Introduction
Diabetic retinopathy (DR) is a sight-threatening,
chronic microvascular complication of diabetes. DR, which accounts
for 5% of all blindness, affects ~5 million patients worldwide and
is characterized by the progressive occlusion of capillaries,
leading to retinal nonperfusion and ischemia (1). In an ischemic retina, the induction of
vascular endothelial growth factor (VEGF) expression mediates the
pathological intraocular proliferation of vessels which
characterizes proliferative diabetic retinopathy (PDR) (2). The majority of diabetic patients
develop varying degrees of retinopathy by 20 years of disease
duration (3). In 2012, there were
~93 million cases of DR globally, 17 million of which were PDR
(4). The pathogenesis of DR is
complex, including inflammation (5),
oxidative stress (6) and advanced
glycation end products (AGEs) (7).
Previous studies have suggested that chronic inflammation and the
immune response promote the development of DR. T helper (Th)17
cells and interleukin (IL)-17 participate in the immune response
and are associated with the development and progression of DR
(8,9); however, this remains controversial as
previous studies have demonstrated a positive association between
IL-17 and DR (8,10), whereas others have demonstrated a
negative association (9–12).
Sirtuin 1 (SIRT1) is a nicotinamide-adenine
dinucleotide (NAD)+-dependent histone deacetylase
associated with various fundamental physiological processes,
including oxidative stress, glucose metabolism, DNA stability,
aging and tumorigenesis (13–15).
Previous studies have demonstrated that SIRT1 may be associated
with the pathogenesis of DR (16,17);
however, the underlying mechanisms are yet to be elucidated.
Furthermore, as previous studies have stated that SIRT1 is capable
of modulating the production of IL-17 (18,19), the
authors of the present study hypothesized that SIRT1 functions
through the regulation of IL-17 in patients with DR. In order to
test this hypothesis, the present study aimed to evaluate the
expression levels of SIRT1 in the retinal fibrovascular membranes
and peripheral blood mononuclear cells (PBMCs) of patients with DR
and analyze the potential association between SIRT1 expression and
serum IL-17 expression levels.
Materials and methods
Patients
A total of 19 patients with PDR were recruited for
the present study between April 2014 and August 2014. All of the
patients had previously been diagnosed with type 2 diabetes (T2D),
according to the World Health Organization (WHO) criteria (20). A total of 20 patients without
diabetes who presented with idiopathic macular epiretinal membranes
whilst waiting for vitrectomy were recruited as control subjects.
The present study was approved by the Clinical Research Ethics
Committee of the First Affiliated Hospital of Chongqing Medical
University (Chongqing, China) and followed the tenets of the
Declaration of Helsinki. Informed consent was acquired from all
participants and detailed demographics of the patients are outlined
in Table I.
 | Table I.Characteristics of subjects. |
Table I.
Characteristics of subjects.
| Characteristics | Total | Patients with
PDR | Control subjects |
|---|
| Number | 39 | 19 | 20 |
| Gender
(male/female) | 18/21 | 10/9 | 8/12 |
| Average age
(years) | – | 59.5 | 65.8 |
| Average duration of
type 2 diabetes (years) | – | 14 | – |
Immunohistochemistry
Vitrectomy was performed on all the participants.
Fibrovascular membrane samples from patients with PDR and
epiretinal membrane samples from the controls were excised during
the surgery, fixed in 4% paraformaldehyde, embedded in paraffin,
and cut into 5-µm sections. Briefly, 10% goat serum (Beyotime
Institute of Biotechnology, Haimen, China) was used to block
nonspecific binding, and the slides were incubated overnight with
mouse monoclonal SIRT1 primary antibody (1:200; sc-74504; Santa
Cruz Biotechnology, Inc., Dallas, TX, USA). After washing three
times with Tris-buffered saline, biotinylated secondary antibody
(1:150; Santa Cruz Biotechnology, Inc.) was subsequently applied
for 20 min at room temperature and the sections were visualized
using a StrepABC horseradish peroxidase kit (Beyotime Institute of
Biotechnology). Subsequently, goat anti-mouse biotinylated
secondary antibody (1:150; sc-2039; Santa Cruz Biotechnology, Inc.)
was applied for 20 min at room temperature and the sections were
visualized using a StrepABC horseradish peroxidase kit (Beyotime
Institute of Biotechnology). SIRT1 expression levels were
semiquantitatively measured using a light microscope
(magnification, ×200; BX51T-PHD-J11; Olympus Corporation, Tokyo,
Japan), to generate an immunoreactive score (IRS) (21). Negative expression was defined by an
IRS score of 0, low expression levels were defined by an IRS score
of 1–5, whereas high expression was denoted by an IRS score of
6–12.
Circulating IL-17 measurements
Circulating expression levels of IL-17 in the sera
of patients with PDR and the controls were determined using
enzyme-linked immunosorbent assay (ELISA; R&D Systems, Inc.,
Minneapolis, MN, USA), according to the manufacturer's
protocol.
PBMC culture
PBMC culture was performed as previously described
(22). Briefly, fasting blood
samples were harvested from all participants using Vacutainer®
tubes supplemented with heparin (BD Biosciences, Franklin Lakes,
NJ, USA). PBMCs were obtained using Ficoll-Hypaque™ density
gradient centrifugation (GE Healthcare, Piscataway, NJ, USA). PBMCs
were cultured in RPMI-1640 medium supplemented with 10% fetal
bovine serum and 1% penicillin/streptomycin (all Invitrogen,
Carlsbad, CA, USA), and incubated at 37°C in an atmosphere
containing 5% CO2 for 24 h. Subsequently,
1×106 PBMCs/ml were cultured on 24-well plates and
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) or western blotting was used to determine the mRNA and
protein expression levels of SIRT1, respectively. In order to
ascertain the effects of an SIRT1 activator, resveratrol, on the
expression levels of IL-17, anti-CD3 (5 µg/ml; 11-0039-41;
eBioscience, Inc., San Diego, CA, USA) and anti-CD28 (1 µg/ml;
11-0289-41; eBioscience, Inc.) mouse monoclonal antibodies were
added with/without 10 µM resveratrol (Sigma-Aldrich, St. Louis, MO,
USA) (23). Resveratrol was stored
as a powder and sterile phosphate-buffered saline solution (PBS)
was added to the powder prior to use. Following 72 h incubation,
the expression levels of IL-17 in the supernatants of the PBMCs
were analyzed using ELISA (R&D Systems, Inc.). The mRNA and
protein expression levels of SIRT1 in the PBMCs from the patients
and controls were analyzed again using the methods described below.
All experiments were repeated in triplicate.
RNA extraction and RT-qPCR
An RNeasy Mini kit (Qiagen GmbH, Hilden, Germany)
was used to extract the total RNA from PBMCs, according to the
manufacturer's protocol. cDNA was synthesized from 1 µg total RNA
using a TaqMan® Reverse Transcription kit (Applied Biosystems;
Thermo Fisher Scientific, Inc., Foster City, CA, USA), according to
the manufacturer's protocol. RT-qPCR was subsequently performed on
an ABI 7500 Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific) using SYBR® Premix Ex Taq™ II (Takara Bio, Inc.,
Otsu, Japan). The following primer sequences were used: β-actin,
forward 5′-GGATGCAGAAGGAGATCACTG-3′ and reverse
5′-CGATCCACACGGAGTACTTG-3′; and SIRT1, forward
5′-CGGAAACATACCTCCACCTGA-3′ and reverse
5′-GAAGTCTACAGCAAGGCGAGCA-3′. The following cycling conditions were
used: One cycle at 95°C for 3 min, and 40 cycles of 95°C for 3 sec
and 58°C for 20 sec, followed by 1 cycle of 95°C for 15 sec, 60°C
for 15 sec and 95°C for 15 sec. SIRT1 expression levels were
normalized to the expression levels of a housekeeping gene,
β-actin. Fold change was calculated using the 2−ΔΔCq
method (24).
Nuclear protein extraction and western
blotting
PBMCs were washed twice with ice-cold PBS and
NE-PER™ Nuclear Extraction reagents (Pierce Biotechnology, Inc.,
Rockford, IL, USA) were used to extract PBMC nuclear proteins,
according to the manufacturer's protocol. Nuclear proteins were
subsequently boiled for 10 min with 5% sodium dodecyl sulfate (SDS)
loading buffer (4:1), separated by 8% SDS-polyacrylamide gel
electrophoresis and transferred to a polyvinylidene difluoride
(PVDF; EMD Millipore, Billerica, MA, USA) membrane. The membrane
was blocked using 5% non-fat milk and rabbit monoclonal anti-SIRT1
(1:2,000; #2496; Cell Signaling Technology, Inc., Danvers, MA, USA)
primary antibody was added to the membrane and incubated for 1 h at
room temperature. The membrane was subsequently washed using PBS
and alkaline phosphatase (ALP) buffer (Beyotime Institute of
Biotechnology) containing 100 mmol/l Tris-HCl prior to incubation
with ALP-conjugated secondary antibody (1:7,500; #7054; Cell
Signaling Technology, Inc., Danvers, MA, USA) for 1 h at room
temperature. Following this, 10 ml ALP buffer, 66 µl
5-bromo-4-chloro-3-indolyl phosphate (Beyotime Institute of
Biotechnology) and 33 µl nitro blue tetrazolium chloride (Beyotime
Institute of Biotechnology) were mixed, added to the membrane and
incubated at 37°C. ddH2O was added once the protein
bands were clear and ImageJ software, version 1.43 (National
Institutes of Health, Bethesda, MA, USA) was used to quantify the
protein levels. β-actin housekeeping protein was used for
normalization.
Statistical analysis
One-way analysis of variance was used to compare the
expression levels of IL-17 in the supernatants of the PBMCs and the
mRNA and protein expression levels of SIRT1 in PBMCs. Between-group
differences were determined using Tukey's test. Student's t-test
was used to compare the expression levels of IL-17 in the sera of
the control and PDR groups, whereas χ2 test was used to
compare the differences in SIRT1 expression levels in the excised
membranes from the controls and patients with PDR. Statistical
tests were performed using GraphPad Prism® 5 (GraphPad Software,
Inc., La Jolla, CA, USA) or SPSS software (SPSS, Inc., Chicago, IL,
USA). Data are expressed as the mean ± standard deviation.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Increased SIRT1 expression levels in
fibrovascular membranes from patients with PDR
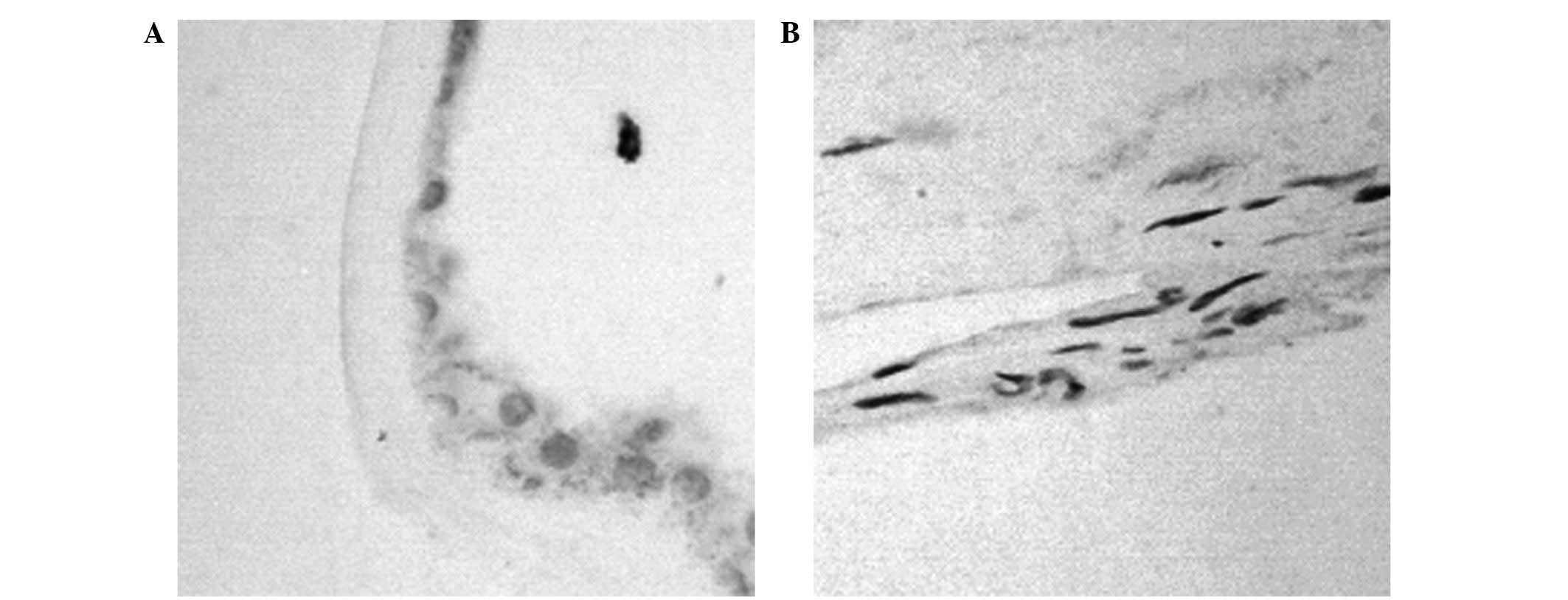
The expression levels of SIRT1 in fibrovascular
(n=19) and epiretinal membranes (n=20) were examined using
immunohistochemical analysis (Fig.
1; Table II). A significant
difference in the expression levels of SIRT1 was demonstrated
between the two groups (χ2=23.85, P <0.001).
 | Table II.Sirtuin 1 (SIRT1) expression levels in
samples excised from patients with proliferative diabetic
retinopathy (PDR) and non-diabetic control subjects. |
Table II.
Sirtuin 1 (SIRT1) expression levels in
samples excised from patients with proliferative diabetic
retinopathy (PDR) and non-diabetic control subjects.
|
|
| SIRT1 expression
levels |
|
|
|---|
|
|
|
|
|
|
|---|
| Sample | Case | Negative | Low | High | χ2 | P-value |
| Fibrovascular
membranes from patients with PDR | 19 | 2 | 5 | 12 | 23.85 | <0.001 |
| Epiretinal
membranes from control subjects | 20 | 16 | 3 | 1 |
|
|
IL-17 expression levels increase in
the sera and PBMC supernatants of patients with PDR
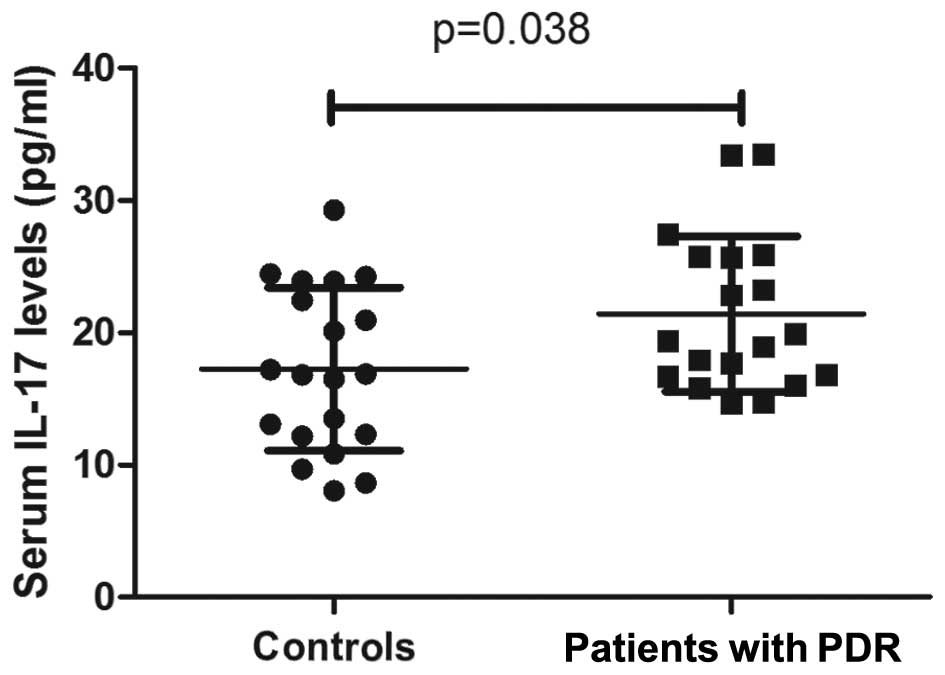
IL-17 expression levels were significantly increased
in the sera from patients with PDR (21.4±5.9 pg/ml), as compared
with the control group (17.3±6.2 pg/ml; P=0.038; Fig. 2). Furthermore, the expression levels
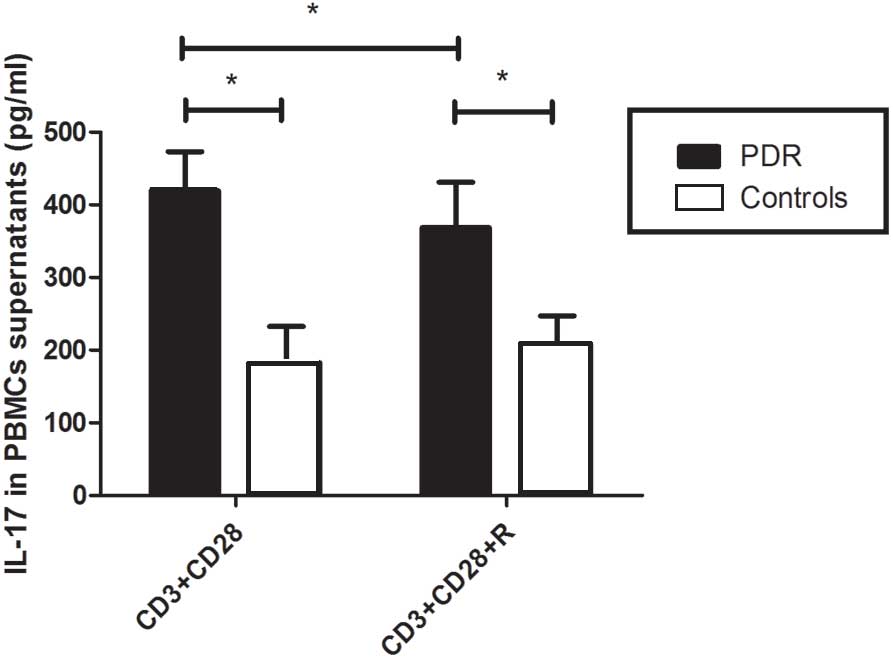
of IL-17 in the supernatants of cultured PBMCs were significantly
increased in patients with PDR (419.3±53.7 pg/ml), as compared with
the control group (182.5±50.3 pg/ml; P<0.05; Fig. 3).
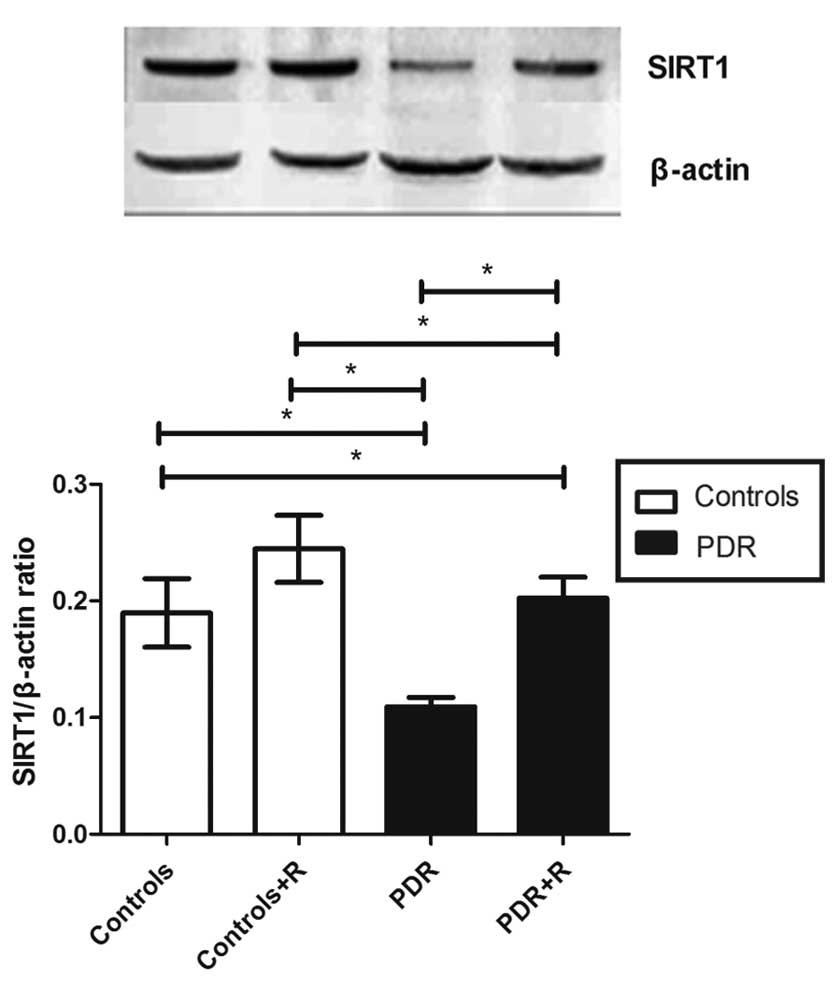
SIRT1 mRNA and protein expression
levels decrease in patients with PDR
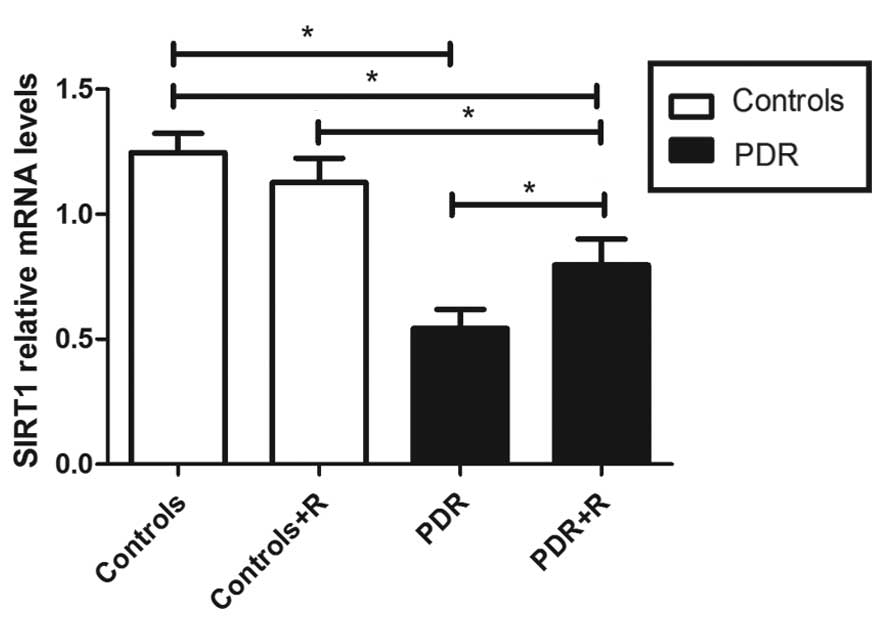
The mRNA expression levels of SIRT1 in the PBMCs of
patients with PDR were significantly reduced, as compared with the
control group (0.54±0.08 vs. 1.24±0.08; P<0.05; Fig. 4). The protein expression levels of
SIRT1 were consistent with these mRNA results. In the PBMCs of
patients with PDR that did not receive resveratrol stimulation,
SIRT1 protein expression levels were significantly reduced, as
compared with those from control subjects (0.11±0.01 vs. 0.19±0.03;
P<0.05; Fig. 5).
SIRT1 activation inhibits IL-17
production by PBMCs in patients with PDR
In order to explore the effects of resveratrol on
the expression levels of IL-17 in PBMCs, PBMCs were incubated with
anti-CD3, anti-CD28 and 10 µM resveratrol for 72 h, and the mRNA
and protein expression levels of SIRT1 were subsequently
determined. The results demonstrated that resveratrol activated the
expression of SIRT1 mRNA and protein in patients with PDR (mRNA
with vs. without resveratrol, 0.80±0.10 vs. 0.54±0.08; protein with
vs. without resveratrol, 0.20±0.02 vs. 0.11±0.01; both P<0.05;
Figs. 4 and 5). However, SIRT1 expression levels
remained lower in the PBMCs of patients with PDR following
stimulation with resveratrol (P<0.05), as compared with those
from the control group. SIRT1 expression levels in the controls
were not significant affected by resveratrol administration
(P>0.05; Figs. 4 and 5). IL-17 expression levels in the PBMC
supernatants from patients with PDR were inhibited by resveratrol
(with vs. without resveratrol, 368.5±62.72 vs. 419.3±53.7 pg/ml),
and they were not altered in the control subjects (with vs. without
resveratrol, 207.6±39.5 vs. 182.5±50.3 pg/ml; supernatant with vs.
without resveratrol, 0.20±0.02 vs. 0.11±0.01; both P>0.05;
Fig. 3).
Discussion
The results of the present study indicated that
serum IL-17 expression levels were increased in patients with PDR,
as compared with non-diabetic control subjects with idiopathic
macular epiretinal membranes. PBMCs exhibited increased expression
levels of IL-17 in patients with PDR, whereas SIRT1 mRNA and
protein expression levels were decreased in the PBMCs of patients
with PDR. Furthermore, increased expression levels of SIRT1 were
detected on the fibrovascular membranes of samples harvested from
patients with PDR. These results suggested an imbalance in IL-17
and SIRT1, which may contribute to the pathogenesis of DR;
therefore, SIRT1 may have protective effects in PDR.
IL-17, which is secreted by various cells including
Th17 cells, is a key cytokine responsible for the recruitment,
activation and migration of neutrophils. Furthermore, IL-17 is
capable of inducing nonimmune cells, including endothelium and
epithelium cells, to secrete proinflammatory factors (25). Previous studies have demonstrated
that IL-17 has a pathological role in inflammatory and autoimmune
diseases, as elevated levels of serum IL-17 have been detected in
patients with diabetes (26),
rheumatoid arthritis (27),
psoriasis (28), multiple sclerosis
(29) and systemic lupus
erythematosus (30). Furthermore,
IL-17 is capable of promoting angiogenesis by directly acting on
endothelial cells and via other lymphokines with angiogenic
properties (31). IL-17 is also
capable of promoting the expression of VEGF, which is crucial in
the development of PDR (32). In the
present study, IL-17 expression levels were elevated in the sera
and PBMCs of patients with PDR, which was consistent with previous
results (11). The results of the
present study also demonstrated that patients with PDR and T2D
suffer from systemic inflammation, as IL-17 is capable of inducing
the secretion of inflammatory factors in the endothelium, which
subsequently disrupts tight junctions and the blood-retinal barrier
(33); therefore, the increased
expression levels of IL-17 in patients with PDR leads to retinal
damage. Local expression levels of IL-17 in the vitreous fluid or
retina should be investigated in future studies.
Previous studies have implicated SIRT1 in the
regulation of inflammatory responses (34,35), in
particular, it has been demonstrated that SIRT1 is capable of
modulating IL-17 production (19,36);
however, whether SIRT1 regulates IL-17 signaling in patients with
DR remains unknown. In the present study, SIRT1 expression levels
were reduced in the PBMCs of patients with PDR and, following
treatment of the PBMCs with a SIRT1 activator, resveratrol, SIRT1
expression levels were upregulated. Consistent with previous
studies (18,19), IL-17 expression levels were inhibited
by SIRT1 activation in the present study; however, in contrast with
the present hypothesis that SIRT1 expression levels may be
downregulated in the fibrovascular membranes of patients with PDR,
SIRT1 expression was upregulated, which is consistent with the
findings of Maloney et al (37). This may be due to a protective
feedback mechanism in the retina; however, the precise underlying
mechanism remains unclear and requires further study. Therefore,
the results of present study indicated that SIRT1 may have a
protective effect against DR.
There were a number of limitations to the present
study. Although IL-17 and SIRT1 expression levels were compared
between patients with PDR and non-diabetic controls, the
associations between the two factors and the duration of PDR were
not evaluated due to limitations in the number of patients.
Furthermore, SIRT1 activity may reflect the function of SIRT1,
however, measuring SIRT1 activity using a fluorescent SIRT1
enzymatic assay may yield artifacts and is therefore not considered
to be reliable by the majority of researchers (38). As an alternative, SIRT1 mRNA and
protein expression levels were measured.
In conclusion, the present study demonstrated that
IL-17 expression levels were increased in the serum of patients
with PDR. In addition, IL-17 expression was upregulated and SIRT1
expression levels were decreased in the PBMCs of patients with PDR.
Stimulation of SIRT1 may inhibit the production of IL-17 in
patients with PDR. The molecular mechanisms underlying this are
complex and an improved understanding of this interplay may
elucidate a new therapeutic target for the treatment of PDR.
Acknowledgements
This study was supported by National Key Clinical
Specialities Construction Program of China.
References
|
1
|
Hendrick AM, Gibson MV and Kulshreshtha A:
Diabetic retinopathy. Prim Care. 42:451–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang X, Bao S, Lai D, Rapkins RW and
Gillies MC: Intravitreal triamcinolone acetonide inhibits breakdown
of the blood-retinal barrier through differential regulation of
VEGF-A and its receptors in early diabetic rat retinas. Diabetes.
57:1026–1033. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Frank RN: Diabetic retinopathy. N Engl J
Med. 350:48–58. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yau JW, Rogers SL, Kawasaki R, Lamoureux
EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund
J, et al: Meta-Analysis for Eye Disease (META-EYE) Study Group:
Global prevalence and major risk factors of diabetic retinopathy.
Diabetes Care. 35:556–564. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cheung CM, Vania M, Ang M, Chee SP and Li
J: Comparison of aqueous humor cytokine and chemokine levels in
diabetic patients with and without retinopathy. Mol Vis.
18:830–837. 2012.PubMed/NCBI
|
|
6
|
Giacco F and Brownlee M: Oxidative stress
and diabetic complications. Circ Res. 107:1058–1070. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Milne R and Brownstein S: Advanced
glycation end products and diabetic retinopathy. Amino Acids.
44:1397–1407. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu H, Cai M and Zhang X: Effect of the
blockade of the IL-23-Th17-IL-17A pathway on streptozotocin-induced
diabetic retinopathy in rats. Graefes Arch Clin Exp Ophthalmol.
253:1485–1492. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Afzal N, Zaman S, Asghar A, Javed K,
Shahzad F, Zafar A and Nagi AH: Negative association of serum IL-6
and IL-17 with type-II diabetes retinopathy. Iran J Immunol.
11:40–48. 2014.PubMed/NCBI
|
|
10
|
Takeuchi M, Sato T, Tanaka A, Muraoka T,
Taguchi M, Sakurai Y, Karasawa Y and Ito M: Elevated levels of
cytokins associated with Th2 and Th17 cells in vitreous fluid of
proliferative diabetic retinopathy patients. PLoS One.
10:e01373582015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nadeem A, Javaid K, Sami W, Zafar A, Jahan
S, Zaman S and Nagi A: Inverse relationship of serum IL-17 with
type-II diabetes retinopathy. Clin Lab. 59:1311–1317.
2013.PubMed/NCBI
|
|
12
|
Afzal N, Zaman S, Shahzad F, Javaid K,
Zafar A and Nagi AH: Immune mechanisms in type-2 diabetic
retinopathy. J Pak Med Assoc. 65:159–163. 2015.PubMed/NCBI
|
|
13
|
Longo VD and Kennedy BK: Sirtuins in aging
and age-related disease. Cell. 126:257–268. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li T, Zhang J, Feng J, Li Q, Wu L, Ye Q,
Sun J, Lin Y, Zhang M, Huang R, et al: Resveratrol reduces acute
lung injury in a LPS-induced sepsis mouse model via activation of
Sirt1. Mol Med Rep. 7:1889–1895. 2013.PubMed/NCBI
|
|
15
|
Sung B, Chung JW, Bae HR, Choi JS, Kim CM
and Kim ND: Humulus japonicus extract exhibits antioxidative
and anti-aging effects via modulation of the AMPK-SIRT1 pathway.
Exp Ther Med. 9:1819–1826. 2015.PubMed/NCBI
|
|
16
|
Balaiya S, Khetpal V and Chalam KV:
Hypoxia initiates sirtuin1-mediated vascular endothelial growth
factor activation in choroidal endothelial cells through hypoxia
inducible factor-2α. Mol Vis. 18:114–120. 2012.PubMed/NCBI
|
|
17
|
Zheng Z, Chen H, Li J, Li T, Zheng B,
Zheng Y, Jin H, He Y, Gu Q and Xu X: Sirtuin 1-mediated cellular
metabolic memory of high glucose via the LKB1/AMPK/ROS pathway and
therapeutic effects of metformin. Diabetes. 61:217–228. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gardner PJ, Joshi L, Lee RW, Dick AD,
Adamson P and Calder VL: SIRT1 activation protects against
autoimmune T cell-driven retinal disease in mice via inhibition of
IL-2/Stat5 signaling. J Autoimmun. 42:117–129. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Park YD, Kim YS, Jung YM, Lee SI, Lee YM,
Bang JB and Kim EC: Porphyromonas gingivalis
lipopolysaccharide regulates interleukin (IL)-17 and IL-23
expression via SIRT1 modulation in human periodontal ligament
cells. Cytokine. 60:284–293. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alberti KG and Zimmet PZ: Definition,
diagnosis and classification of diabetes mellitus and its
complications. Part 1: Diagnosis and classification of diabetes
mellitus provisional report of a WHO consultation. Diabetic Med.
15:539–553. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Remmele W and Stegner HE: Recommendation
for uniform definition of an immunoreactive score (IRS) for
immunohistochemical estrogen receptor detection (ER-ICA) in breast
cancer tissue. Der Pathologe. 8:138–140. 1987.(In German).
PubMed/NCBI
|
|
22
|
Wang C, Tian Y, Ye Z, Kijlstra A, Zhou Y
and Yang P: Decreased interleukin 27 expression is associated with
active uveitis in Behçet's disease. Arthritis Res Ther.
16:R1172014. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Howitz KT, Bitterman KJ, Cohen HY, Lamming
DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL,
et al: Small molecule activators of sirtuins extend
Saccharomyces cerevisiae lifespan. Nature. 425:191–196.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Park H, Li Z, Yang XO, Chang SH, Nurieva
R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q and Dong C: A distinct
lineage of CD4 T cells regulates tissue inflammation by producing
interleukin 17. Nat Immunol. 6:1133–1141. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sumarac-Dumanovic M, Jeremic D, Pantovic
A, Janjetovic K, Stamenkovic-Pejkovic D, Cvijovic G, Stevanovic D,
Micic D and Trajkovic V: Therapeutic improvement of glucoregulation
in newly diagnosed type 2 diabetes patients is associated with a
reduction of IL-17 levels. Immunobiology. 218:1113–1118. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zizzo G, De Santis M, Bosello SL, Fedele
AL, Peluso G, Gremese E, Tolusso B and Ferraccioli G: Synovial
fluid-derived T helper 17 cells correlate with inflammatory
activity in arthritis, irrespectively of diagnosis. Clin Immunol.
138:107–116. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lynde CW, Poulin Y, Vender R, Bourcier M
and Khalil S: Interleukin 17A: Toward a new understanding of
psoriasis pathogenesis. J Am Acad Dermatol. 71:141–150. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Esendagli G, Kurne AT, Sayat G, Kilic AK,
Guc D and Karabudak R: Evaluation of Th17-related cytokines and
receptors in multiple sclerosis patients under interferon β-1
therapy. J Neuroimmunol. 255:81–84. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wong CK, Lit LC, Tam LS, Li EK, Wong PT
and Lam CW: Hyperproduction of IL-23 and IL-17 in patients with
systemic lupus erythematosus: Implications for Th17-mediated
inflammation in auto-immunity. Clin Immunol. 127:385–393. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Numasaki M, Fukushi J, Ono M, Narula SK,
Zavodny PJ, Kudo T, Robbins PD, Tahara H and Lotze MT:
Interleukin-17 promotes angiogenesis and tumor growth. Blood.
101:2620–2627. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Suryawanshi A, Veiga-Parga T, Reddy PB,
Rajasagi NK and Rouse BT: IL-17A differentially regulates corneal
vascular endothelial growth factor (VEGF)-A and soluble VEGF
receptor 1 expression and promotes corneal angiogenesis after
herpes simplex virus infection. J Immunol. 188:3434–3446. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Y, Yang P, Li F and Kijlstra A: The
effects of Th17 cytokines on the inflammatory mediator production
and barrier function of ARPE-19 cells. PLoS One. 6:e181392011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lee SI, Min KS, Bae WJ, Lee YM, Lee SY,
Lee ES and Kim EC: Role of SIRT1 in heat stress- and
lipopolysaccharide-induced immune and defense gene expression in
human dental pulp cells. J Endod. 37:1525–1530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim YS, Lee YM, Park JS, Lee SK and Kim
EC: SIRT1 modulates high-mobility group box 1-induced
osteoclastogenic cytokines in human periodontal ligament cells. J
Cell Biochem. 111:1310–1320. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Beier UH, Wang L, Bhatti TR, Liu Y, Han R,
Ge G and Hancock WW: Sirtuin-1 targeting promotes Foxp3+
T-regulatory cell function and prolongs allograft survival. Mol
Cell Biol. 31:1022–1029. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Maloney SC, Antecka E, Granner T,
Fernandes B, Lim LA, Orellana ME and Burnier MN Jr: Expression of
SIRT1 in choroidal neovascular membranes. Retina. 33:862–866. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Pacholec M, Bleasdale JE, Chrunyk B,
Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis
P and Pabst B: SRT1720, SRT2183, SRT1460, and resveratrol are not
direct activators of SIRT1. J Biol Chem. 285:8340–8351. 2010.
View Article : Google Scholar : PubMed/NCBI
|