Introduction
Random pattern skin flaps are common for wound
repair and reconstruction. Skin flap grafting is widely used in
plastic surgery to repair defects resulting from trauma, congenital
disease and tumor excision. In clinical practice, flap necrosis,
which may be caused by lack of adequate nutrient blood supply, is a
serious problem (1,2). Therefore, exploring possible targets
for therapeutic intervention to reduce flap necrosis and increase
ischemic tissue survival is important.
A number of studies have been conducted to improve
random pattern skin flap survival. For example, angiogenic growth
factors, such as vascular endothelial cell growth factor (VEGF)
improved skin flap survival by enhancing the revascularization of
ischemic tissues (3,4). VEGF is a specific mitogen for
endothelial cells and it stimulates vasculogenesis and
angiogenesis. It can promote vascular endothelial cell
proliferation and migration, promote angiogenesis, extend the life
of the vascular endothelial cells and increase vascular
permeability (5). In addition to
VEGF, it has also been reported that hyperbaric oxygen (HBO) could
increase the tolerance of tissues to ischemia and thus improve the
survival probability (2,6,7). HBO
could protect the microcirculation through interfering with the
deleterious activity of neutrophils (8). The effects of VEGF and HBO in improving
the survival of a random skin flap are already known. However,
information regarding the effect of the combination of VEGF and HBO
is unclear.
Considering the fact that HBO could increase the
expression of VEGF (9) and both
could promote angiogenesis (3,4,10), it was hypothesized that dual
treatment of VEGF-loaded microspheres and HBO would result in
improved random pattern skin flap survival. To test this
hypothesis, the effects of HBO, VEGF loaded microspheres, and HBO
plus VEGF on the survival of random skin flaps in rats were
analyzed.
Materials and methods
Ethics statement
The study was approved by the ethics committee of
Huai'an Hospital Affiliated of Xuzhou Medical College and Huai'an
Second People's Hospital (Huai'an, China). All procedures strictly
followed the recommendations in the Guide for the Care and Use of
Laboratory Animals of the National Institutes of Health.
Animals and materials
Forty Male Sprague-Dawley (SD) rats (weight, 200–250
g; age, 2–3 moths) were obtained from the laboratory animal center
of Xuzhou Medical College (Xuzhou, China). VEGF was purchased from
Boyun Biotech Co., Ltd. (Shanghai, China). Mice anti-rat VEGF
antibodies (sc-7269) were purchased from Santa Cruz Biotechnology
Inc. (Santa Cruz, CA, USA). The goat anti-mouse secondary antibody
and goat-anti-rabbit secondary antibody (R125) were purchased from
Boyun Biotech Co., Ltd. (Shanghai, China). Antigen retrieval was
achieved by microwaving (10 min, 700 W) in citrate buffer solution
at pH 6 (BY02072; Boyun Liyuan Biotechnology Co., Ltd., Shanghai,
China). HBO chamber was designed by the Wuhan Ship Development and
Design Institute (Wuhan, China).
Preparation of VEGF-loaded
microspheres
For microsphere preparation, 1.2 g gelatin (Type B,
225 Bloom Sigma-Aldrich, St. Louis, MO, USA) was firstly dissolved
in 4 ml double-distilled water in a water bath at 55°C. The gelatin
solution was added dropwise to 30 ml liquid paraffin (containing 10
g/l Span-80; Sigma-Aldrich), which was preheated to 55°C, to form a
water in oil emulsion by stirring (450 rpm for 10 min).
Subsequently, the emulsion was chilled at 4°C and gelatin
microspheres were formed in the aqueous phase, then 0.1 ml
glutaraldehyde (250 g/l) precooled at 4°C was added. The resulting
microspheres were filtered and rinsed in acetone, isopropyl alcohol
and diethyl ether to remove the remaining oil on their surfaces.
Finally, the rinsed gelatin microspheres were dried in a vacuum
oven. Encapsulation of VEGF was then achieved by diffusional
loading. Briefly, phosphate-buffered saline (PBS) solution
containing bovine serum albumin (0.1% w/v, 200 µl) and VEGF (1 mg/1
ml) added to sterilized microspheres (20 mg) and left for 24 h.
Subsequently, the loaded microparticles were washed, centrifuged at
10,000 × g and sterilized. To investigate the VEGF release, 10 mg
microspheres were placed in a tube containing 100 ml PBS at 37°C.
The tube was continuously shaken using a JJ21 electronic mixer (135
rpm; Changzhou Guohua Electric Appliance Co., Ltd., Changzhou,
China) and at particular time intervals (every 24 h for 7 days) the
samples were centrifuged at 10,000 × g, supernatant aliquots were
collected and replaced with an equal volume of PBS. The
concentration of VEGF in the supernatant was measured with
commercial enzyme-linked immunosorbent assay kits (R&D Systems,
Inc., Minneapolis, MN, USA). The release tests were conducted in
triplicate.
Flap model and experimental
design
Under sterile conditions, all rats were anesthetized
with 10% chloral hydrate (3 ml/kg; Sigma-Aldrich) by
intraperitoneal injection. The dorsal region was shaved and
disinfected with povidone iodine (PI) solution (Sigma-Aldrich).
Random dorsal skin flaps were elevated using the Improved McFarlane
flap method as described previously (11,12). A
rectangular area (3×9 cm2) was outlined on the back of
the rats and the sacral arteries were systematically sectioned. The
flap was completely separated from the underlying fascia and then
immediately sutured back to the donor bed using 4-0 sutures (Ailee
Co., Ltd., Busan, South Korea). Regions around the incision were
disinfected with iodine PI solution and smeared with aureomycin
ointment (Sigma-Aldrich). For subsequent analysis, the flap area
was divided into three zones of equal size: The proximal area I the
intermediate area II and the distal area III (13). The 40 rats were randomly divided into
four groups. The VEGF group received 3 ml microsphere PBS solution
(2 µg/ml) after the elevation of skin flap. The microspheres were
delivered in two sites, one site was 3 cm distant from the distal
end and the other was 6 cm distant from the distal end, and each
site received a 1.5-ml injection. For the HBO group, the rats were
firstly injected with 3 ml saline. After 30 min the rats were then
moved to the HBO chamber (2.5 standard atmospheric pressure, oxygen
concentration over 98%) for 30 min. After 8 h, the rats were moved
into the HBO chamber again for 30 min. The dual treatment group was
firstly injected with 3 ml VEGF microsphere solution. After 30 min,
the rats were moved to the HBO chamber for 30 min (twice per day at
8 h intervals). The control group only received 3 ml saline daily.
All treatments were performed consecutively every 24 h for 7 days.
All rats were housed individually and fed standard rat chow and
water ad libitum. Each rat was given a neck collar to
prevent self-mutilation (14).
General observation and percentage of
necrosis
On the seventh day, the flap area was photographed
using a BX51 optical microscope (Olympus Corporation, Tokyo, Japan)
and compared with the record on the first day. The necrotic area
was defined according to the dark color and eschar formation. The
photos were captured by the software Image-Pro Plus 6.0 (Media
Cybernetics, Inc., Rockville, MD, USA). For each group, the mean
and standard deviation of the necrotic area percentage were
calculated.
Histology
After the rats were sacrificed with an overdose of
chloral hydrate, flap tissues from all zones were conventionally
dehydrated, embedded, sectioned and stained (hematoxylin and
eosin). Tissue condition, such as the thickness of granulation
tissue, tissue edema, necrosis, hyperplasia of capillaries, blood
vessels and inflammatory cell infiltration of each layer was
observed under the BX51 light microscope (magnification, x100). In
addition, the microvessel number per unit area (per mm2)
was also determined as an indicator of the microvascular density
(MVD) (15).
Immunohistochemistry for VEGF
expression
Immunohistochemical staining was conducted for VEGF
using the streptavidin-peroxidase method (16). Positive VEGF expression in each flap
was observed under an inverted microscope and analyzed the image
through the software Image-Pro Plus 6.0. The integral absorbance
(IA) value was detected as an indicator of VEGF expression.
Statistical analysis
All results are expressed as the mean ± standard
deviation for continuous variables. The data were analyzed using
SPSS software, version 17.0 (SPSS Inc., Chicago, IL, USA).
P<0.05 was considered to indicate a statistically significant
difference. Statistical analysis consisted of a comparison of means
of each group by using analysis of variance. Tukey's multiple
comparison test was applied when appropriate.
Results
Microsphere characterization and VEGF
release
The obtained microspheres were spherical, smooth and
non-porous. Over 85% microspheres had a diameter of 10–28 µm.
Loading VEGF did not induce any change in the morphology or size
range. The ability of the microspheres to provide a sustained
delivery of VEGF was assessed in preliminary experiments. VEGF
release displayed an initial burst on the first day (25%), followed
by a sustained release of ~10% from days 1–7. On the seventh day,
~92% of the VEGF had been released from the microspheres in
total.
Gross observation and percentage of
necrotic area
Seven days following treatment, the necrotic
sections tended to fuse, scab and harden. The boundaries between
necrotic and survival areas were stable. The necrotic area was
black, rigid, and glabrous and it did not bleed when cut with a
scalpel. The survival area was pink-white, tender, grew fine hair
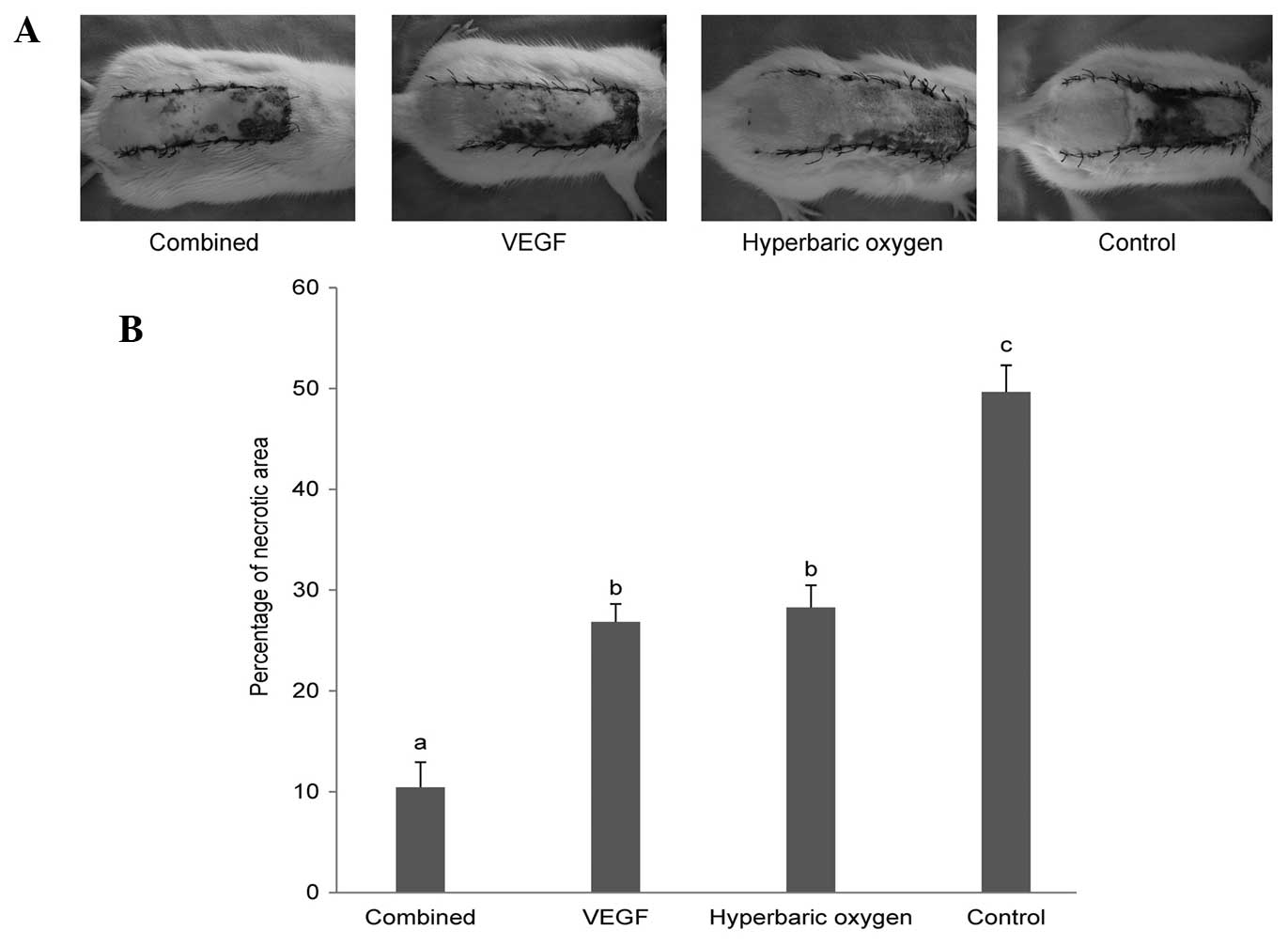
and it bled when cut (Fig. 1A).
The results of the necrotic area percentage are
presented in Fig. 1B. The mean
percentage of necrotic flap area in the control group (49.66±2.64%)
was significantly enlarged compared with that of the dual treatment
group (10.44±2.48%), the VEGF group (26.85±1.77%) and the HBO group
(28.27±2.21%). No significant difference was detected between the
VEGF group and the HBO group. The mean necrotic flap area
percentage of the dual treatment group was significantly smaller
than that of the VEGF group and the HBO group.
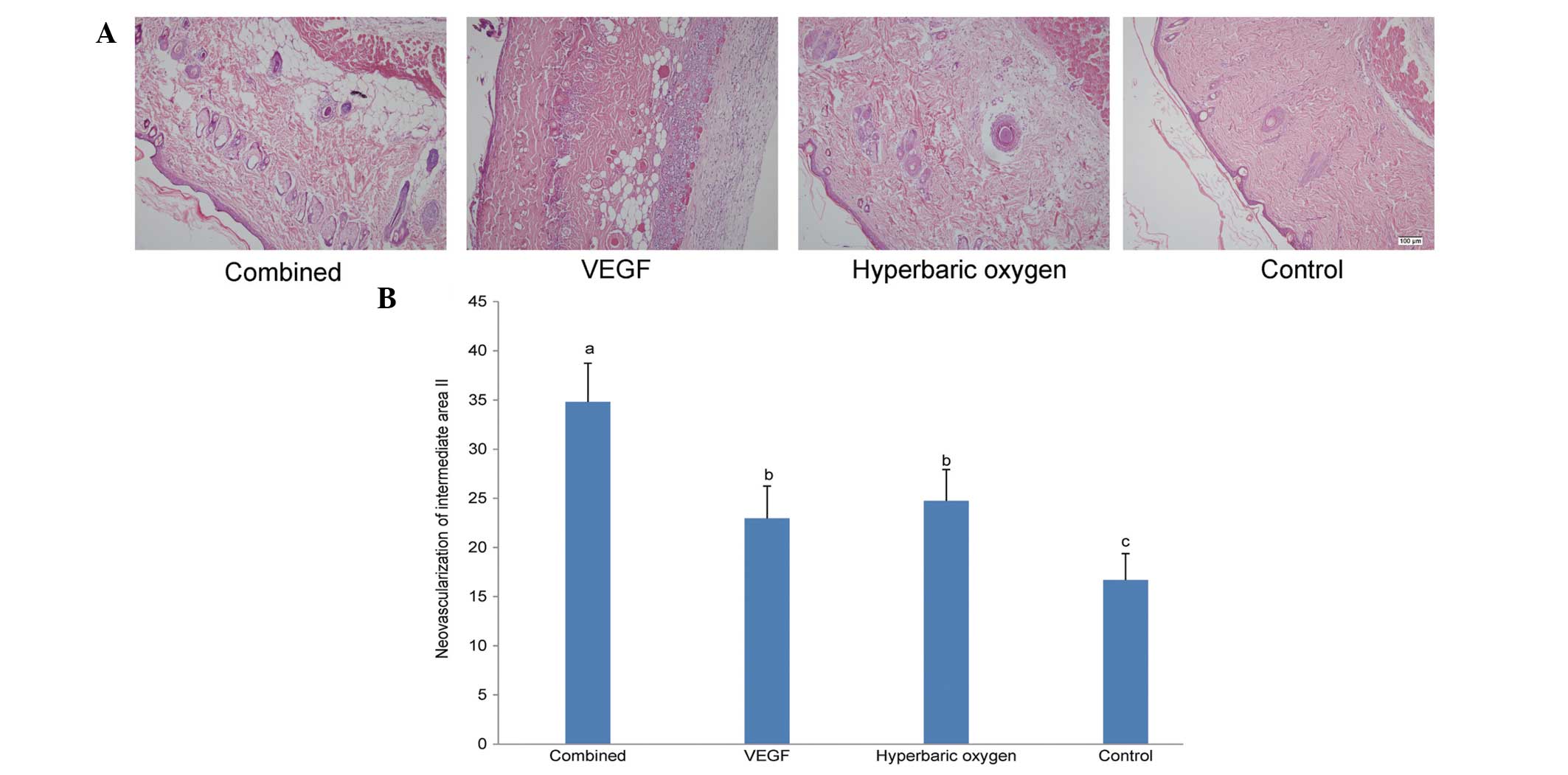
Histology
The morphological images of all groups on the
seventh day following treatment is shown in Fig. 2A. The neovascularization results of
intermediate area II for the four groups are illustrated in
Fig. 2B. Consistent with the
necrotic area percentage analysis, the neovascularization of the
dual treatment group (34.81±3.93/mm2) was significantly
higher than that of other groups. No significant difference was
detected between the VEGF group (22.96±3.29/mm2) and the
HBO group (24.74±3.19/mm2). In addition, the
neovascularization of the controls (16.68±2.69/mm2) was
lower than that of others.
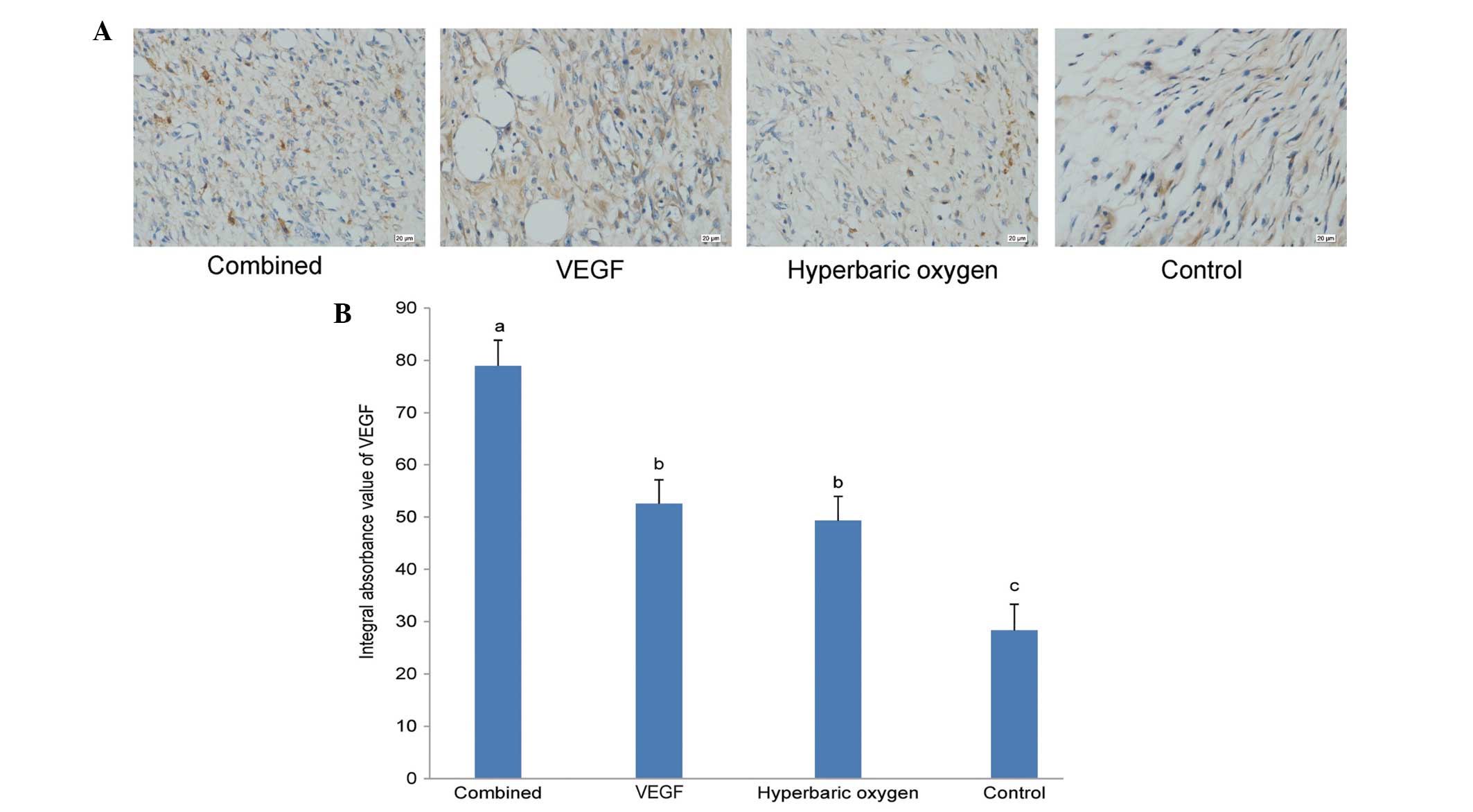
Immunohistochemistry for VEGF
Seven days after the treatments, the results of
immunohistochemical staining of the four groups were as follows
(Fig. 3A). The IA values of VEGF of
the dual treatment group, VEGF group, the HBO group and the
controls were 78.97±4.90, 52.54±4.55, 49.32±4.62 and 28.33±4.98,
respectively. Statistical analysis results corroborated the results
of the histological analysis and necrotic area percentage analysis
(Fig. 3B).
Discussion
In the present study, it was observed that VEGF or
HBO administered after surgery improved the survival of random skin
flaps. This is consistent with the results of previous studies
(2–4,17). There
was no significant difference between the VEGF group and the HBO
group. However, a combination therapy with VEGF and HBO led to
improvement in the average survival compared with treatment with
VEGF or HBO alone, suggesting that these agents act
synergistically. For the combination group, the subcutaneous
tissues had no obvious edema, congestion or neutrophil invasion.
The number of novel blood vessels and the expression of VEGF of the
combination group was significantly greater than that in the other
groups. The control group treated with saline alone exhibited the
highest percentage of necrotic area, the fewest number of novel
blood vessels and the lowest VEGF expression.
A hypoxic environment has been shown to improve the
VEGF expression (18–20). In damaged histiocytes, HBO stimulates
the expression of hypoxia inducible factor 1 (HIF-1α), which binds
to the VEGF transcription initiation site and promotes VEGF
transcription and translation (19,20). The
upregulation of VEGF by HBO intervention may enhance its effect of
promoting angiogenesis and vascularization. In addition, since the
effect of VEGF administration is limited by the existing numbers of
endothelial cells, increasing the number of endothelial cells may
enhance its effects. A hypoxic environment has also been shown to
promote the proliferation of endothelial cells (21). Thus, the enhanced effect of the
combination of VEGF and HBO on the improvement of random skin flap
survival may be explain the above described association of HBO with
VEGF.
In conclusion, HBO or VEGF intervention can improve
skin flap survival in rats. In addition, combination of VEGF and
HBO did improve random skin flap survival to a greater extent than
VEGF or HBO alone, suggesting that these two agents do potentiate
one another. The results suggest that the combination of
VEGF-loaded microspheres and hyperbaric oxygen may be a promising
therapeutic intervention for the reduction of flap necrosis.
Acknowledgements
This study was supported by a grant from the Project
of Science and Technology Bureau (no. HAS2012002).
References
|
1
|
Matsumura H, Yoshizawa N, Vedder NB and
Watanabe K: Preconditioning of the distal portion of a rat
random-pattern skin flap. Br J Plast Surg. 54:58–61. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Richards L, Lineaweaver WC, Stile F, Zhang
J and Zhang F: Effect of hyperbaric oxygen therapy on the tubed
pedicle flap survival in a rat model. Ann Plast Surg. 50:51–56.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Padubidri A and Browne E Jr: Effect of
vascular endothelial growth factor (VEGF) on survival of random
extension of axial pattern skin flaps in the rat. Ann Plast Surg.
37:604–611. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kryger Z, Zhang F, Dogan T, Cheng C,
Lineaweaver WC and Buncke HJ: The effects of VEGF on survival of a
random flap in the rat: examination of various routes of
administration. Br J Plast Surg. 53:234–239. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Khan A, Ashrafpour H, Huang N, et al:
Acute local subcutaneous VEGF165 injection for augmentation of skin
flap viability: efficacy and mechanism. Am J Physiol Regul Integr
Comp Physiol. 287:R1219–R1229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang T, Gong W, Li Z, et al: Efficacy of
hyperbaric oxygen on survival of random pattern skin flap in
diabetic rats. Undersea Hyperb Med. 34:335–339. 2007.PubMed/NCBI
|
|
7
|
Ulkür E, Karagoz H, Ergun O, Celikoz B,
Yildiz S and Yildirim S: The effect of hyperbaric oxygen therapy on
the delay procedure. Plast Reconstr Surg. 119:86–94. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kranke P, Bennett MH, Martyn-St James M,
Schnabel A and Debus SE: Hyperbaric oxygen therapy for chronic
wounds. Cochrane Database Syst Rev. 4:CD0041232012.PubMed/NCBI
|
|
9
|
Jung S, Wermker K, Poetschik H, Ziebura T
and Kleinheinz J: The impact of hyperbaric oxygen therapy on
serological values of vascular endothelial growth factor (VEGF) and
basic fibroblast growth factor (bFGF). Head Face Med. 6:292010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Hunt TK and Pai MP: The effect of varying
ambient oxygen tensions on wound metabolism and collagen synthesis.
Surg Gynecol Obstet. 135:561–567. 1972.PubMed/NCBI
|
|
11
|
Rinsch C, Quinodoz P, Pittet B, et al:
Delivery of FGF-2 but not VEGF by encapsulated genetically
engineered myoblasts improves survival and vascularization in a
model of acute skin flap ischemia. Gene Ther. 8:523–533. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mcfarlane RM, Deyoung G and Henry RA: The
design of a pedicle flap in the rat to study necrosis and its
prevention. Plast Reconstr Surg. 35:177–182. 1965. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Mandriota SJ, Pyke C, Di Sanza C, Quinodoz
P, Pittet B and Pepper MS: Hypoxia-inducible angiopoietin-2
expression is mimicked by iodonium compounds and occurs in the rat
brain and skin in response to systemic hypoxia and tissue ischemia.
Am J Pathol. 156:2077–2089. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ozkan O and Ozgentas HE: Combination of
rat vest, teeth shortening and nail cutting to prevent
autocannibalization and protect surgical flaps. Plast Reconstr
Surg. 117:16712006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen Z, Wang T, Cai L, et al:
Clinicopathological significance of non-small cell lung cancer with
high prevalence of Oct-4 tumor cells. J Exp Clin Cancer Res.
31:102012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shi ZR, Itzkowitz SH and Kim YS: A
comparison of three immunoperoxidase techniques for antigen
detection in colorectal carcinoma tissues. J Histochem Cytochem.
36:317–322. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
da Rocha FP, Fagundes DJ, Pires JA and da
Rocha FS: Effects of hyperbaric oxygen and N-acetylcysteine in
survival of random pattern skin flaps in rats. Indian J Plast Surg.
45:453–458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daniel RA, Cardoso VK, Gois E Jr, et al:
Effect of hyperbaric oxygen therapy on the intestinal ischemia
reperfusion injury. Acta Cir Bras. 26:463–469. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Y, Liu XH, Qu SD, et al: Hyperbaric
oxygen intervention on expression of hypoxia-inducible
factor-1alpha and vascular endothelial growth factor in spinal cord
injury models in rats. Chin Med J (Engl). 126:3897–3903.
2013.PubMed/NCBI
|
|
20
|
Ijichi H, Taketomi A, Yoshizumi T, et al:
Hyperbaric oxygen induces vascular endothelial growth factor and
reduces liver injury in regenerating rat liver after partial
hepatectomy. J Hepatol. 45:28–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ren P, Kang Z, Gu G, et al: Hyperbaric
oxygen preconditioning promotes angiogenesis in rat liver after
partial hepatectomy. Life Sci. 83:236–241. 2008. View Article : Google Scholar : PubMed/NCBI
|