Introduction
microRNAs (miRNAs) are non-coding RNA molecules,
comprising ~22 nucleotides, that are evolutionarily conserved and
function as sequence-specific regulators of gene expression through
translational repression and/or transcript cleavage (1–4). miRNAs
downregulate the protein expression of target genes through
affecting their translation by binding to the 3′-untranslated
regions (UTRs), or downregulate the mRNA expression of target genes
by inducing the direct degradation of target mRNAs. Since the first
miRNA, lin-4, was identified in Caenorhabditis elegans in
1993 (5), >24,521 miRNAs have
been identified, of which all are included in the miRBase database
(6,7). Previous studies have demonstrated that
miRNAs play an important role in cell growth, differentiation,
apoptosis, fat metabolism, morphogenesis and viral infection
(8,9). In recent years, an increasing number of
studies have investigated the role of miRNAs in cancer. These
studies have revealed that specific miRNAs exhibit an aberrant
expression profile in tumor tissue, and are involved in various
aspects of tumorigenesis, including growth, apoptosis, metastasis
and particularly angiogenesis (10–14).
Furthermore, a number of studies have indicated that alterations in
miRNA expression may be involved in the regulation of the cellular
response to hypoxia (15–18).
Hypoxia is associated with various
pathophysiological events, including cancer, lung and
cardiovascular diseases (19). In
mammals, the hypoxia-dependent changes, on a gene expression level,
are primarily mediated by the α-subunits of hypoxia-inducible
transcription factors (HIFs). HIF-1α expression is maintained at
lower level by proteasomal degradation during normoxia, while the
degradation of HIF-1α is inhibited under hypoxia (20). A previous study revealed that HIF-1α
was able to regulate the expression profile of a number of miRNAs,
and certain miRNAs were also able to affect the protein expression
level of HIF-1α (21). For example,
in the study by Cha et al, miR-519c was demonstrated to be a
hypoxia-independent regulator of HIF-1α, functioning through the
direct binding to the 3′-UTR of the HIF-1α gene and leading to
reduced tumor angiogenesis (21).
The aim of the present study was to investigate
miR-18a expression levels and the effect of miR-18a on cell
apoptosis and invasion in gastric carcinoma cells subjected to
hypoxic conditions. Furthermore, the target gene of miR-18a was
identified based on the results of a luciferase assay and the
detection of mRNA and protein expression levels. Subsequently, the
expression levels of apoptosis-associated genes were detected in
the cell lines with miR-18a overexpression.
Materials and methods
Cell culture and hypoxia exposure
Human gastric carcinoma cell lines, MGC-803 and
HGC-27, were purchased from the China Center for Type Culture
Collection (Wuhan, China). MGC-803 and HGC-27 cells were cultured
in RPMI 1640 media supplemented with 10% fetal bovine serum
(HyClone, Logan, UT, USA). To expose the cells to hypoxia, the
cells were cultured in a Billups-Rotenburg chamber
(Billups-Rothenberg, Inc., Del Mar, CA, USA) with 94%
N2, 1% O2 and 5% CO2 at 37°C for a
certain time.
miRNA mimics transient
transfection
miR-18a mimics and negative control were purchased
from Guangzhou RiboBio Co., Ltd. (Guangzhou, China). The cells were
plated to 50% confluency and transfected with 200 nM miR-18a mimics
or negative control using Lipofectamine 2000 (Invitrogen Life
Technologies, Carlsbad, CA, USA), according to the manufacturer's
instructions. At 24 or 48 h after transfection, the cells were
harvested for the further experiments.
Cell apoptosis assay
At 48 h after transfection, the cells were harvested
and 500-µl samples were added to fluorescence-activated cell
sorting tubes. The solutions were mixed with 25 ng/ml fluorescein
isothiocyanate-labeled annexin V and 10 mg/ml propidium iodide, and
incubated for 15 min at room temperature in the dark. Subsequently,
the cells were analyzed by flow cytometry (BD Biosciences, San
Jose, CA, USA).
Cell invasion
MGC-803 and HGC-27 cells were transfected with the
miR-18a-5p mimics, HIF-1α siRNA or negative control, cultivated for
24 h and transferred to the top of Matrigel-coated chambers
(24-well insert; 8-µm pore size; BD Biosciences) in serum-free RPMI
1640 medium. Medium containing 20% fetal calf serum was added to
the lower chamber as a chemoattractant. Following incubation for 24
h, the non-invaded cells were removed from the upper well with
cotton swabs, while the invaded cells were fixed with 4%
paraformaldehyde, stained with hematoxylin, and photographed
(magnification, x200) in five independent fields for each well,
using an optical microscope (Olympus Corporation, Tokyo, Japan).
Each test was performed in triplicate.
Reverse transcription quantitative
polymerase chain reaction (PCR)
Total RNA was extracted using TRIzol reagent
(Invitrogen Life Technologies), according to the manufacturer's
instructions. For cDNA synthesis, 2 µg total RNA was mixed with 500
ng oligo(dT) primers (Promega Corporation, Madison, WI, USA) or
miRNA specific primers. The samples were incubated at 65°C for 10
min with 5 µl 5X first-strand buffer, 2 µl dNTP (5 mM), 20 units
RNasin (Takara Bio, Inc., Otsu, Japan), 1 µl M-MLV reverse
transcriptase (Promega Corporation) and distilled water to a total
volume of 25 µl. The quantitative PCR assay mixture contained 12.5
µl 2X SYBR Green PCR Mix (Fermentas; Thermo Fisher Scientific), 0.3
µM gene-specific forward and reverse primers, and 1 µl cDNA
template, which was made up to a final volume of 25 µl with
distilled water. Thermal cycling parameters were set as follows:
Initial activation at 95°C for 5 min, followed by 35 amplification
cycles of denaturation at 94°C for 30 sec, annealing at 58°C for 30
sec and extension at 72°C for 15 sec. The levels of gene expression
were calculated by relative quantification against GAPDH or U6
snRNA, which were used as the internal controls, with U6 snRNA used
as an internal reference gene for the qPCR detection of MiR-18a.
All samples were amplified in triplicate, and the data analysis was
conducted using MxPro qPCR system software (Stratagene, La Jolla,
CA, USA).
Western blot analysis
MGC-803 and HGC-27 cells (2×106) were
collected and washed twice with ice-cold phosphate-buffered saline
(PBS). The cell pellets were suspended in radioimmunoprecipitation
assay lysis buffer for 30 min on ice, after which the lysates were
centrifuged at 12,000 × g at 4°C for 20 min. Equal amounts of
protein in the supernatant were isolated by 12% SDS polyacrylamide
gel electrophoresis and transferred onto a polyvinylidene
difluoride membrane (Millipore Corporation, Billerica, MA, USA).
The membranes were blocked for 1 h at 37°C with 5% non-fat milk,
and subsequently incubated with mouse monoclonal anti-HIF-1α
(1:500; #359702, BioLegend, Inc., San Diego, CA, USA), anti-Bax
(1:1,000; Abcam, Cambridge, MA, USA), Bcl-2 (1:1,500; #ab117115,
Abcam), caspase 3 (1:1,000; #ab2171, Abcam), caspase 9 (1:1,000;
#ab28131, Abcam) and GAPDH (1:400; #sc-365062, Santa Cruz
Biotechnology, Inc., Santa Cruz, CA, USA) antibodies in 5% non-fat
milk for 1 h at 37°C. After washing in PBS with 0.5% Tween 20
(PBST), the membrane was incubated with a rabbit anti-mouse
horseradish peroxidase-conjugated secondary antibody (1:4,000;
#ab6728, Abcam) at room temperature for 1 h. After further washing
with PBST, the membrane was assayed with enhanced chemiluminescence
with a chemiluminescence HRP subtrate (Millipore Corporation) and
visualized on X-ray films.
Vector construction and luciferase
reporter assay
To construct a luciferase reporter vector, the
wild-type 3′-UTR or the mutant 3′-UTR of HIF-1α, which contained
putative binding sites for miR-18a-5p, were subcloned into the
psiCHECK-2 vector (Promega Corporation). MGC-803 cells were plated
at a density of 5×104 cells per well in 24-well plates.
The following day, psiCHECK-2 luciferase vectors, which included
the 3′-UTR of HIF-1α and miR-18a-5p mimics or negative control
oligonucleotides, were transfected into the cells using
Lipofectamine 2000. At 48 h after transfection, the luciferase
assays were performed using a dual luciferase reporter assay system
(Promega Corporation).
Statistical analysis
Statistical analysis was performed using the SPSS
19.0 software package (IBM SPSS, Armonk, NY, USA). All numerical
data were analyzed using the Student's t-test, and all the
tests performed were two-sided. P<0.05 was considered to
indicate a statistically significant difference.
Results
Hypoxia downregulates the expression
of miR-18a
To investigate the function of miR-18a in gastric
carcinoma cells under hypoxic conditions, the expression level of
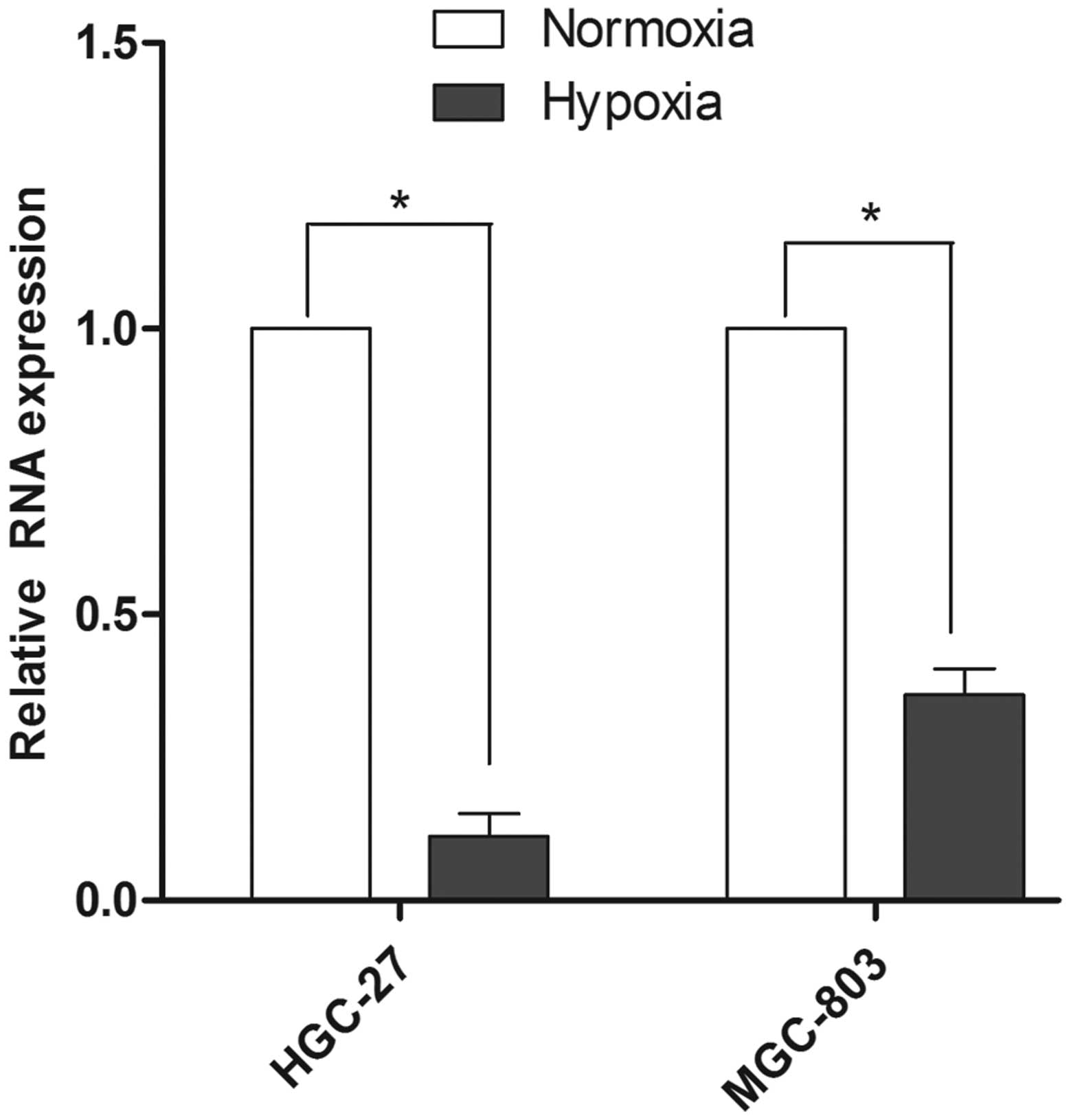
miR-18a in MGC-803 and HGC-27 cell lines was initially detected. As
shown in Fig. 1, the expression
level of miR-18a was downregulated in the two cell lines subjected
to hypoxic conditions. In the HGC-27 cells, the miR-18a expression
level under hypoxic conditions was 89% lower compared with the
expression under normoxic conditions. Furthermore, in the MGC-803
cell line, the miR-18a expression level under hypoxic conditions
was 64% lower compared with the expression under normoxic
conditions.
miR-18a regulates cell apoptosis under
hypoxic conditions
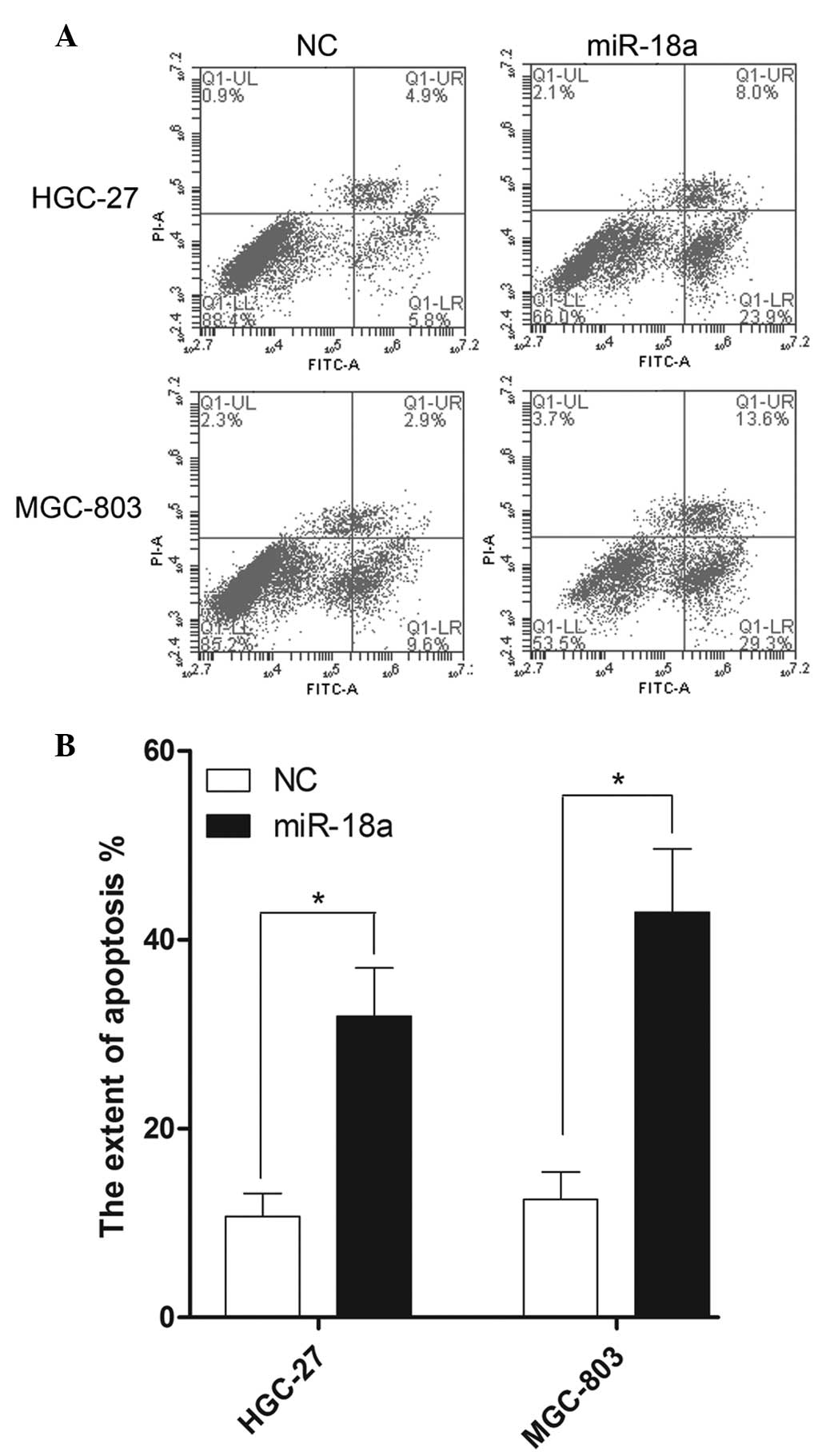
The apoptosis rate of the gastric carcinoma cells
was investigated using flow cytometry following subjection to
hypoxic conditions for 24 h. As shown in Fig. 2A, miR-18a markedly affected the rate
of apoptosis in the HGC-27 and MGC-803 cell lines. Early and late
apoptosis rates (lower right and upper right quadrants of the FITC
graphs, respectively; Fig. 2A) for
the HGC-27 cells were 5.8 and 4.9%, respectively, transfected with
negative control (NC) mimics. In addition, early and late apoptosis
rates for the MGC-803 cells were 9.6 and 2.9%, respectively,
transfected with NC mimics. By contrast, in the cells
overexpressing miR-18a subjected to hypoxic conditions, the early
and late apoptosis rates of HGC-27 cells were 23.9 and 8.0%,
respectively. In addition, the early and late apoptosis rates of
the MGC-803 cells were 29.3 and 13.6%, respectively, in the cells
subjected to hypoxic conditions and miR-18a overexpression. As
shown in Fig. 2B, the total
apoptosis rates of HGC-27 and MGC-803 cells increased 2.98-fold and
3.43-fold following miR-18a mimics transfection, respectively.
miR-18a regulates the invasion
capacity of cells under hypoxic conditions
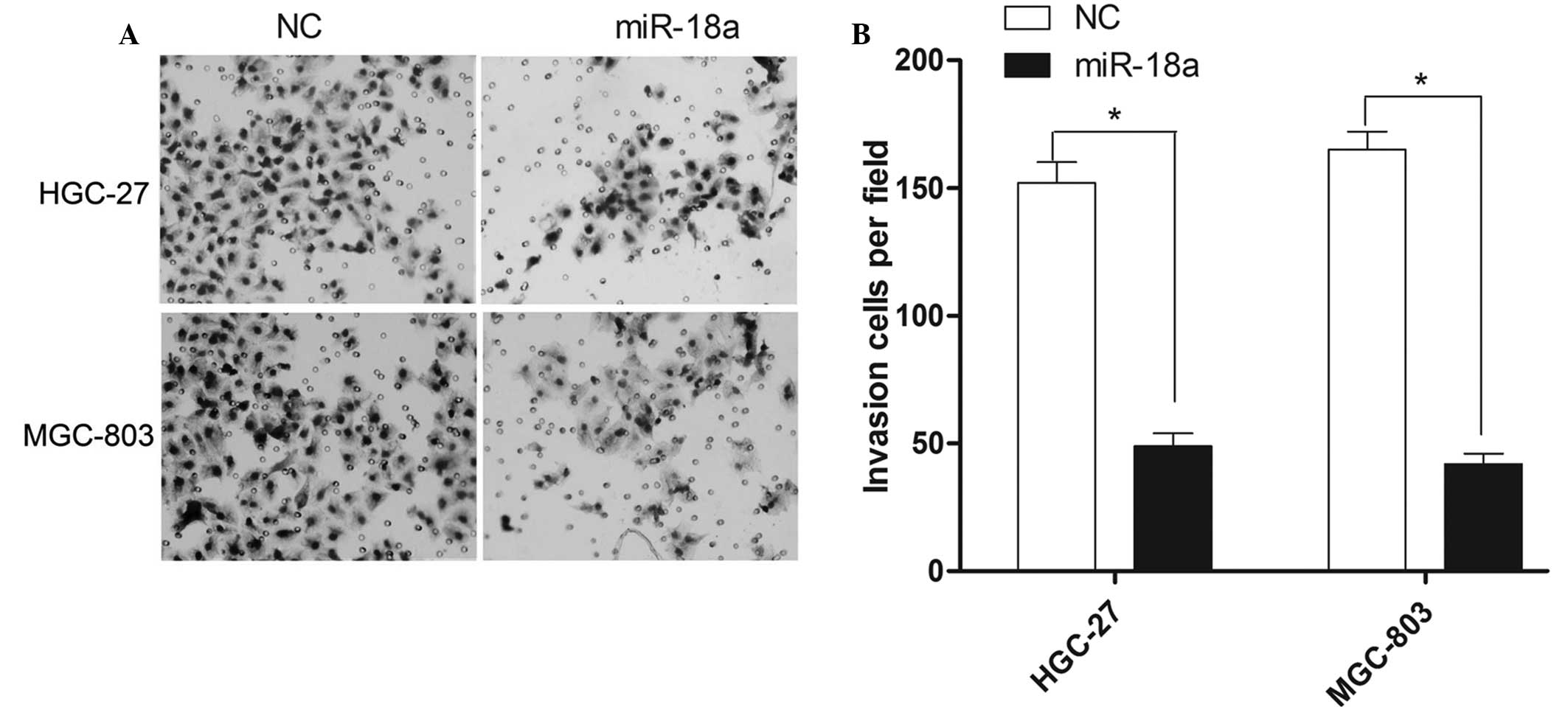
The invasion ability of gastric carcinoma cells was
investigated using a Transwell assay following the induction of
hypoxic conditions for 24 h. As shown in Fig. 3A, miR-18a markedly affected the
invasion ability of the HGC-27 and MGC-803 cells. The numbers of
HGC-27 cells that had invaded the chamber following transfection
with the negative control mimics or the miR-18a mimics were 152±8
and 49±5, respectively. Furthermore, the numbers of invasive
MGC-803 cells following transfection with the negative control or
miR-18a mimics were 165±7 and 42±4, respectively. As shown in
Fig. 3B, the total number of
invasive cells in the HGC-27 and MGC-803 cell lines decreased by
66.76 and 74.55% following miR-18a mimics transfection,
respectively.
HIF-1α is a direct target of
miR-18a
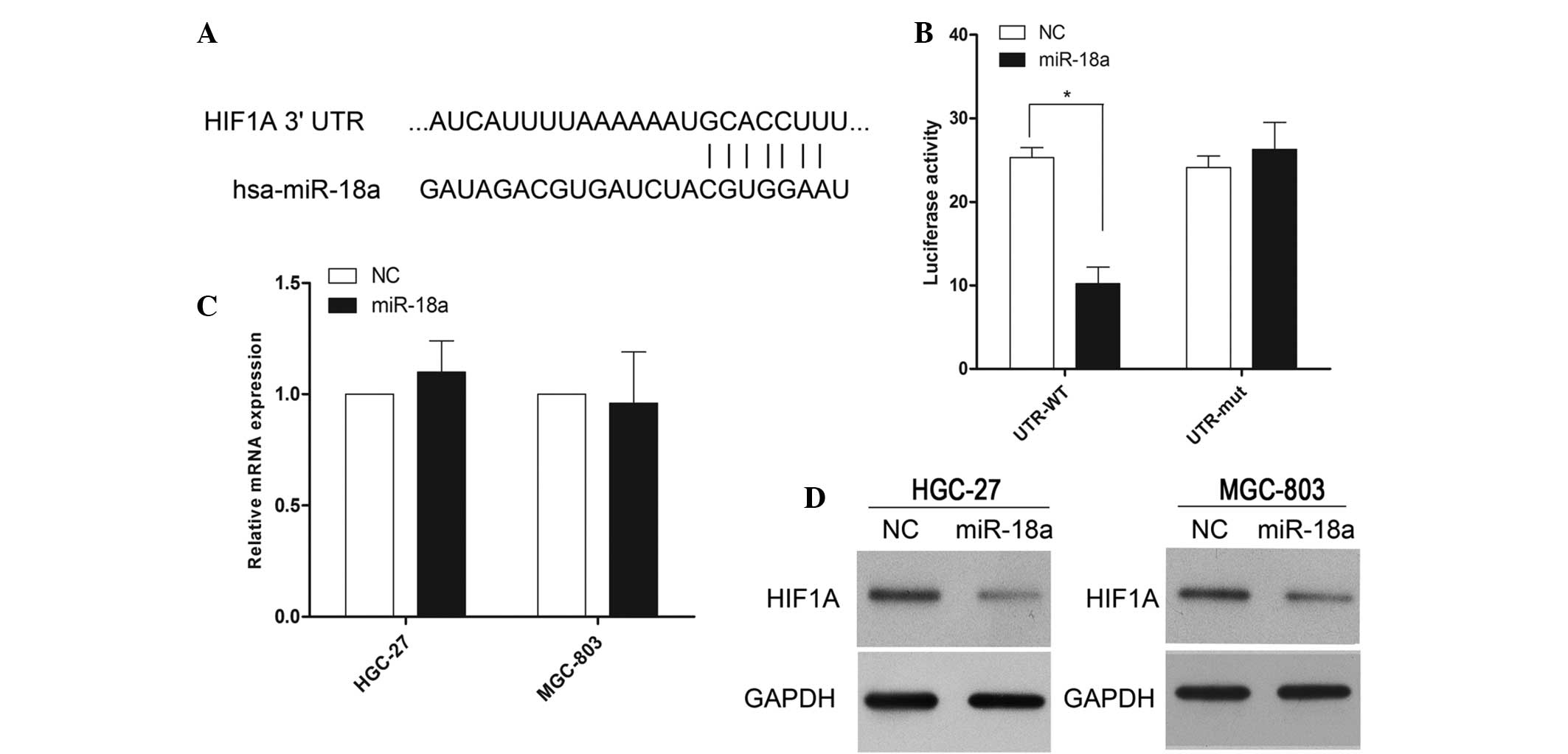
To understand the mechanisms through which miR-18a
induces cell apoptosis and inhibits tumor invasion, several
computational methods were used to identify miR-18a targets in
humans, and the predicted duplex formation between the 3′-UTR of
HIF-1α and miR-18a is shown in Fig.
4A. Among the ~275 targets predicted using the TargetScan 6.2
software program, HIF-1α had been previously implicated in the
regulation of cell apoptosis and/or invasion, which was of
particular interest since the expression of HIF-1α may be induced
under hypoxic conditions. To investigate whether HIF-1α was a
direct target of miR-18a, a luciferase reporter assay was
conducted. The results revealed that miR-18a inhibited the activity
of the firefly luciferase with the wild-type 3′-UTR of HIF-1α. By
contrast, miR-18a exhibited no effect on the activity of the
firefly luciferase with mutant type 3′-UTR of HIF-1α (Fig. 4B). Subsequently, the effect of
miR-18a overexpression on the mRNA and protein expression levels of
HIF-1α were investigated. Although miR-18a overexpression did not
result in the degradation of HIF-1α mRNA (Fig. 4C), miR-18a overexpression did reduce
the activity of the luciferase reporter gene fused to the wild-type
HIF-1α 3′-UTR, indicating that miR-18a targets HIF-1α through
translational inhibition. In accordance with these results, a clear
reduction in the level of endogenous HIF-1α protein expression was
observed in the MGC-803 and HGC-27 cells overexpressing miR-18
(Fig. 4D).
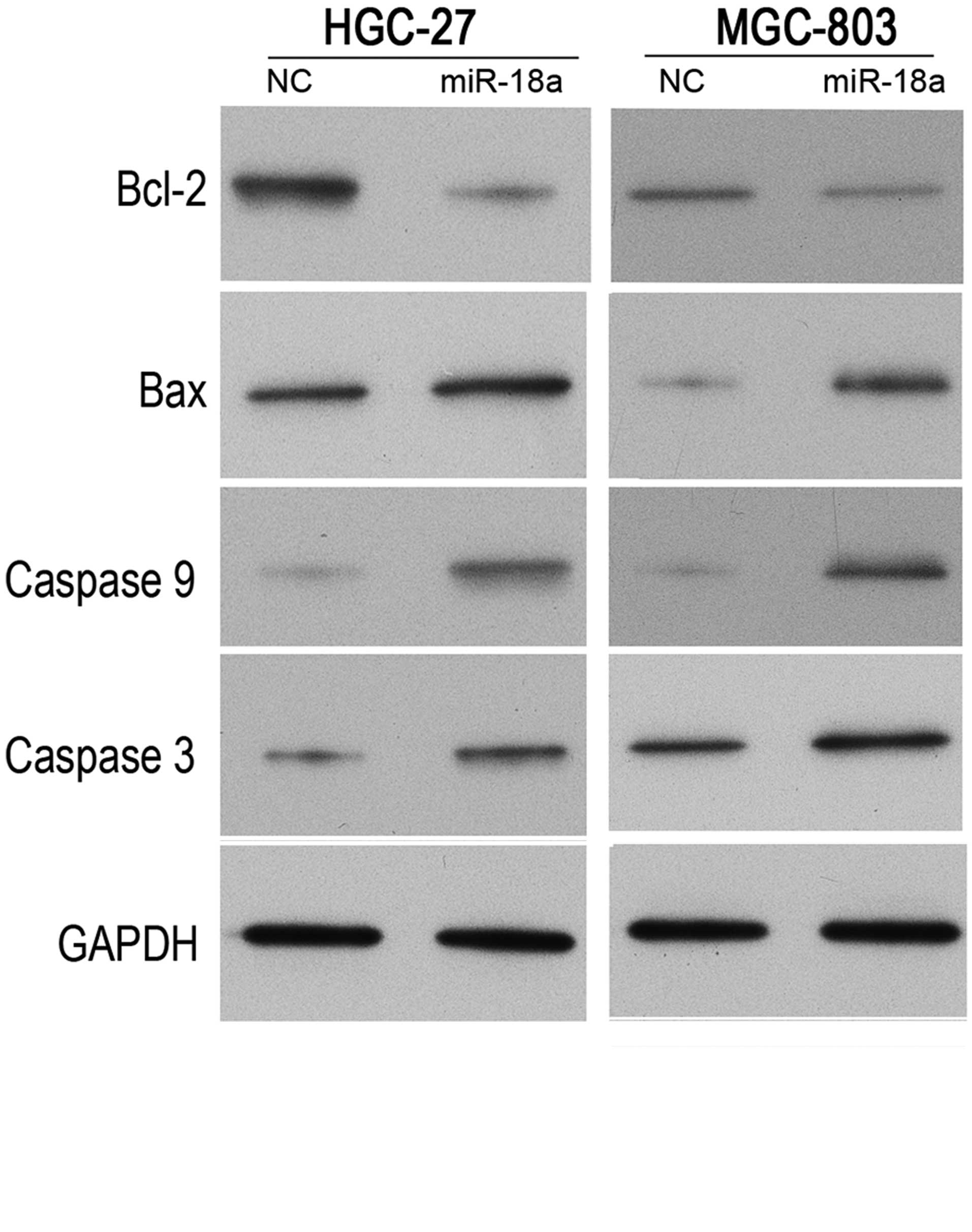
miR-18a regulates the expression of
Bax, Bcl-2, caspase 3 and caspase 9 under hypoxic conditions
To investigate the possible mechanisms underlying
the effects of miR-18a on cell apoptosis, the implication of
miR-18a in a typical signaling pathway of apoptosis was evaluated.
Thus, the protein expression levels of Bcl-2, Bax, caspase 3 and
caspase 9 were detected in cells subjected to hypoxic conditions
following transfection with the miR-18a mimics or negative control
mimics. As shown in Fig. 5, Bcl-2
protein expression levels were downregulated following the
induction of miR-18a overexpression, while Bax protein expression
levels were upregulated in the MGC-803 and HGC-27 cells. In
addition, two important genes in the apoptosis signaling pathway
were analyzed. The protein expression levels of caspase 3 and
caspase 9 were demonstrated to be upregulated following the
induction of miR-18a overexpression. These results indicated that
the apoptosis signaling pathway may be activated by miR-18a under
hypoxic conditions.
Discussion
In present study, miR-18a was initially found to be
downregulated in the MGC-803 and HGC-27 gastric carcinoma cell
lines subjected to hypoxic conditions. Hypoxia is an essential
feature of a tumorous microenvironment. When cancer cells are
placed in hypoxic conditions, a variety of signaling pathways that
regulate proliferation, angiogenesis and cell death become
activated. However, cancer cells have adapted to these pathways,
allowing tumors to survive and even grow under hypoxic conditions
(22). Thus, the identification of
key factors that are able to inhibit the adaptation of cancer cells
to hypoxic conditions is important. Previous studies have revealed
that alterations in miRNA expression levels may be involved in the
regulation of the cellular response to hypoxia (15–18). For
example, the expression levels of miR-210 and miR-373 have been
shown to be induced by HIF-1α, while miR-20b, miR-199a and miR-424
have been demonstrated to regulate the expression profile of HIF-1α
under hypoxic conditions (16). In
the present study, the expression of miR-18a markedly changed under
hypoxic conditions in the gastric carcinoma cells; thus, miR-18a
was hypothesized to play a role in the regulation of gastric
carcinoma cell behavior. In addition, the results demonstrated that
miR-18a overexpression was able to inhibit the cell invasion
ability and promote cell apoptosis in MGC-803 and HGC-27 cells
subjected to hypoxic conditions. To the best of our knowledge, the
present study is the first to report that miR-18a is able to
regulate cell invasion and apoptosis under hypoxic conditions.
miRNAs exert a regulatory role on target genes.
Through bioinformatics analysis, HIF-1α was identified as a
possible target gene of miR-18a. Subsequently, the results of the
luciferase assay, quantitative PCR and western blot analysis
confirmed that HIF-1α was an indeed a target gene of miR-18a.
HIF-1α is an oxygen-dependent transcriptional activator. HIF-1
induces the transcription of >60 proteins, and these proteins
are able to increase oxygen availability by promoting
erythropoiesis and angiogenesis, which activates genes involved in
glucose transport and metabolism (23,24).
Based on the important role of miR-18a in hypoxic conditions and
the association between miR-18a and HIF-1α, miR-18a was
hypothesized to affect cell invasion and apoptosis through
HIF-1α.
To further investigate the mechanism underlying the
regulation of cell apoptosis by miR-18a, the expression levels of
apoptosis-associated genes were detected. Following the induction
of miR-18a overexpression, Bcl-2 protein expression levels were
downregulated, while the protein expression levels of Bax, caspase
3 and caspase 9 were upregulated in the MGC-803 and HGC-27 cell
lines. These results demonstrated that miR-18a overexpression was
able to activate the mitochondrial apoptosis pathway (25,26).
Thus, miR-18a was hypothesized to induce apoptosis through the
HIF-1α/mitochondrial apoptosis pathway.
However, the present study had a number of potential
limitations. The results demonstrated that miR-18a inhibited
apoptosis through the HIF-1α/mitochondrial apoptosis pathway.
However, other potential regulatory pathways between HIF-1α and the
mitochondrial apoptosis pathway were not assessed in the present
study. In addition, the mechanism underlying miR-18a-induced
regulation of cell invasion was not investigated. Therefore, future
studies should focus their attention on solving these
limitations.
In conclusion, the results of the present study
indicated that miR-18a was downregulated and was able to affect
cell apoptosis and invasion in MGC-803 and HGC-27 cell lines under
hypoxic conditions. Furthermore, HIF-1α was identified as a
potential target gene of miR-18a. In addition, miR-18a
overexpression was able to activate the mitochondrial apoptosis
pathway. Thus, it is hypothesized that miR-18a may induce apoptosis
via the HIF-1α/mitochondrial apoptosis pathway.
Acknowledgements
This study was supported by a grant from the Science
and Technology Planning Project of Putian City [no. 2012S02
(1)].
References
|
1
|
Zhang J, Du YY, Lin YF, Chen YT, Yang L,
Wang HJ and Ma D: The cell growth suppressor, miR-126, targets
IRS-1. Biochem Biophys Res Commun. 377:136–140. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Luzi E, Marini F, Sala SC, Tognarini I,
Galli G and Brandi ML: Osteogenic differentiation of human adipose
tissue-derived stem cells is modulated by the miR-26a targeting of
the SMAD1 transcription factor. J Bone Miner Res. 23:287–295. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu B, Chapman EJ, Yang Z, Carrington JC
and Chen X: Transgenically expressed viral RNA silencing
suppressors interfere with microRNA methylation in
Arabidopsis. FEBS Lett. 580:3117–3120. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lee RC, Feinbaum RL and Ambros V: The
C. elegans heterochronic gene lin-4 encodes small RNAs with
antisense complementarity to lin-14. Cell. 75:843–854. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Griffiths-Jones S, Saini HK, van Dongen S
and Enright AJ: miRBase: Tools for microRNA genomics. Nucleic Acids
Res. 36:(Database issue). D154–D158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kozomara A and Griffiths-Jones S: miRBase:
annotating high confidence microRNAs using deep sequencing data.
Nucleic Acids Res. 42:(Database issue). D68–D73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Giraldez AJ, Cinalli RM, Glasner ME, et
al: MicroRNAs regulate brain morphogenesis in zebrafish. Science.
308:833–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Guo H, Ingolia NT, Weissman JS and Bartel
DP: Mammalian microRNAs predominantly act to decrease target mRNA
levels. Nature. 466:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tsai WC, Hsu PW, Lai TC, et al:
MicroRNA-122, a tumor suppressor microRNA that regulates
intrahepatic metastasis of hepatocellular carcinoma. Hepatology.
49:1571–1582. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xiong Y, Fang JH, Yun JP, Yang J, Zhang Y,
Jia WH and Zhuang SM: Effects of microRNA-29 on apoptosis,
tumorigenicity and prognosis of hepatocellular carcinoma.
Hepatology. 51:836–845. 2010.PubMed/NCBI
|
|
12
|
Xu T, Zhu Y, Xiong Y, Ge YY, Yun JP and
Zhuang SM: MicroRNA-195 suppresses tumorigenicity and regulates
G1/S transition of human hepatocellular carcinoma cells.
Hepatology. 50:113–121. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Anand S: A brief primer on microRNAs and
their roles in angiogenesis. Vasc Cell. 5:22013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seok JK, Lee SH, Kim MJ and Lee YM:
MicroRNA-382 induced by HIF-1α is an angiogenic miR targeting the
tumor suppressor phosphatase and tensin homolog. Nucleic Acids Res.
42:8062–8072. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chan SY and Loscalzo J: MicroRNA-210: A
unique and pleiotropic hypoxamir. Cell Cycle. 9:1072–1083. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Loscalzo J: The cellular response to
hypoxia: Tuning the system with microRNAs. J Clin Invest.
120:3815–3817. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kulshreshtha R, Ferracin M, Wojcik SE, et
al: A microRNA signature of hypoxia. Mol Cell Biol. 27:1859–1867.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kulshreshtha R, Davuluri RV, Calin GA and
Ivan M: A microRNA component of the hypoxic response. Cell Death
Differ. 15:667–671. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Adams JM, Difazio LT, Rolandelli RH, et
al: HIF-1: A key mediator in hypoxia. Acta Physiol Hung. 96:19–28.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang LE, Gu J, Schau M and Bunn HF:
Regulation of hypoxia-inducible factor 1alpha is mediated by an
O2-dependent degradation domain via the
ubiquitin-proteasome pathway. Proc Natl Acad Sci USA. 95:7987–7992.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cha ST, Chen PS, Johansson G, et al:
MicroRNA-519c suppresses hypoxia-inducible factor-1alpha expression
and tumor angiogenesis. Cancer Res. 70:2675–2685. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Harris AL: Hypoxia - a key regulatory
factor in tumour growth. Nat Rev Cancer. 2:38–47. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Semenza GL: HIF-1 and tumor progression:
Pathophysiology and therapeutics. Trends Mol Med. 8 (Suppl
4):62–67. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Semenza GL: Targeting HIF-1 for cancer
therapy. Nat Rev Cancer. 3:721–732. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Eskes R, Desagher S, Antonsson B and
Martinou JC: Bid induces the oligomerization and insertion of Bax
into the outermitochondrial membrane. Mol Cell Biol. 20:929–935.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Miramar MD, Costantini P, Ravagnan L, et
al: NADH oxidase activity of mitochondrial apoptosis-inducing
factor. J Biol Chem. 276:16391–16398. 2001. View Article : Google Scholar : PubMed/NCBI
|