implicated in vascular
inflammation in atherogenesis, starting from the initiation of
fatty streak formation to lesion progression and plaque rupture
(12). ROS are established to damage
the fundamental biomolecules in cells including DNA, proteins and
lipids (13). A previous report
demonstrated that oxidative modification of low-density lipoprotein
(LDL) is a key mechanism rendering lipoproteins atherogenic
(14). Furthermore, it has been
reported that lipid peroxidation produces unsaturated aldehydes
including acrolein and malondialdehyde (MDA), which exert toxic
effects due to their reactivity with nucleophile compounds and
their ability to produce protein and DNA adducts without prior
metabolic activation (15). These
aldehydes are considered to function as mediators of inflammation
and vascular dysfunction (15).
On the other hand, there are several antioxidant
systems grouped as enzymatic and non-enzymatic antioxidant systems.
Antioxidant enzymes include catalase and glutathione peroxidase
(GPx), superoxide dismutase and glutathione reductase (GR), while
glutathione (GSH), vitamins A, E and C and uric acid are major
non-enzymatic antioxidants (16).
The intent of the present study was to determine the
oxidative and antioxidative markers in patients with CAD and to
compare these parameters between patient and healthy volunteer
groups. It also aimed to compare oxidant/antioxidant status in
chronic CAD patients with single, double or triple vessel
stenosis.
Materials and methods
Patients
The study sample consisted of 90 subjects who were
divided into three equal groups: patients with acute coronary
syndrome (ACS), patients with chronic CAD and healthy subjects as
controls. Each group comprised of 30 subjects (20 male and 10
female aged 40–70 years). ACS subjects were selected from patients
hospitalized at the Coronary Care Unit (CCU) of Modares Hospital in
Tehran, Iran, due to angina pectoris or acute myocardial
infarction. CAD patients were selected from patients referred to
the Angiography Unit of Modares Hospital and healthy subjects from
Modares Hospital were included in the study as controls. All
patients were enrolled from June to November, 2012. All patients
signed informed consent forms agreeing to their participation in
the study. No subject had any other disease or was taking
medications. The study was conducted following approval by the
Ethics Committee of Shahid Beheshti University of Medical Sciences
(Tehran, Iran; approval no. IR.SBMU.REC.1387.134). Clinical and
laboratory data of the patients and controls are presented in
Table I.
 | Table I.Biochemical parameters of patients and
control subjects included in the study. |
Table I.
Biochemical parameters of patients and
control subjects included in the study.
|
| Study group
(n=30/group) |
|---|
|
|
|
|---|
| Parameter | ACS patients | Chronic CAD
patients | Healthy controls |
|---|
| Age | 65 | 63 | 61 |
| Cholesterol,
mg/dl |
246±21 |
194±22 |
178±16 |
| Triglyceride,
mg/dl |
222±21 |
162±15 |
129±9 |
| Creatine kinase,
IU/l |
295±20 |
118±15 |
94±11 |
| Creatine kinase-MB,
IU/l |
63±10 |
345±7 |
20±8 |
| High-density
lipoprotein, mg/dl |
43±7 |
48±5 |
52±5 |
| Low-density
lipoprotein, mg/dl |
158±22 |
105±25 |
100±8 |
| Diastolic blood
pressure, mmHg |
155±8 |
145±4 |
127±9 |
| Systolic blood
pressure, mmHg |
111±7 |
96±8 |
85±7 |
Sample collection
Blood samples (10 ml) were collected into EDTA
sterile plastic tubes. All samples were centrifuged at 2,000 × g
for 10 min at 4°C, and were maintained at −70°C until measurement
of plasma total antioxidant capacity (TAC). For determination of
GPx activity as well as GSH and MDA levels, the obtained packed red
blood cells (pRBC) were washed with normal saline and
phosphate-buffered saline (PBS), respectively, and then stored at
−70°C until further analysis.
Measurement of erythrocyte GSH
concentration
GSH level was measured using 5,
5′-dithiobis-(2-nitrobenzoate) (DTNB; Merck KGaA, Darmstadt,
Germany) according to the spectrophotometric method described by
Beutler et al (16). Briefly,
0.2 ml of pRBCs was mixed with 8 ml PBS (0.2 M; pH 7.4) and
centrifuged at 25,000 × g for 5 min at 4°C. The sample was then
mixed with 0.5 ml DTNB. For each GSH test, 0.1 ml pRBC suspension
was mixed with 0.9 ml distilled water to provide hemolysate.
Subsequently, the mixture was vortexed and absorbance was measured
at 412 nm to detect the GSH content. The GSH levels were expressed
per gram of hemoglobin (Hb; µmol/gHb). The quantity of GSH was
determined by the known molar extinction coefficient of GSH
(1.36×104 mol−1cm−1) (16).
Determining the susceptibility of RBC
to oxidative stress
Plasma MDA is a naturally occurring product of lipid
peroxidation usually measured based on levels of thiobarbituric
acid (TBA) reactive substances or lipid peroxides (17). pRBCs were diluted (1:8 v/v) with 0.9%
saline. According to a method reported by Stocks and Dormandy for
induction of oxidative conditions, the RBCs were incubated with 4
mM H2O2 at 37°C, in a shaking thermostatic
bath for 120 min, either in the absence or presence of 2 mM sodium
azide (as a potent inhibitor of RBC catalase; Merck KGaA). A ‘zero
time’ sample was obtained by terminating the reaction [with
arsenite-trichloroacetic acid (TCA) solution (Merck KGaA)]
immediately following addition of 4 mM H2O2.
This sample was also treated by H2O2 either
in the absence or presence of 2 mM sodium azide. Healthy control
samples were also incubated in the same conditions to assess the
extent of spontaneous oxidation of RBC. Following addition of
arsenite-TCA solution and centrifugation at 4,400 × g at 4°C for 5
min, 2 ml supernatant was added to TBA-containing tubes. All tubes
were then placed in a 100°C bath for 15 min, and finally, the
absorbance of the samples was measured spectrophotometrically at
535 nm. 1,1,3,3-tetraethoxy-propane was used as a standard. The
results were reported as nmol/gHb. The concentration of blood Hb
was measured by the cyanmethemoglobin method described by Amatuzio
et al (18). The percentage of
MDA release from erythrocyte membranes was calculated by the
following formula: Concentration of MDA without sodium
azide/concentration of MDA with sodium azide ×100 (19).
Determination of erythrocyte GPx
activity
The GPx activity of erythrocytes was measured by the
modified method of Andersen et al (20), which is based on spectrophotometric
monitoring of the decrease in absorbance of NADPH (Fluka Chemie
GmbH; Sigma-Aldrich; Merck KGaA) at 340 nm in the presence of GR
(Sigma-Aldrich; Merck KGaA). This method is based on GPx oxidizing
GSH to oxidized GSH, which is then reduced by GR; finally,
oxidation of NADPH to NADP+ leads to the steady decrease
in absorbance of NADPH (21). GPx
activities of erythrocytes were expressed in U/gHb in
hemolysate.
TAC of plasma
TAC of plasma was measured by the method of Miller
et al (22) using a Total
Antioxidant Status kit (Randox Laboratories Ltd., Crumlin, UK).
According to this method, 2,2′-azino-bis
(3-ethylbenzothiazoline-6-sulfonate) (ABTS; Merck KGaA) is
incubated with metmyoglobin (Merck KGaA) and
H2O2 to form the radical cation
ABTS+•, the absorbance of which can be measured at 600
nm. In this method, the capacity of plasma antioxidants to inhibit
this reaction is measured and compared with
6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox;
Merck KGaA) as a standard. Briefly, 1 ml chromogen (601 µM
metmyoglobin and 610 µM ABTS) was added to 50 µl plasma sample and
mixed with 350 µl of 2,500 µM H2O2. The
mixture was incubated at 37°C for 3 min and its absorbance at 600
nm read. Values were expressed in mmol/l.
Statistical analysis
Data were expressed as the mean ± standard error of
the mean. The data were analyzed using SPSS version 19.0 software
(IBM Corp., Armonk, NY, USA). To compare the difference between the
three groups, data were analyzed by one-way analysis of variance
(ANOVA). Scheffe's test was used post hoc to assess the
significance of differences among the groups. Additionally, to
assess the association between the number of stenotic vessels and
oxidative status in chronic CAD patients, the 30 patients with
chronic CAD were divided into three equal groups (n=10/group) as
follows: patients with one stenotic vessel, patients two stenotic
vessels and patients with three vessel stenoses. The 30 healthy
subjects were included in the analysis as the control group, and
ANOVA and Scheffe's test were used as above. In all analyses
P<0.05 was considered to indicate statistical significance.
Results
Comparison of erythrocyte GSH levels
between the three subject groups
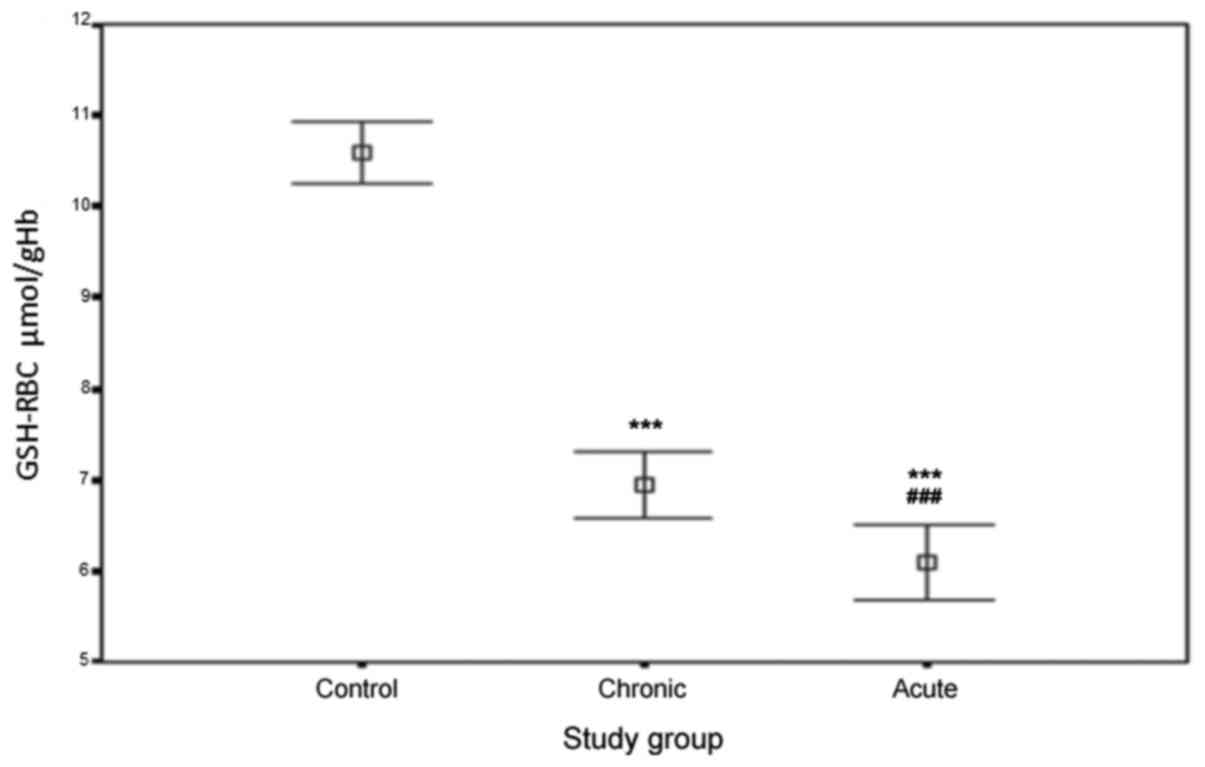
Fig. 1 depicts the
level of GSH measured in RBCs from healthy controls and the groups
of patients, where the capacity to overcome an oxidative stress has
been evaluated. The concentrations of erythrocyte GSH in the three
groups were as follows: ACS patients, 6.14±0.88 µmol/gHb; chronic
CAD patients, 6.91±0.73 µmol/gHb and healthy controls, 10.63±0.48
µmol/gHb. As the results indicate, the levels of erythrocyte GSH in
ACS and chronic CAD patients was significantly lower than in
healthy controls (P<0.0001). In addition, the level in ACS
patients was significantly lower compared with in chronic CAD
patients (P<0.0001).
Comparison of RBC susceptibility to
oxidative stress in the three study groups
Table II presents the
levels of MDA measured in RBCs of the two patient groups and of
healthy controls following incubation with
H2O2 for 120 min and at zero time, either in
the presence or absence of sodium azide. MDA levels in the presence
of sodium azide at the incubation time of 120 min were as follows:
in ACS patients, 988±52 nmol/gHb; in chronic CAD patients, 873±48
nmol/gHb; and in healthy controls, 409±24 nmol/gHb. As can be
observed, these levels of MDA in ACS and chronic CAD patients were
markedly higher than in the control group (P<0.05). Furthermore,
the MDA level in ACS patients was significantly higher than that in
chronic CAD patients (P<0.05). The levels of MDA in the absence
of sodium azide were as follows: in ACS patients, 633±39 nmol/gHb;
in chronic CAD patients, 490±33 nmol/gHb and in healthy controls,
173±19 nmol/gHb, which revealed notable difference between the
patient and control groups, and between the ACS and chronic CAD
cases. A similar pattern of data were obtained with the zero time
samples, revealing markedly high levels of MDA in ACS and chronic
CAD patients compared with in the controls, and higher MDA levels
in ACS patients in comparison with chronic CAD patients. Table III presents the percentage of MDA
release from erythrocytes in patients and controls after the
120-min incubation and at zero time. The percentage of MDA release
from erythrocytes, both at zero time and 120 min, was significantly
increased in the ACS and chronic CAD patients compared with in the
healthy controls (P<0.05). In addition, this percentage was
considerably higher in the ACS patients when compared with that in
the chronic CAD patients (P<0.05).
 | Table II.Levels of MDA in the presence or
absence of sodium azide at zero time and 120 min after incubation
with H2O2. |
Table II.
Levels of MDA in the presence or
absence of sodium azide at zero time and 120 min after incubation
with H2O2.
|
| MDA-SA,
nmol/gHb | MDA-SA,
nmol/gHb | MDA, nmol/gHb | MDA, nmol/gHb |
|---|
|
|
|
|
|
|
|---|
| Group
(n=30/group) | 120 min | Zero time | 120 min | Zero time |
|---|
| Control |
409±24 |
246±20 |
173±19 |
108±9 |
| Chronic CAD
patients |
873±48b |
463±28 |
490±33 |
286±22 |
| ACS patients |
988±52a |
512±37 |
633±39 |
312±26 |
 | Table III.Percentage of MDA release from
erythrocytes at zero time and 120 min after incubation with 4 mM
H2O2. |
Table III.
Percentage of MDA release from
erythrocytes at zero time and 120 min after incubation with 4 mM
H2O2.
| Group
(n=30/group) | % MDA release (zero
time) | % MDA release (120
min) |
|---|
| Control |
35.0±5.00 |
42.0±4.80 |
| Chronic CAD
patients |
42.0±4.70a |
57.0±9.90 |
| ACS patients |
51.0±6.40b |
64.0±9.30 |
Evaluation of erythrocyte GPx activity
in patient groups and healthy controls
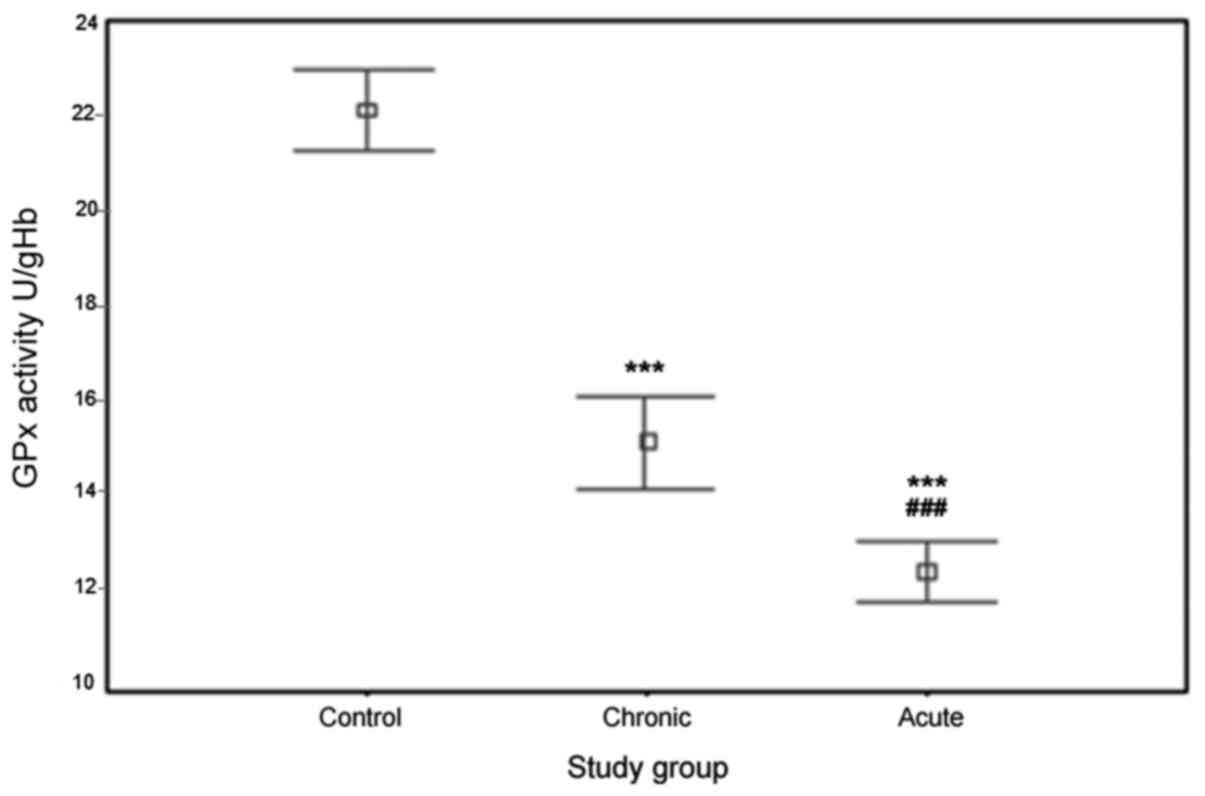
The results of erythrocyte GPx activity in the
ACS/chronic CAD patients and controls are depicted in Fig. 2. GPx activity values in the three
groups were 12.52±1.76 U/gHb for ACS patients, 15.14±2.52 U/gHb for
chronic CAD patients and 22.12±2.12 U/gHb for controls. As
illustrated in the Figure, the activity of erythrocyte GPx of ACS
and chronic CAD patients was significantly lower than that in
healthy controls (P<0.0001). Furthermore, the activity of this
enzyme in ACS patients was markedly lower than that in chronic CAD
patients (P<0.0001).
Comparison of plasma TAC in patient
and control groups
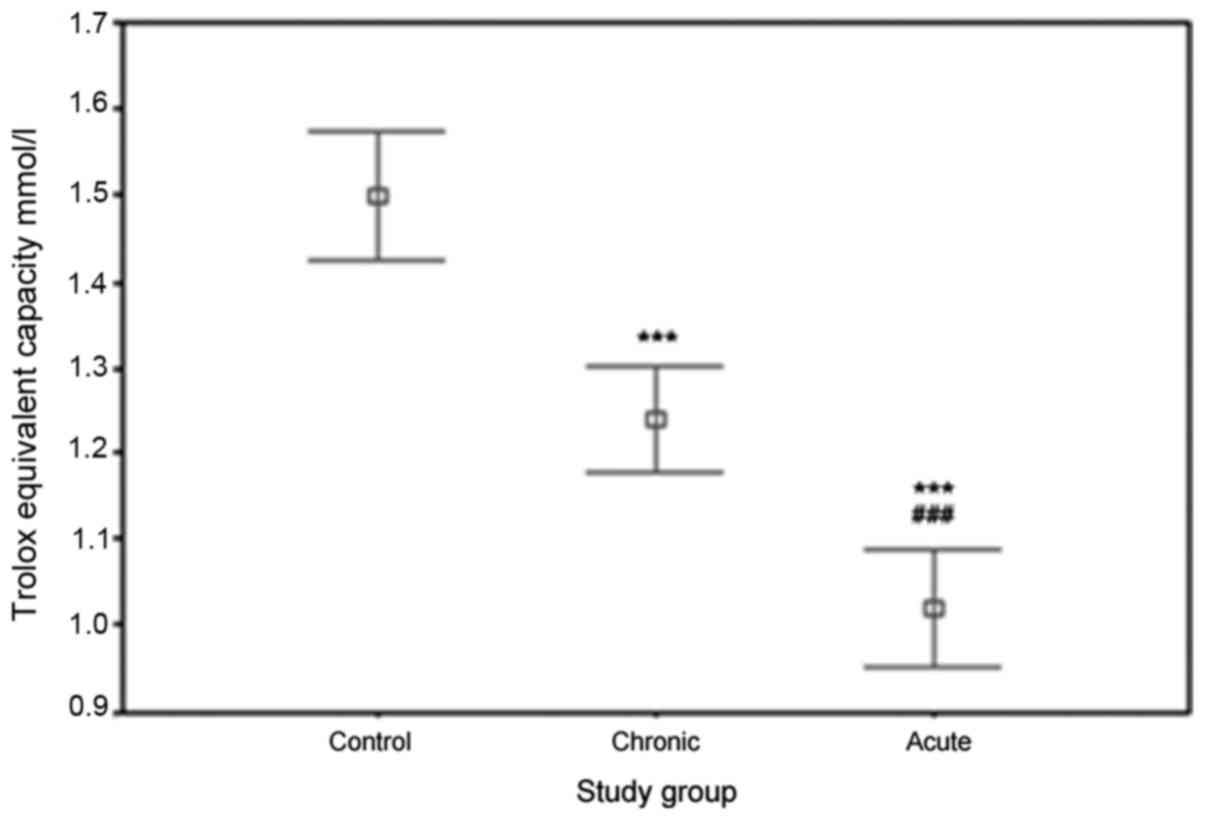
Significant differences were observed between the
TAC of plasma in the ACS, chronic CAD and healthy control groups.
The values of plasma TAC were as follows: in ACS patients,
1.02±0.18 mmol/l; in chronic CAD patients, 1.24±0.16 mmol/l; and in
controls, 1.52±0.23 mmol/l. Fig. 3
illustrates the significant reduction in the TAC of plasma in ACS
and chronic CAD patients compared with that in controls
(P<0.0001). A significant reduction in the plasma TAC of ACS
patients compared with that of chronic CAD patients was also
revealed (P<0.0001).
Evaluation of association between the
number of stenotic vessels and oxidative status in chronic CAD
patients
The results of oxidative and antioxidative
parameters measured in these three groups are presented in Table IV. From these data a significant
increase in MDA (120 min) level (P<0.05) and notable decreases
in GSH concentration, TAC and erythrocyte GPx activity were
observed in patients with triple-vessel disease compared with the
patients with double and single-vessel disease and healthy
controls. Additionally, an augmented level of MDA (P<0.05) and
reduced GSH and TAC levels as well as GPx activity were identified
in patients with double stenotic vessels in comparison with the
single stenosis and control groups. Similar data were observed with
single stenotic patients as compared with healthy subjects.
 | Table IV.Association between the number of
stenotic vessels and oxidative status in chronic coronary artery
disease patients. |
Table IV.
Association between the number of
stenotic vessels and oxidative status in chronic coronary artery
disease patients.
| Group
(n=30/group) | MDA nmol/gHb | Erythrocyte GPx,
U/gHb | Erythrocyte GSH
µmol/gHb | TAC mmol/l |
|---|
| Control |
409±41 |
22.1±2.20 |
10.6±0.48 |
1.5±0.20 |
| Patients with
one-vessel diseasen |
660±28a |
17.5±1.10 |
7.8±1.20 |
1.12±0.12 |
| Patients with
two-vessel diseasen |
900±35b |
14.7±1.60 |
6.7±0.48 |
1.06±0.16 |
| Patients with
three-vessel diseasen |
991±43c |
13.9±3.70 |
6.3±0.53 |
0.15±0.91 |
Discussion
The main purposes of the present investigation were
to compare plasma oxidative status and RBC susceptibility to
oxidative stress in patients with CAD and healthy controls. It was
identified that the levels of erythrocyte GSH in ACS and chronic
CAD patients was markedly lower than in healthy controls.
Furthermore, the level was also lowered in ACS patients as compared
with in chronic CAD patients. Similar results have been reported by
other groups with regard to significant decreases in the level of
GSH in CAD patients (23–25).
The present findings indicated that both the levels
of MDA and the percentage of MDA release from erythrocytes in ACS
and chronic CAD patients were significantly increased compared with
in the healthy controls. In addition, these two parameters in ACS
patients were significantly higher than in chronic CAD patients.
These data suggest that erythrocyte membranes in ACS patients are
more readily oxidized in comparison to those in chronic CAD
patients and healthy subjects; and furthermore that the
susceptibility of erythrocyte membranes to oxidation in chronic CAD
patients is higher than that in healthy subjects. Generally,
increases in MDA level and the percentage of MDA release from
erythrocytes in patients appeared to be due to decreased
erythrocyte GSH content relative to that in healthy subjects. Since
a high concentration of polyunsaturated fatty acids in the
phospholipid membrane of RBCs may lead to more extensive oxidation
of the membrane lipids (26), it is
necessary to consider the differences in erythrocytes membrane
composition between patients groups and controls.
The present findings are also in accordance with a
number of studies reporting an increased level of MDA in CAD and
myocardial infarction patients compared with healthy control groups
(25,27,28). Amaki
et al (29) identified that
the serum levels of circulating MDA-modified LDL in patients with
CAD were significantly higher than in patients without CAD,
indicating the use of this parameter as a diagnostic marker for
advanced atherosclerosis.
The results of the present study revealed that
patients with ACS and chronic CAD exhibited lower erythrocyte GPx
and TAC activity compared with healthy controls. Decreased
erythrocyte GPx and TAC activity in the patient groups appeared to
be correlated with induced oxidative conditions, leading to
extensive oxidative stress and increased susceptibility of the
erythrocyte membrane to this oxidant status.
These present data are also concordant with the
results of Serdar et al (30),
who reported decreased activities of antioxidant enzymes including
erythrocyte GPx and some other antioxidant enzymes as well as a
decreased concentration of antioxidant factors in patients with
CAD. In another study, Serdar et al (31) investigated the correlation between
total sialic acid (TSA) concentration in serum and antioxidative
and oxidative markers including plasma MDA, paraoxonase, GPx,
vitamin C and vitamin E in CAD patients. They identified a positive
correlation between TSA and these parameters. Furthermore, their
study revealed a significant reduction of antioxidant parameters in
the patients with CAD. Different groups have also reported an
imbalance in the levels of peroxiredoxin-1 (an antioxidant enzyme)
and GPx in the blood of patients with CAD, and existence of a
direct correlation between low GPx activity and high levels of ROS
in ACS patients (32,33). Collectively, these findings suggest a
potential contribution of inefficient protection against
oxidant-mediated damage to increased clinical risk of CAD. Further
study has revealed marked increases in the levels of oxidized-LDL
and GPx expression and activity in ACS patients in comparison with
patients with stable CAD and healthy controls (34). According to this previous study, GPx
level may be upregulated in response to an alteration in oxidative
stress during ACS. According to the current data, patients with
triple-vessel stenosis had significantly increased levels of MDA
and notably decreased GSH, TAC and GPx activities in comparison
with patients with double and single vessel disease and healthy
controls. These parameters also exhibited the same patterns in
double vessel disease patients when compared with the single vessel
disease patients and controls. Such findings suggest that the
number of narrowed vessels may have positive correlation with
deteriorated oxidative condition in chronic CAD patients; the
greater the number of stenotic vessels, the higher the oxidative
stress induced in these patients.
It was apparent that ACS patients had deteriorated
oxidant and antioxidant conditions compared with chronic CAD
patients and healthy controls. The significant differences between
ACS and chronic CAD patients suggested that the chronic form of
disease had greater probability of improvement in antioxidative
processes to ameliorate oxidative stress. Meanwhile, acute syndrome
likely has insufficient time to enhance the protective processes
and adaptive mechanisms.
This present study sheds light on the association
between acute and chronic conditions in CAD and the oxidative
status of affected patients. The results indicate that the chronic
form of disease is more adapted to oxidative stress in comparison
to the acute form, indicating the need to reduce oxidative status
in ACS patients. However, in both ACS and chronic CAD patients,
high erythrocyte membrane susceptibility, low antioxidant capacity
and decreased function of antioxidative systems were detected
compared with in the healthy controls. Therefore, the use of
exogenous antioxidants may have potential therapeutic benefits in
reducing oxidant status in these patients. However, there remains a
need for detailed study of other key pathways involved in
atherogenesis, including those associated with pro-inflammatory
markers.
Acknowledgements
Not applicable.
Funding
The present study was supported by internal funding
from Shahid Beheshti Univesity of Medical Sciences, Tehran,
Iran.
Availability of data and materials
All datasets used or analyzed during this study are
available from the corresponding authors on reasonable request.
Authors' contributions
AB designed the study and aided in writing the
manuscript. SR assisted in drafting the manuscript and revising it
critically for intellectual content. AD performed the experiments.
HS was the co-supervisor together with AB and aided in collecting
the samples and interpreting the results. FKB aided with manuscript
writing and interpreting the data.
Ethics approval and consent to
participate
Written informed consent was obtained and signed by
all patients who agreed to participate in the study.
Patient consent for publication
The patients provided written informed consent for
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pathak LA, Shirodkar S, Ruparelia R and
Rajebahadur J: Coronary artery disease in women. Indian Heart J.
69:532–538. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wong ND: Epidemiological studies of CHD
and the evolution of preventive cardiology. Nat Rev Cardiol.
11:276–289. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hansson GK: Inflammation, atherosclerosis,
and coronary artery disease. N Engl J Med. 352:1685–1695. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Badimon L and Vilahur G: Thrombosis
formation on atherosclerotic lesions and plaque rupture. J Intern
Med. 276:618–632. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zaman AG, Helft G, Worthley SG and Badimon
JJ: The role of plaque rupture and thrombosis in coronary artery
disease. Atherosclerosis. 149:251–266. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wick G and Grundtman C: Inflammation and
Atherosclerosis. I. Springer-Verlag Wien; Austria: 2011
|
|
7
|
Cervantes Gracia K, Llanas-Cornejo D and
Husi H: CVD and oxidative stress. J Clin Med. 6:222017. View Article : Google Scholar
|
|
8
|
Vogiatzi G, Tousoulis D and Stefanadis C:
The role of oxidative stress in atherosclerosis. Hellenic J
Cardiol. 50:402–409. 2009.PubMed/NCBI
|
|
9
|
Bonomini F, Tengattini S, Fabiano A,
Bianchi R and Rezzani R: Atherosclerosis and oxidative stress.
Histol Histopathol. 23:381–390. 2008.PubMed/NCBI
|
|
10
|
Stocker R and Keaney JF: Role of oxidative
modifications in atherosclerosis. Physiol Rev. 84:1381–1478. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Madamanchi NR, Hakim ZS and Runge MS:
Oxidative stress in atherogenesis and arterial thrombosis: The
disconnect between cellular studies and clinical outcomes. J Thromb
Haemost. 3:254–267. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Valko M, Rhodes CJ, Moncol J, Izakovic M
and Mazur M: Free radicals, metals and antioxidants in oxidative
stress-induced cancer. Chem Biol Interact. 160:1–40. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shao B and Heinecke JW: HDL, lipid
peroxidation, and atherosclerosis. J Lipid Res. 50:599–601. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lee SE and Park YS: Role of lipid
peroxidation-derived α, β-unsaturated aldehydes in vascular
dysfunction. Oxid Med Cell Longev. 2013:6290282013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ahmadinejad F, Geir Møller S,
Hashemzadeh-Chaleshtori M, Bidkhori G and Jami M-S: Molecular
Mechanisms behind Free Radical Scavengers Function against
Oxidative Stress. Antioxidants. 6:512017. View Article : Google Scholar
|
|
16
|
Beutler E, Duron O and Kelly BM: Improved
method for the determination of blood glutathione. J Lab Clin Med.
61:882–888. 1963.PubMed/NCBI
|
|
17
|
Kadiiska MB, Gladen BC, Baird DD, Germolec
D, Graham LB, Parker CE, Nyska A, Wachsman JT, Ames BN, Basu S, et
al: Biomarkers of oxidative stress study II: Are oxidation products
of lipids, proteins, and DNA markers of CCl4 poisoning? Free Radic
Biol Med. 38:698–710. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Amatuzio DS, Grande F and Wada S: The
cyanmethemoglobin method for hemoglobin determination. Minn Med.
45:378–381. 1962.PubMed/NCBI
|
|
19
|
Cynamon HA, Isenberg JN and Nguyen CH:
Erythrocyte malondialdehyde release in vitro: A functional measure
of vitamin E status. Clin Chim Acta. 151:169–176. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andersen HR, Nielsen JB, Nielsen F and
Grandjean P: Antioxidative enzyme activities in human erythrocytes.
Clin Chem. 43:562–568. 1997.PubMed/NCBI
|
|
21
|
Sonet J, Bierla K, Bulteau A-L, Lobinski R
and Chavatte L: Comparison of analytical methods using enzymatic
activity, immunoaffinity and selenium-specific mass spectrometric
detection for the quantitation of glutathione peroxidase 1. Anal
Chim Acta. 1011:11–19. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Miller NJ, Rice-Evans C, Davies MJ,
Gopinathan V and Milner A: A novel method for measuring antioxidant
capacity and its application to monitoring the antioxidant status
in premature neonates. Clin Sci (Lond). 84:407–412. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Damy T, Kirsch M, Khouzami L, Caramelle P,
Le Corvoisier P, Roudot-Thoraval F, Dubois-Randé JL, Hittinger L,
Pavoine C and Pecker F: Glutathione deficiency in cardiac patients
is related to the functional status and structural cardiac
abnormalities. PLoS One. 4:e48712009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kaur K, Bedi G, Kaur M, Vij A and Kaur I:
Lipid peroxidation and the levels of antioxidant enzymes in
coronary artery disease. Indian J Clin Biochem. 23:33–37. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pamplona R: Membrane phospholipids,
lipoxidative damage and molecular integrity: A causal role in aging
and longevity. Biochim Biophys Acta. 1777:1249–1262. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar E, Mukherjee R, Senthil R,
Parasuraman S and Suresh B: Evaluation of oxidative stress and
antioxidant status in patients with cardiovascular disease in rural
populations of the nilgiris, South India. ISRN pharmacol.
2012:9410682012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bhat MA, Mahajan N and Gandhi G: Oxidative
stress status in coronary artery disease patients. Int J Life Sc Bt
Pharm Res. 1:236–243. 2012.
|
|
28
|
Surekha RH, Srikanth BB, Jharna P,
Ramachandra RV, Dayasagar RV and Jyothy A: Oxidative stress and
total antioxidant status in myocardial infarction. Singapore Med J.
48:137–142. 2007.PubMed/NCBI
|
|
29
|
Amaki T, Suzuki T, Nakamura F, Hayashi D,
Imai Y, Morita H, Fukino K, Nojiri T, Kitano S, Hibi N, et al:
Circulating malondialdehyde modified LDL is a biochemical risk
marker for coronary artery disease. Heart. 90:1211–1213. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Serdar Z, Aslan K, Dirican M, Sarandöl E,
Yeşilbursa D and Serdar A: Lipid and protein oxidation and
antioxidant status in patients with angiographically proven
coronary artery disease. Clin Biochem. 39:794–803. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Serdar Z, Yeşilbursa D, Dirican M,
Sarandöl E and Serdar A: Sialic acid and oxidizability of lipid and
proteins and antioxidant status in patients with coronary artery
disease. Cell Biochem Funct. 25:655–664. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Vnukov VV, Sidorov RV, Milutina NP,
Ananyan AA, Gvaldin DY, Sagakyants AB, Shlyk IF and Talalaev EP:
Concentration of proinflammatory cytokines, peroxiredoxin-1 and
glutathione peroxidase activity in the blood plasma of patients
with coronary artery disease undergoing coronary artery bypass
grafting. Adv Gerontol. 30:269–275. 2017.(In Russian). PubMed/NCBI
|
|
33
|
Holley AS, Miller JH, Larsen PD and
Harding SA: Relationship between glutathione peroxidase, platelet
reactivity, and reactive oxygen species in an acute coronary
syndrome population. Ann Clin Lab Sci. 46:639–644. 2016.PubMed/NCBI
|
|
34
|
Holley A, Pitman J, Miller J, Harding S
and Larsen P: Glutathione peroxidase activity and expression levels
are significantly increased in acute coronary syndromes. J Investig
Med. 65:919–925. 2017. View Article : Google Scholar : PubMed/NCBI
|