Introduction
Astragalus membranaceus has been used
historically in China for its apparent medicinal functions.
Astragalus membranaceus in isolation, combined with other
drugs or as a food supplement may promote health and immune balance
(1). Modern pharmacological research
has indicated that Astragalus membranaceus has various
effects including in regulating the immune system, antioxidant
function, in promoting hematopoiesis and in regulating metabolism,
among others, and that it could protect endothelial cells subjected
to hypoxia-reperfusion by inhibiting lipid peroxidation and
enhancing scavenging of oxygen free radicals (2). Studies have also suggested that
Astragalus membranaceus may regulate the immune system,
inhibit cell mitosis and inhibit the growth of mouse leukemia and
lymphadenoma tumor cells by activating mouse B cells and macrophage
cells (2,3). The biological activity of Astragalus
membranaceus may be due to the functions of its composite
proteins, polysaccharides and flavonoids. However, there are few
studies on the biological activities of the putative functional
proteins of Astragalus membranaceus, and its mechanism of
action remains unclear. Therefore, it is of interest to study the
effects of proteins extracted from Astragalus membranaceus
in terms of potential Antitumor and anti-antibacterial
mechanisms.
Lectin has various biological functions, which have
become increasingly recognized by previous studies (4–8). It is a
type of non-immune glycoprotein that can interact with carbohydrate
in a reversible and specific manner. Lectin contains a variable
amino acid sequence, and is widely distributed in microbes, virus'
and plants (9). The specificity of
lectin extends not only to sugar-binding activity but also to
function, structure and tissue expression (10). Certain studies have revealed that
lectin exhibits various biological activities, including
insecticidal activity (4), antifungal
action (5), Antitumor effects
(6), antivirus action (7) and immunomodulatory effects (8). Furthermore, it has been identified that
lectin could be applied in host defence and in the treatment of
various diseases including cancer (11,12).
At present, to the best of our knowledge, there are
no reports on the abstraction or functions of lectin from the seeds
of Astragalus membranaceus. Therefore, in the present study,
Astragalus membranaceus lectin (AML) was abstracted,
purified and resolved, and its biological activities examined to
provide experimental support for the application of Astragalus
membranaceus as a therapeutic remedy.
Materials and methods
Materials and samples
The seeds of Astragalus membranaceus were
obtained from Shanxi Academy of Agricultural Sciences (Taiyuan,
China). Human blood samples (1 ml each of two type A samples, two
type B samples, two type AB samples and two type O; all 8 subjects
were male, aged 20-40 years old and disease-free) were collected by
the Chinese Medicine Hospital of Shanxi Province (Taiyuan, China).
Experimental animals were supplied by the animal laboratory centre
of Shanxi Medical University (Taiyuan, China). A total of 5 male
Japanese White rabbits (8 months old, weight 4-5 kg), 10 C57 male
mice (5 weeks old, weight 20-22g) and 10 SD male rats (2.5 months
old, weight 350-380 g) were used to collect blood samples, and were
housed in a specific pathogen-free (SPF) room under controlled
temperature and humidity. A 12-h light/dark cycle was maintained. A
HiTrap SP XL anion exchange column and Superdex G25 gel filtration
column were purchased from GE Healthcare (Uppsala, Sweden). Fetal
bovine serum (FBS) and RPMI-1640 medium were sourced from
Sigma-Aldrich (Merck KGaA, Darmstadt, Germany). All solvents used
in high-performance liquid chromatography were of chromatography
grade, and all other reagents were of the highest purity
available.
All subjects whose blood was collected gave sign
informed consent for the use of their samples for research
purposes. All human and animal protocols were performed according
to the Guide for the Care and Use of Laboratory Animals (13) and approved by the Ethics Committees of
the Chinese Medicine Hospital of Shanxi Province (Taiyuan, China)
and the Chinese Academy of Medical Sciences (Beijing, China).
Extraction and purification of
AML
The seeds of Astragalus membranaceus were
ground and treated with acetate buffer (pH 5.0) for 24 h at 4°C,
and then centrifuged at 10,000 × g and 4°C for 30 min.
(NH4)2SO4 was added into the
supernatant to 80% saturation and stirred for 4 h at 4°C, followed
by centrifugation at 12,000 × g and 4°C for 30 min. The supernatant
was discarded and the remaining pellet was precipitated after
dissolving with 20 mM Tris-HCl buffer (pH 7.0), and then
centrifuged at 10,000 × g and 4°C for 30 min. The supernatant was
used to confirm protein content and hemagglutination activity. A
two-step chromatography method was used to collect AML. Crude
astragalus seed extracts dissolved in 2 ml 20 mM Tris-HCl buffer
(pH 7.0) were loaded onto a HiTrap SP XL column, then loaded onto a
Superdex G-25 column and eluted by 0.5 M NaCl in 20 mM
phosphate-buffered saline (PBS), at a flow rate of 0.5 ml/min, in
an AKTA™ Explore protein purification system (GE Healthcare) at
room temperature. The bicinchoninic acid assay method was used to
detect the level of AML.
Polyacrylamide gel electrophoresis
(SDS-PAGE)
According to the method reported by Laemmli
(14), the separation gel and spacer
gel concentrations were 12.5 and 4.0% respectively. A total of 15
µl protein was loaded per lane. The gel was stained with Coomassie
brilliant blue R250 for 1 h at room temperature, and then the
formula weight of the protein band was confirmed by a Gel imaging
analysis system (Bio-Rad Gel DocXR with Quantity One v4.62
software; Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Analysis of coagulation activity of
AML
Rabbit blood (0.5 ml) was collected via the
auricular vein, and mouse and rat blood (0.5 ml) were collected via
orbital puncture. The coagulation activity of AML was detected in
microtitration V plates (25 µl). The AML (1 mg/ml) was mixed with
25 µl of 2% whole blood in normal saline, and then incubated at
room temperature until the cells were all deposited in the wells of
the blank group (blood samples not mixed with AML). Agglutination
activity was determined from the detection of agglutinated
erythrocytes by naked eye, and 1/2n (with n being the
number of wells exhibiting an agglutination reaction) was presented
as the agglutination titer (15).
Detection of PH and thermal stability
of AML
To detect the influence of pH on the AML, AML was
dissolved in Cl at pH 2.4-3.0 and NaOH at pH 11.7-12.2. Incubation
was performed for 30 min at room temperature to neutralize the
solution, and coagulation activity was detected by the above
method. To detect the thermal stability of AML, the protein (1
mg/ml) was incubated at different temperatures (25-80°C) for 30
min, then placed immediately on ice for 5 min, and its coagulation
activity was detected.
Detection of metal ion stability of
AML
A 20 mM EDTA-AML solution (1 mg/ml AML initial
concentration) was dialyzed and its coagulation activity was
detected. Following dialysis, the solution was added into 10 mM
Cu2+, Mg2+ or Zn2+, and its
coagulation activity was detected.
Detection of sugar content of AML
The sugar content of 1 mg/ml AML was detected by the
phenol-sulfuric acid method (16),
and its optical density (OD) value was measured at 490 nm.
Anhydrous glucose was chosen as the standard. A total of 0.5 ml
sample containing 2-25 µg sugar was selected, mixed with 0.3 ml 5%
phenol solution followed by 1.8 ml concentrated sulfuric acid, and
its OD value was detected at 490 nm. Sugar content of AML was
determined from the standard curve. The sugar content was the
abscissa, and the OD value the ordinate.
Detection of amino acid content of
AML
A total of 1 mg/ml AML was dissolved in 6 M HCl,
hydrolyzed at 100°C for 24 h, and analyzed using a Germany
Manmerbor A300 Automatic amino acid analyser (MembraPure GmbH,
Hennigsdorf, Germany).
Antibacterial activity of AML
Serial dilution of liquid nutrient medium was
conducted to detect the antibacterial activity of AML, and the
medial lethal dose of AML against bacteria was screened according
to preliminary results (unpublished). Using four sterile tubes, 1
ml broth culture (tryptone 10 g/l, yeast extract 5 g/l, NaCl 10
g/l) and 1 ml AML solution were mixed in one tube, from which 1 ml
solution was isolated added into the second tube until the fourth
tube; the final concentrations of AML were 25, 50, 100 and 200
µg/ml. A tube containing 1 ml broth culture and 1 ml aseptic water
was used as a control. Into each tube 50 µl bacterial liquid
(106 cells/ml in broth culture) containing Bacillus
dysenteriae (B. dysenteriae), Staphylococcus
aureus (S. aureus) or Escherichia coli (E.
coli) (all from China General Microbiological Culture
Collection Center, Beijing, China) was added, mixed and cultured in
a 37°C incubator. A sixth tube was designated a zero tube, into
which 1 ml broth culture, 1 ml aseptic water and 50 µl normal
saline were added. After 24 h, the absorbance value of each tube
was observed at 600 nm. Mean absorbance was calculated from three
replicate measurements. The half maximal inhibitory concentration
(IC50) of AML to each test bacteria was calculated
according to the formula
IC50=Ilg-1{Xm-I[ΣP-(3-Pm-Pn)/4]} (17), where Xm: the logarithmic value of the
maximum concentration; i: the logarithmic value of the ratio of
maximum dose to current dose; ΣP: the sum of the growth inhibition
rate of each group; Pm: the maximum positive reaction rate; Pn: the
minimum positive reaction rate.
Antitumor activity of AML
A Cell Counting Kit-8 (CCK-8) assay (Dojindo
Molecular Technologies, Inc., Kumamoto, Japan) was used to analyze
the antitumor activity of AML. SGC-7901, HepG2, H22 and HL-7702
cells (BeNa Culture Collection, Beijing, China) in logarithmic
growth phase were selected to prepare cell suspensions of
1×106 cell/ml, which were seeded into 96-well plates,
with three repeats per cell sample. The cells were cultured in a 5%
CO2, 37°C incubator for 2 h, after which PBS was added
with different concentrations of AML (6.25, 12.50, 25.00 and 50.00
µg/ml). The cells were further cultured in the 37°C, 5%
CO2 incubator for 12, 20 and 24 h respectively, after
which 10 µl CCK-8 was added and cells were incubated for another 4
h prior to measurement at 570 nm. The IC50 of AML to
each cell line was calculated according to the above formula.
Results
Abstraction and identification of
AML
Following rough abstract, ammonium sulfate
precipitation and dialysis, the crude proteins were purified by an
AKTA Explore system with HiTrap SP XL and Superdex G25 columns.
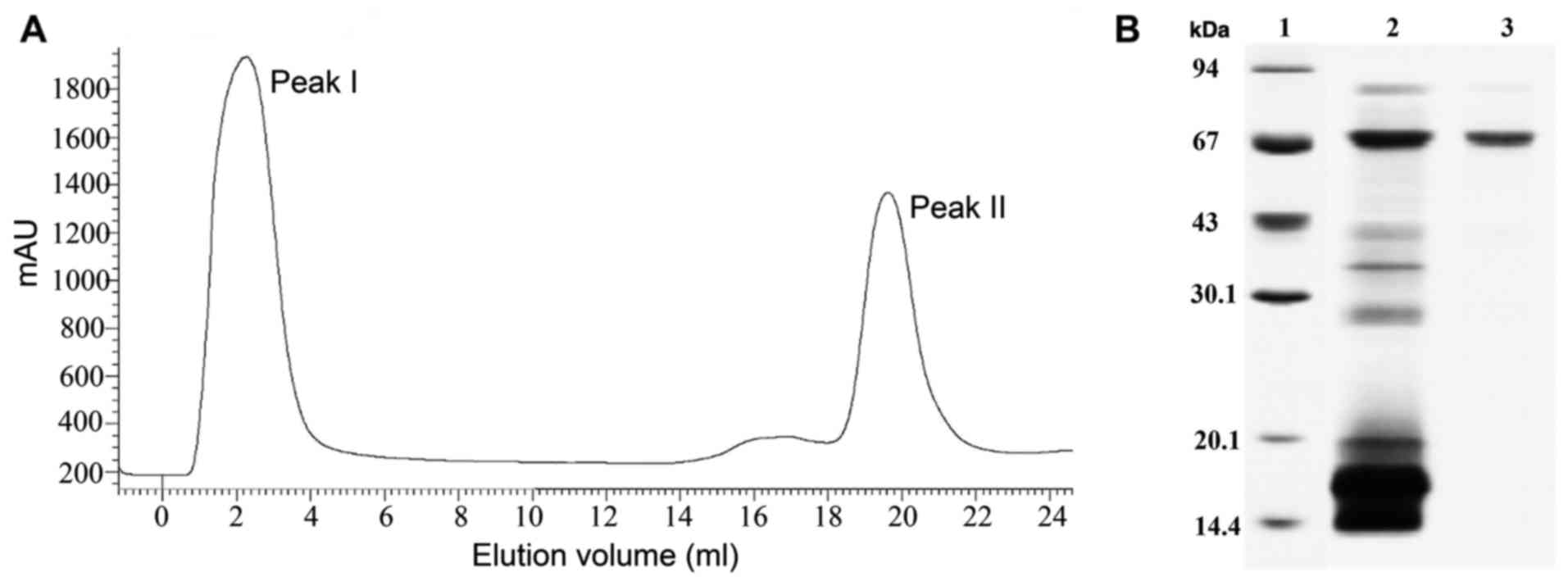
Proteins were obtained weighted 67 kDa by SDS-PAGE (Fig. 1).
Detection of agglutination titer
The hemagglutination effect of AML on human and
animal erythrocytes was as follows: Human (A type),
1/29; human (B type), 1/28; human (AB type),
1/28; human (O type), 1/210; rat,
1/29; mouse, 1/28; rabbit, 1/210.
At the initial concentration of 1 mg/ml, 1/28=3.91
µg/ml, 1/29=1.95 µg/ml and 1/210=0.97 µg/ml
(data not shown).
PH and thermal stability of AML
The AML exhibited minor thermal stability: When it
was heated to 64°C, it exhibited normal coagulation activity; while
the temperature increase from 64 to 70°C caused a marked decline in
activity, and activity was no longer apparent at 80°C. By contrast,
AML exhibited considerable pH stability, with normal coagulation
activity observed at pH 2-13. At pH 0-1, activity declined to ~50%
and at pH 14 was undetectable (data not shown).
Influence of metalions on the
agglutination activity of AML
Following dialysis of AML using 20 mM EDTA, no
apparent changes were observed in its agglutination activity. Its
activity was also unchanged following the addition of 20 mM
Cu2+, Mg2+ or Zn2+; the titers of
agglutination activity were 1/210 for Cu2+,
1/210 for Mg2+ and 1/29 for
Zn2+. Initial concentration was 1 mg/ml;
1/29=1.95 µg/ml and 1/210=0.97 µg/ml.
Therefore, the agglutination activity of AML had no apparent
association with the presence of the three metal ions (data not
shown).
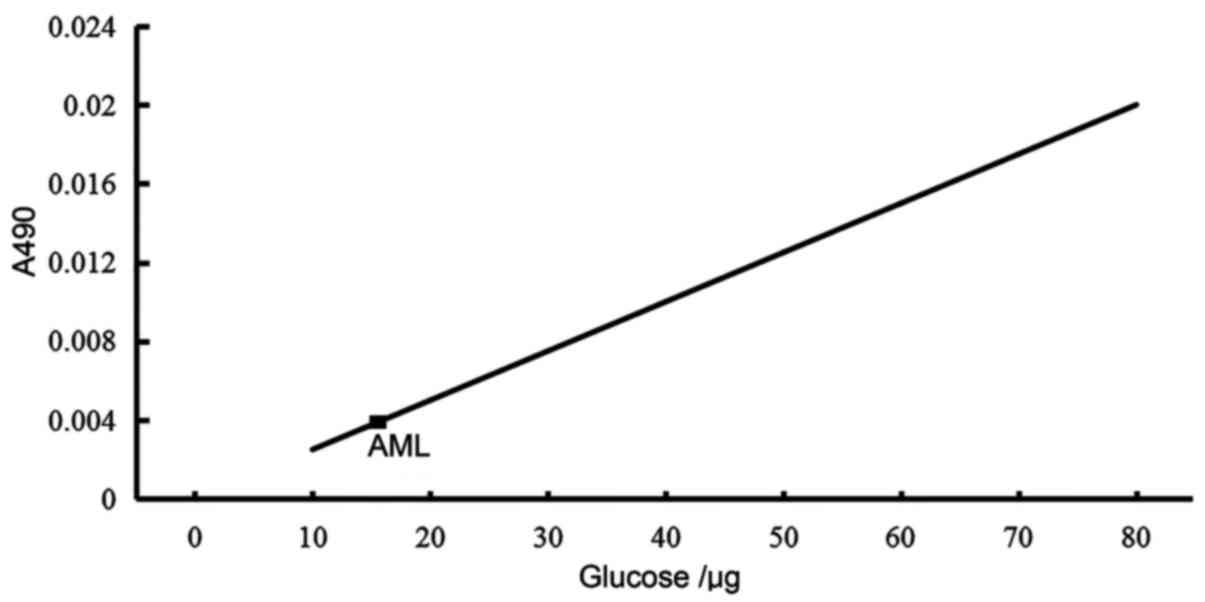
Sugar content of AML
The sugar content of AML was determined to be 16.4%
(Fig. 2).
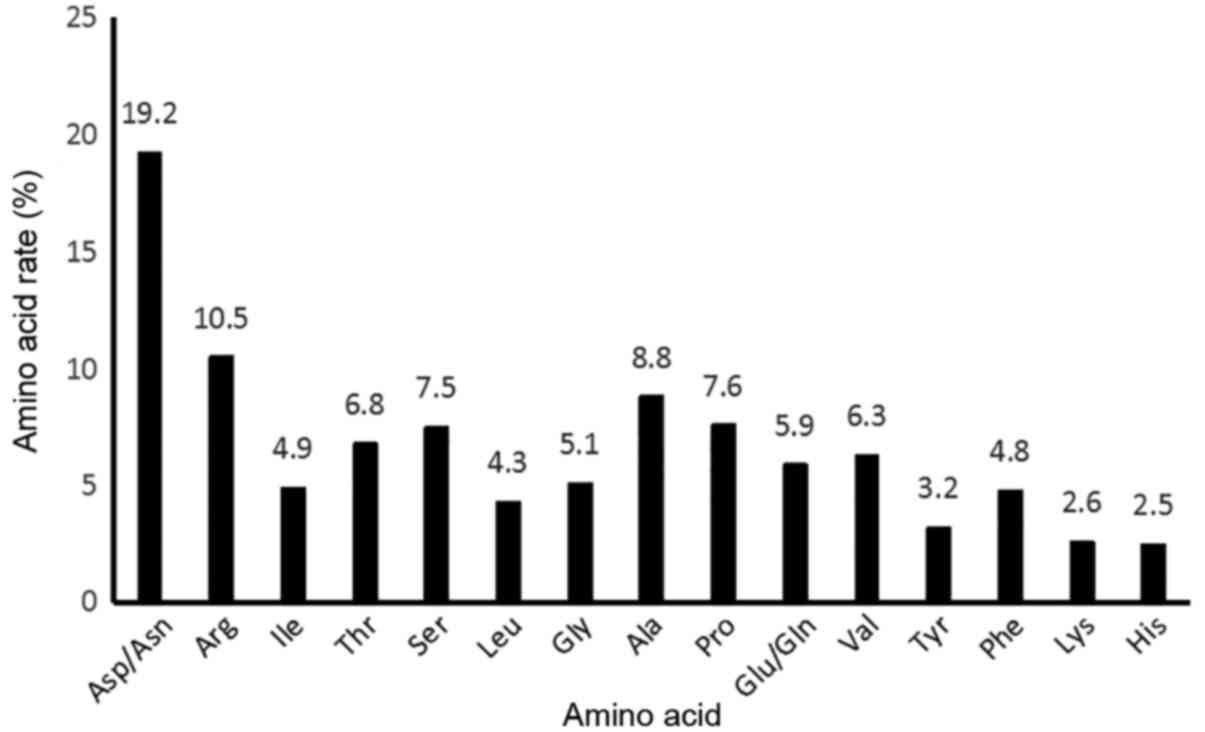
Amino acid content of AML
AML contained 15 types of amino acid, among which
the majority were charged and polar amino acids, accounting for
~70%, while the remaining 30% were hydrophobic amino acids. The
content of Asp/Asn and Arg, as amino acids associated with immunity
(18), was relatively high, nearing
30% (Fig. 3).
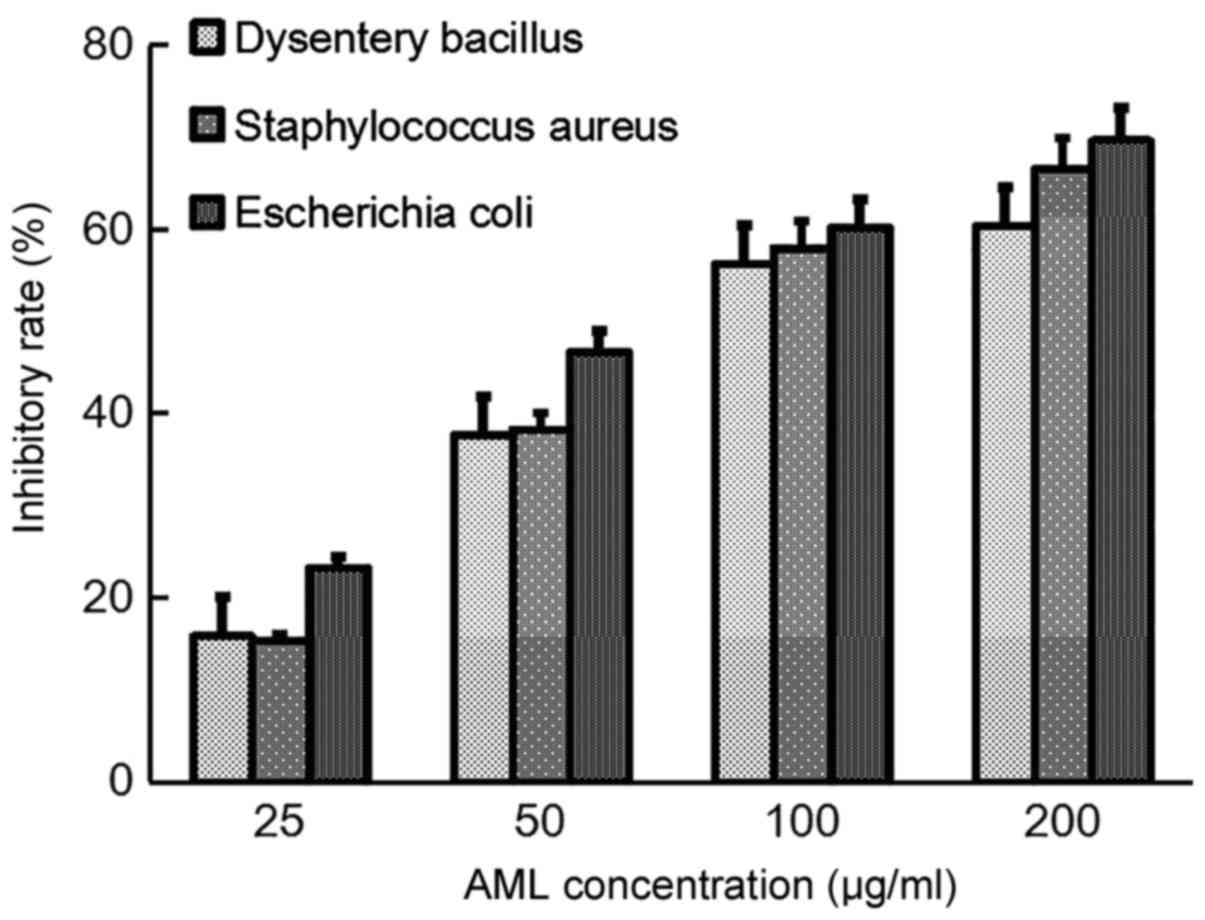
Antibacterial activity of AML
The inhibition ratios of different concentrations of
AML indicated a dose-dependent effect on the three test bacteria.
The IC50 for B. dysenteriae, S. aureus and E.
coli were 85.4, 80.2 and 65.3 µg/ml, respectively (Fig. 4).
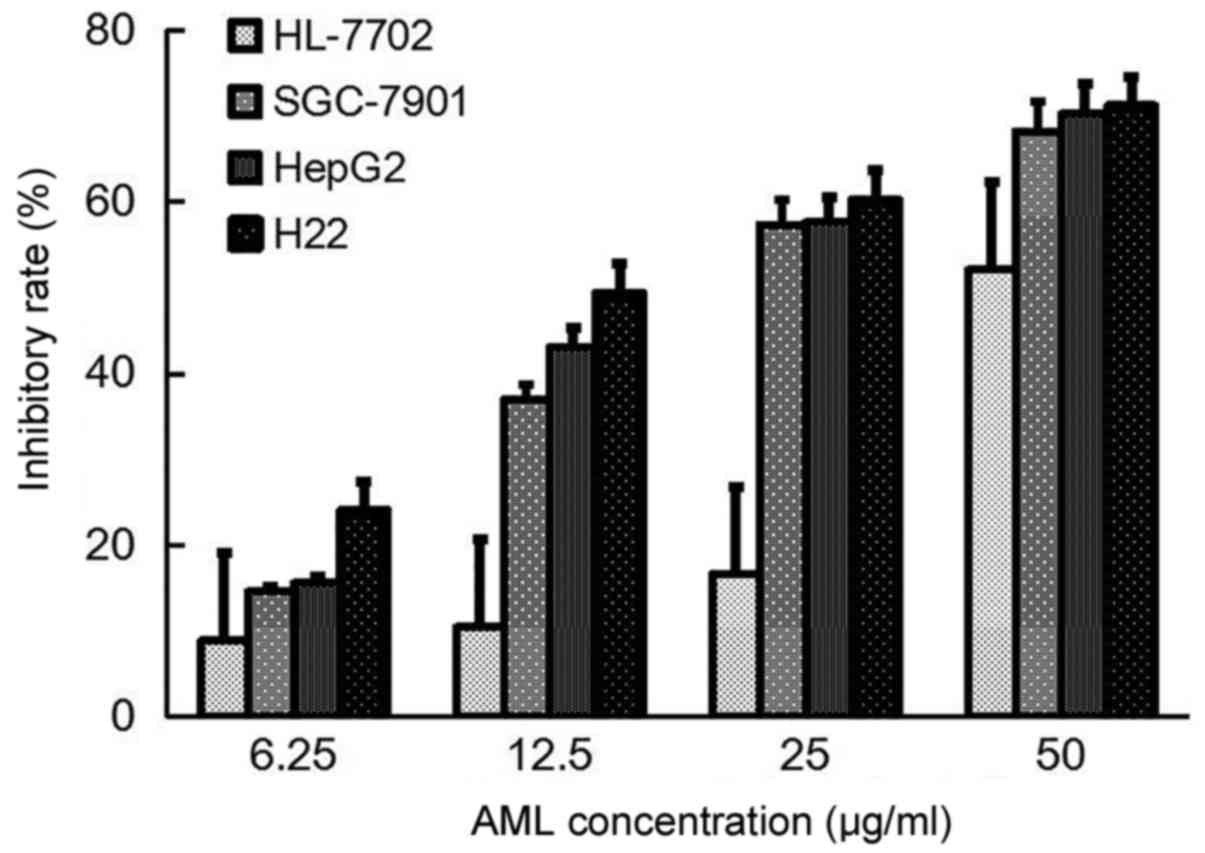
Antitumor activity of AML
The inhibition ratios of different concentrations of
AML suggested a dose-dependent effect on the three tumor cell lines
and normal liver cells. The IC50s for SGC-7901, HepG2,
H22 and HL-7702 were 19.6, 19.6, 15.5 and 45.1 µg/ml, respectively.
Therefore, compared with its effect on the tumor cells, AML
exhibited lower cytotoxicity against normal cells, which confirmed
that AML had inhibitory effects on the three tumor cell lines
(Fig. 5).
Discussion
In the present study, AML was extracted, separated
and purified from the seeds of Astragalus membranaceus via
buffer extraction, ammonium sulfate precipitation, dialysis and
laminar flow analysis with HiTrap SP XL ion exchange and Superdex
G25 solvent resistant columns. Its molecular weight was analyzed
using SDS-PAGE and determined to be ~67 kDa; furthermore, its sugar
content was 16.4% which differed from that reported previously of
10.7% (19) and it contained 15 types
of amino acid, of which the majority were charged and polar amino
acids (~70%), with the remaining portion being hydrophobic amino
acids (~30%). The content of Asp/Asn and Arg, associated with
immunity, was relatively high and nearing 30%. The properties of
AML including coagulation activity, pH and temperature as well as
metal ion stability were further analyzed, and its antibacterial
and Antitumor activities were studied. Results indicated that AML
exerted stimulatory effects on the agglutination of 4 human blood
types and mouse, rat and rabbit erythrocytes, particularly on human
blood type O and rabbit erythrocytes. The AML exhibited resistance
to three types of metal iron, and its thermal denaturation
temperature was over 64°C. AML had preserved total hemagglutination
activity at pH 2-13, despite previous studies indicating that
hemagglutination activity gradually weakened when pH >9
(20,21); the current data indicates that this
novel lectin has a more stable pH than previously proposed, as a
suitable precondition for later drug development. Additionally, AML
exhibited inhibitory effects on three test bacteria, including
B. dysenteriae, S. Aureus and E. coli, for which the
corresponding IC50s were all <100 µg/ml. AML also
exerted inhibitory effects on the growth of SGC-7901, HepG2 and H22
cells, contrasting to its less toxic effect on HL-7702 cells,
although its antitumor mechanism was unclear and requires further
study.
Acknowledgements
Not applicable.
Funding
The current work was supported by the Natural
Science Foundation of Shanxi Province (grant no. 20170110) and the
Scientific Research Project of the Health Planning Committee of
Shanxi (grant no. 201601083).
Availability of data and materials
All data described in the article are available upon
request from the corresponding author.
Authors' contributions
CZB contributed to conception and design of the
study; to acquisition, analysis and interpretation of the data; and
to drafting of the manuscript. JQH, XLH and MLF each contributed to
acquisition of the data and to revision of the manuscript. MLF
contributed to analysis and interpretation of the data as well as
to revision of the manuscript.
Ethics approval and consent to
participate
The study protocol complied with the Guide for the
Care and Use of Laboratory Animals and was approved by the Ethics
Committees of the Chinese Medicine Hospital of Shanxi Province
(Taiyuan, China). Written informed consent was obtained from all
subjects.
Consent for publication
All participants involved in this research agreed to
the publication of any associated data and accompanying images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jin M, Zhao K, Huang Q and Shang P:
Structural features and biological activities of the
polysaccharides from Astragalus membranaceus. Int J Biol Macromol.
64:257–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao BM, Xu W, Dai H, Tu P, Li Z and Gao
XM: A study on the immune receptors for polysaccharides from the
roots of Astragalus membranaceus, a Chinese medicinal herb. Biochem
Biophys Res Commun. 320:1103–1111. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cho WC and Leung KN: In vitro and in vivo
Antitumor effects of Astragalus membranaceus. Cancer Lett.
252:43–54. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Vandenborre G, Smagghe G and Van Damme EJ:
Plant lectins as defense proteins against phytophagous insects.
Phytochemistry. 72:1538–1550. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Vasconcelos MA, Cunha CO, Arruda FV,
Carneiro VA, Mercante FM, do Nascimento Neto LG, de Sousa GS, Rocha
BA, Teixeira EH, Cavada BS, et al: : Lectin from Canavalia
brasiliensis seeds (ConBr) is a valuable biotechnological tool to
stimulate the growth of Rhizobium tropici in vitro. Molecules.
17:5244–5254. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Balzarini J, Neyts J, Schols D, Hosoya M,
Van Damme E, Peumans W and De Clercq E: The mannose-specific plant
lectins from Cymbidium hybrid and Epipactis helleborine and the
(N-acetylglucosamine)n-specific plant lectin from Urtica dioica are
potent and selective inhibitors of human immunodeficiency virus and
cytomegalovirus replication in vitro. Antiviral Res. 18:191–207.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zuo Z, Fan H, Wang X, Zhou W and Li L:
Purification and characterization of a novel plant lectin from
Pinellia ternata with antineoplastic activity. Springerplus.
1:132012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peumans WJ and Van Damme EJ: Lectins as
plant defense proteins. Plant Physiol. 109:347–352. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kaltner H, García Caballero G, Ludwig AK,
Manning JC and Gabius HJ: From glycophenotyping by (plant) lectin
histochemistry to defining functionality of glycans by pairing with
endogenous lectins. Histochem Cell Biol. 149:547–568. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu XQ, Wu H, Yu HL, Zhao TF, Pan YZ and
Shi RJ: Purification of a lectin from Arisaema erubescens (Wall.)
schott and its pro-inflammatory effects. Molecules. 16:9480–9494.
201111 View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye XY, Ng TB, Tsang PW and Wang J:
Isolation of a homodimeric lectin with antifungal and antiviral
activities from red kidney bean (Phaseolus vulgaris) seeds. J
Protein Chem. 20:367–375. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Heinrich EL, Welty LA, Banner LR and
Oppenheimer SB: Direct targeting of cancer cells: A multiparameter
approach. Acta Histochem. 107:335–344. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bruce A: Guide for the Care and Use of
Laboratory Animals. National Academies Press; Washington: pp.
85–123. 1996
|
|
14
|
Laemmli UK: Cleavage of structural
proteins during the assembly of the head of bacteriophage T4.
Nature. 227:680–685. 1970. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Qin XZ, Yang XP and Chen J: Hemagglutinins
of higher fungi in Altay. Xinjiang Nongye Kexue. 53:142–148.
2016.(In Chinese).
|
|
16
|
Dubois M, Gilles KA, Hamilton JK, Rebers
PA and Smith F: Colorimetric method for determination of sugars and
related substances. Anal Chem. 28:350–356. 1956. View Article : Google Scholar
|
|
17
|
Chou TC: The median-effect principle and
the combination index for quantitation of synergism and antagonism.
Synergism and Antagonism in Chemotherapy. Chou TC and Rideout DC:
Academic Press; San Diego, CA: pp. 61–102. 1991
|
|
18
|
Barbul A, Sisto DA, Wasserkrug HL and
Efron G: Arginine stimulates lymphocyte immune response in healthy
human beings. Surgery. 90:244–251. 1981.PubMed/NCBI
|
|
19
|
Zhu LF, Yan QJ, Jiang ZQ and Huang LH:
Isolation and purification of a lectin from roots of Astragalus
membranaceus. Chin Tradit Herbal Drugs. 41:714–717. 2010.
|
|
20
|
Kaur A, Singh J, Kamboj SS, Sexana AK,
Pandita RM and Shamnugavel M: Isolation of an
N-acetyl-D-glucosamine specific lectin from the rhizomes of Arundo
donax with antiproliferative activity. Phytochemistry.
66:1933–1940. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Vaz AF, Costa RM, Melo AM, Oliva ML,
Santana LA, Silva-Lucca RA, Coelho LC and Correia MT: Biocontrol of
Fusarium species by a novel lectin with low ecotoxicity isolated
from Sebastiania jacobinensis. Food Chem. 119:1507–1513. 2010.
View Article : Google Scholar
|