Introduction
Henoch-Schönlein Purpura (HSP) is a systemic
leukocytoclastic vasculitis mediated by immunoglobulin A (IgA)
(1) and characterized by a series of
clinical features, including non-thrombocytopenic palpable purpura,
abdominal pain, arthritis and kidney damage (2). At present, the exact causes of HSP is
unclear. The condition most commonly associated with HSP is an
upper respiratory infection; however, complications of surgical
interventions, digestive perforation and massive gastrointestinal
bleeding can also be due to HSP (3).
HSP is known to be the most common form of vasculitis in children;
more than 90% of HSP cases are <10 year-old pediatric patients
(4). However, this condition also
occurs in adults. The diagnosis of HSP is made on the basis of
clinical manifestations, laboratory examinations and
histopathological biopsy (5). The
diagnostic criteria for this disease, however, vary from patient to
patient. Treatment with antihistamines and anti-inflammatory and
antispasmodic drugs can effectively relieve the symptoms. Since
numerous organs can be involved in HSP, the outcome and prognosis
depends on the lesions in those organs, particularly the kidney in
pediatric patients (6). Adults who
present with HSP are more likely to experience more severe
complications, such as purpuric rash, abdominal pain, arthralgia,
digestive perforation, massive gastrointestinal bleeding and
nephritis; however, the induction of HSP following trauma is rarely
reported in adults (2,7). The present study reports the case of a
40 year-old man, who was diagnosed with HSP following a
high-voltage electrical burn injury. Patient consent was obtained
from the patient's family.
Case report
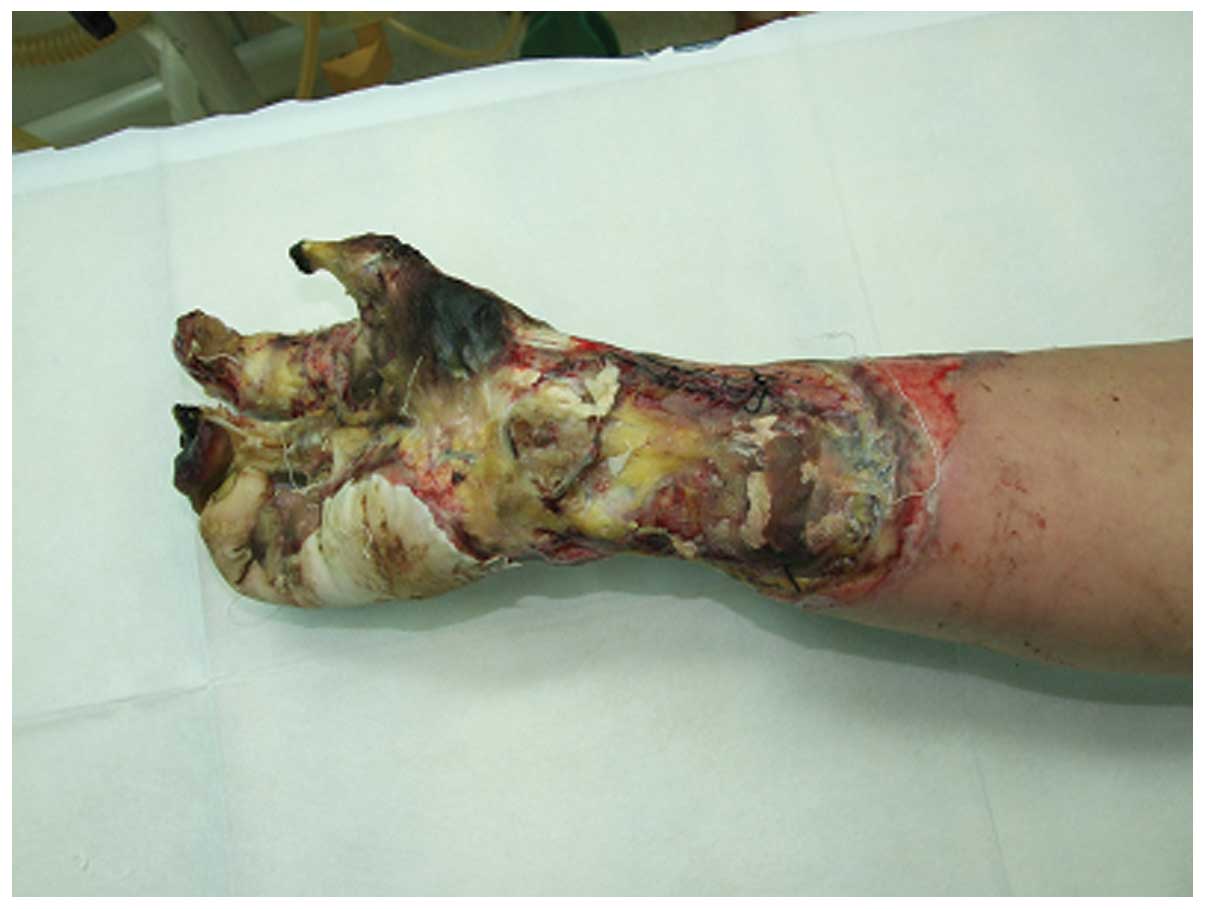
A 40 year-old man who suffered from a high-voltage
burn injury affecting 2% of the total body surface area, with
severe injury of the right hand (Fig.
1), was admitted to the Department of Burns and Plastic Surgery
of the 175th Hospital of PLA, Affiliated Southeast Hospital of
Xiamen University (Zhangzhou, China). Informed consent was obtained
from the patient prior to the study. The patient underwent
one-stage debridement surgery and was then moved to the intensive
care unit and received antibiotic and intravenous fluid support.
Following the first surgery, the patient was subjected to three
additional surgical procedures in the space of 2 weeks, and
followed a routine postoperative course, which consisted of the
administration of antibiotics and the frequent changing of
dressings. In addition, the laboratory examinations showed no
evident abnormalities. On week 3 following hospitalization,
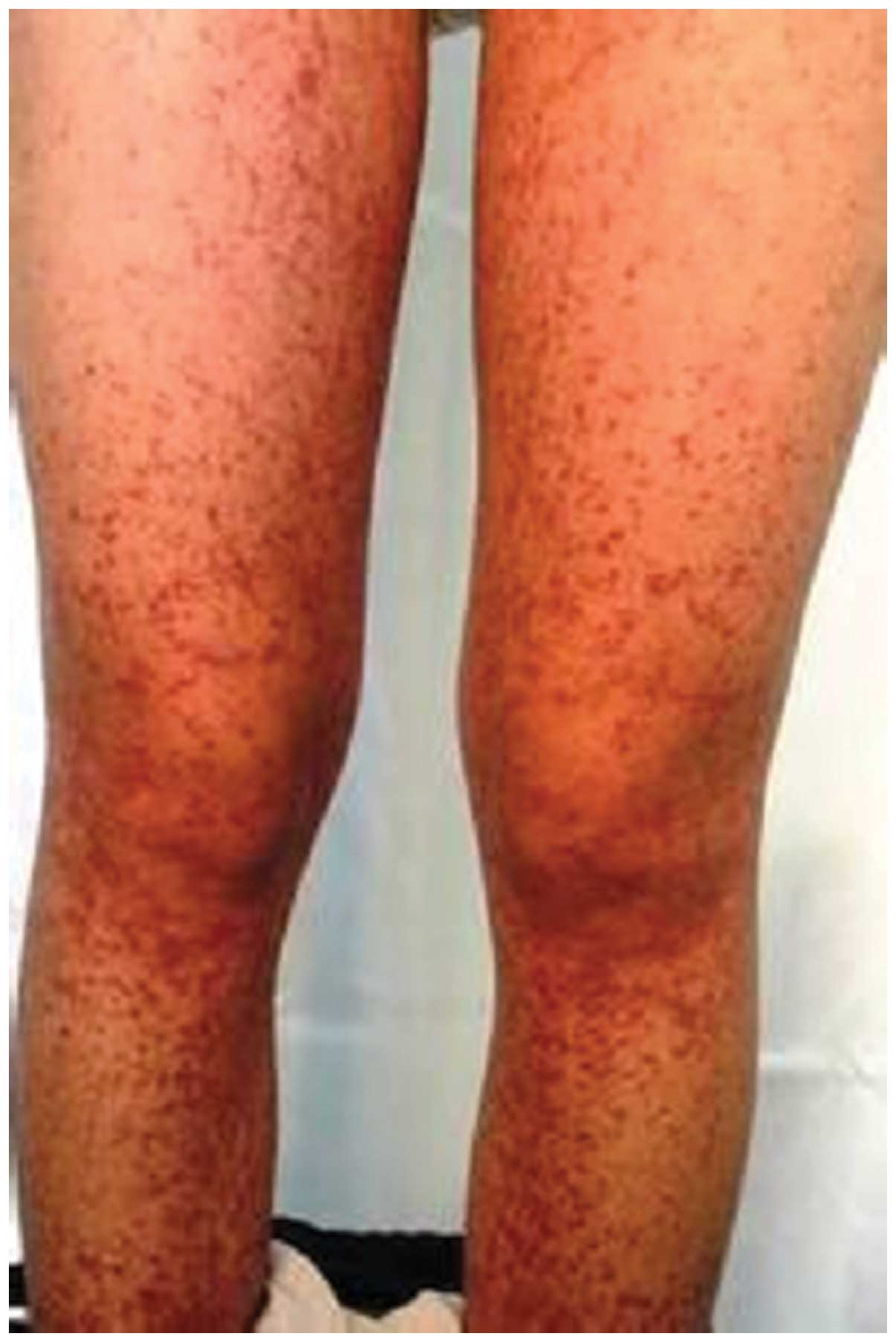
however, an interspersed erythema appeared on the patient's body.
No other abnormalities were observed upon physical examination.
Follow-up showed that the clinical symptoms, such as abdominal
pain, arthralgia and purpuric rash (Fig.
2), improved gradually over the course of 3 days. On the basis
of these symptoms, HSP was considered as a possible diagnosis and
further examinations were performed. Prior to receiving the results
of urine and stool routine tests (including a fecal occult blood
test), the patient was treated with oral loratadine tablets (10
mg/day) and a single intramuscular injection of 5 mg anisodamine.
On the fifth day of the symptoms, the fecal occult blood test
results came back positive, with a high volume of blood detected in
the stool, which confirmed the diagnosis of HSP. The urine test
results were normal. These results, in combination with increases
in serum IgA (11.6 g/l) and complements C3 (9.6 g/l) and C4 (7.6
g/l) levels, led to the conclusion that the diagnosis of HSP was
correct, despite the fact that the kidney function test was normal.
With the injection of Solu-Medrol® (methylprednisolone sodium
succinate; 40 mg/day) and oral administration of omeprazole
magnesium (40 mg/day), the abdominal pain was alleviated and the
purpuric rash gradually decreased. Following a 2-week treatment,
the symptoms were resolved, and on week 5 following admission, the
patient had completely recovered and was discharged. At the 3-, 6-
and 12-month follow-ups, the results of all laboratory tests,
including kidney and liver function and urine and stool tests, were
normal.
Discussion
HSP was first described in 1837 (8,9) and is
an autoimmune acute leucocytoclastic vasculitis, which most
commonly involves the skin, joints, kidney and gastrointestinal
system (10). HSP may be related to
streptococcal infections, viral infections, medicine taken, food
sensitivity and insect bites (9),
and it mainly affects <10 year-old pediatric patients (4). The prevalence is lower in adults,
compared with pediatric patients; however, HSP should not be
ignored in adults, particularly during hospitalization. The
diagnostic criteria of HSP, described by the American College of
Rheumatology (11) and the
International Consensus Conference, 2006 (12,5) are
based on clinical manifestations, such as arthralgia/arthritis,
diffuse abdominal pain, hematuria/proteinuria and purpura nephritis
and pathohistological findings of leukocytoclastic vasculitis and
IgA-immune deposits in vessel walls and/or glomeruli. Not every
patient, however, presents with all aforementioned symptoms, which
makes the establishment of standard criteria challenging. Briefly,
it has been shown that cutaneous manifestations are presented in
70% of HSP cases (13), renal
involvement in 20–60% (14) and
gastrointestinal symptoms in up to 85% (10). Timely urine routine and stool occult
blood tests are required, particularly when the symptoms are not
apparent, in order to avoid misdiagnosis or missed diagnosis. In
the present case, all necessary laboratory examinations had been
concluded within a day of admission, which helped prevent serious,
even fatal, HSP complications, such as pulmonary hemorrhage or
myocardial infarction (15).
Effective therapy is of paramount importance. Analgesics or
non-steroidal anti-inflammatory drugs are used as first-line
treatment for the relief of arthralgia (10) and antihistamines for the treatment of
purpuric rash. Glucocorticoids, such as methylprednisolone, are
commonly used for the relief of abdominal and joint pain. Severe
symptoms affecting the renal and central nervous systems may lead
to life-threatening conditions, and immunosuppressive agents and
plasmapheresis may be required (10); however, caution is required prior to
the use of glucocorticoids and immunosuppressive agents, as
subsequent infections may occur in cases where the patient has a
wound caused by thermal or electric burn injuries.
The phenomenon of HSP following an electric burn
injury is extremely rare as, to the best of our knowledge, only one
other case has been reported (16).
The association between electrical burn injury and HSP is not
clearly established, but the present case of HSP following an
electric burn injury was not considered by our institution as
purely coincidental. Burn or electrical burn injuries can cause
long-term inflammation through tissue necrosis and frequent changes
in dressings, leading to the production of a considerable quantity
of inflammatory mediators that potentially induce the appearance of
HSP. This hypothesis, although a little far-fetched, provides the
most likely explanation of the mechanism of this phenomenon, and
requires further investigation.
In conclusion, HSP is a multisystem autoimmune
disease, which most commonly involves the skin, joints, kidneys and
gastrointestinal system. In patients with electric burn injury,
this autoimmune disease may result from a long-term inflammation;
therefore, examining the liver and kidney function of those
patients is imperative, in order to decrease the risk of
post-traumatic immune system dysfunction.
References
|
1
|
Cao N, Chen T, Guo ZP, Li MM and Jiao XY:
Elevated serum levels of visfatin in patients with Henoch-Schönlein
purpura. Ann Dermatol. 26:303–307. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Semeena N and Adlekha S: Henoch-Schönlein
purpura associated with gangrenous appendicitis: A case report.
Malays J Med Sci. 21:71–73. 2014.PubMed/NCBI
|
|
3
|
Pan YX, Ye Q, Shang SQ, Mao JH, Zhang T,
Shen HQ and Zhao N: Relationship between immune parameters and
organ involvement in children with Henoch-Schonlein purpura. PLoS
One. 9:e1152612014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Reamy BV, Williams PM and Lindsay TJ:
Henoch-Schönlein Purpura. Am Fam Physician. 80:697–704.
2009.PubMed/NCBI
|
|
5
|
Yang YH, Yu HH and Chiang BL: The
diagnosis and classification of Henoch-Schönlein purpura, An
updated review. Autoimmun Rev. 13:355–358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pillebout E and Verine J: Henoch-Schönlein
purpura in the adult. Rev Med Interne. 35:372–381, (In French).
2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanaka M, Kitadai Y, Kodama M, Sumida T,
Shinagawa K, Yoshioka K, Mitsuoka Y, Masuda H, Hiyama T, Tanaka S,
et al: A case report of anaphylactoid purpura with acute abdominal
pain secondary to trauma caused by traffic accident. Nihon
Shokakibyo Gakkai Zasshi. 105:566–571. 2008.(In Japanese).
PubMed/NCBI
|
|
8
|
Schönlein JL: Allegemeine und specielle
Pathologie und Therapie. 2:Herisau, Switzerland:
Literatur-Comptoir. 1834.(In German).
|
|
9
|
Kraft DM, Mckee D and Scott C:
Henoch-Schönlein purpura, A review. Am Fam Physician. 58:405–408.
1998.PubMed/NCBI
|
|
10
|
Hasija N, Taxak S, Bhardwaj M and Vashist
K: Anesthetic management of a patient with Henoch-Schonlein purpura
for drainage of cervical lymphadenitis, A case report. Saudi J
Anaesth. 8:282–283. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mills JA, Michel BA, Bloch DA, Calabrese
LH, Hunder GG, Arend WP, Edworthy SM, Fauci AS, Leavitt RY and Lie
JT: The American College of Rheumatology 1990 criteria for the
classification of Henoch-Schönlein purpura. Arthritis Rheum.
33:1114–1121. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dillon MJ and Ozen S: A new international
classification of childhood vasculitis. Pediatr Nephrol.
21:1219–1222. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sinclair P: Henoch-schönlein purpura – a
review. Curr Allergy Clin Immunol. 23:116–120. 2010.
|
|
14
|
Narchi H: Risk of long term renal
impairment and duration of follow up recommended for
Henoch-Schonlein purpura with normal or minimal urinary findings: A
systematic review. Arch Dis Child. 90:916–920. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Agraharkar M, Gokhale S, Le L, Rajaraman S
and Campbell GA: Cardiopulmonary manifestations of Henoch-Schönlein
purpura. Am J Kidney Dis. 35:319–322. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang W, Xie WG, Min WX, Wang DY, Zhang J
and Wang SY: Treatment of thoracic and abdominal cavity perforation
complicated by Henoch-Schonlein purpura nephritis in a patient with
high-voltage electric burn. Zhonghua Shao Shang Za Zhi. 29:454–458.
2013.(In Chinese). PubMed/NCBI
|