Introduction
Multiple organ dysfunction syndrome (MODS) is the
leading cause of mortality in critically ill patients (1). MODS is defined as the progressive
deterioration of organ function, which often occurs in patients
with severe sepsis, septic shock, hemorrhagic shock, multiple
trauma, severe burns or pancreatitis (2). The mechanisms underlying the pathology
of MODS are not fully understood (3), therefore it is difficult to develop an
effective therapeutic measure for patients with MODS.
Oxygen inhalation is frequently administered to
critically ill patients. Early goal-directed therapy for MODS aims
to balance tissue oxygen delivery and oxygen demand. Previous
studies have suggested that hyperoxia may have a beneficial effect
against sepsis and sepsis-associated multiple organ damage
(4–8). However, the clinical use of hyperoxia
is limited in critically ill patients due to concerns that it may
exacerbate organ damage by increasing free radical formation.
Oxidative stress is crucially involved in the pathogenesis of MODS,
and overproduction of reactive oxygen species (ROS) may exacerbate
organ damage (9,10). It has been suggested that a low
concentration of molecular hydrogen (H2) exerts a
therapeutic antioxidative effect by selectively reducing hydroxyl
(•OH)radicals, the most cytotoxic ROS, and peroxynitrite
(ONOO−), and effectively protects against numerous
diseases (11–15). Furthermore, our previous study showed
that combination therapy with H2 and hyperoxia can
significantly alleviate organ injury and improve survival rate of
mice with polymicrobial sepsis via reducing oxidative stress and
inflammation (16).
Therefore, these findings strongly indicate that
combination therapy with H2 and hyperoxia may afford a
more potent therapeutic strategy for sepsis. The zymosan
(ZY)-induced generalized inflammation model has been widely used by
other research groups (17), as well
as by our group (8), because ZY, a
substance derived from the cell wall of the yeast Saccharomyces
cerevisiae, can produce systemic inflammation by inducing a
wide range of inflammatory mediators (18). This model has also been used in
previous experimental studies investigating MODS (18). Therefore, the aim of the present
study was to investigate whether combination therapy with
H2 and hyperoxia could produce enhanced efficacy in a
murine model of ZY-induced generalized inflammation.
Materials and methods
Animals
Male Imprinting Control Region (ICR) mice (specific
pathogen-free; age, 6–8 weeks; weight, 20–25 g) provided by the
Laboratory Animal Center of the Academy of Military Science of the
Chinese People's Liberation Army (Beijing, China) were used in all
experiments. Animals were housed at 20–22°C with a 12-h light/dark
cycle. Animals were fed standard chow and water ad libitum.
All experimental protocols were approved by the Institutional
Animal Care and Use Committee of Tianjin Medical University
(Tianjin, China), and were performed in accordance with the
National Institutes of Health guidelines for the use of
experimental animals (19).
ZY-induced generalized inflammation
model
ZY (Sigma-Aldrich, St. Louis, MO, USA) was dissolved
in an isotonic sodium chloride solution (normal saline [NS]) to a
final concentration of 25 mg/ml and was sterilized at 100°C for 80
min. All suspensions were freshly produced prior to use.
Generalized inflammation was induced by an aseptic intraperitoneal
injection of ZY (1 g/kg) (8,18). The same volume of NS was injected via
the same route as a control.
Molecular hydrogen and/or hyperoxia
treatment
The animals were put in a sealed plexiglass chamber
with inflow and outflow outlets (20,21).
H2, O2 or N2 was supplied via a
gas flowmeter, (Yukata Engineering Corp., Tokyo, Japan) and
delivered into the chamber through a tube at a rate of 4 l/min. The
concentrations of O2 and H2 in the chamber
were continuously monitored using a gas analyzer (LB-2, Model 40 M;
Beckman Coulter, Inc., Fullerton, CA, USA) and a commercially
available hydrogen detector (HY-ALERTA™ 500, H2scan Corporation,
Valencia, CA, USA), respectively. CO2 was removed from
the chamber gases using baralyme (Allied Healthcare Products, Inc.,
St. Louis, MO, USA). The mixed gases were maintained at the
following levels: 2% H2, 21% O2 and 77%
N2; 0% H2, 98% O2 and 2%
N2; and 2% H2, 98% O2 and 0%
N2 during the treatment. The animals without
H2 or hyperoxia treatment were exposed to room air in
the chamber.
Experimental design
Experiment one: Effects of H2 and/or
hyperoxia treatment on the survival rate in ZY-challenged mice
A total of 180 animals were randomly allocated into
six groups (n=30 per group): Normal saline (NS), NS + H2
+ O2; ZY; ZY + H2; ZY + O2; and ZY
+ H2 + O2 groups. The treatment
concentrations of hydrogen and hyperoxia were determined based on
our previous studies and preliminary observations (8,21). The
animals in the ZY + O2 and ZY + H2 groups
were exposed to 98% O2 or 2% H2 for 3 h
starting at 1 and 6 h after ZY injection, respectively. The animals
in the NS + H2 + O2 and ZY + H2 +
O2 groups were exposed to 98% O2 and 2%
H2 at the same time points. As a control, the animals
from the NS and ZY groups were given room air treatment at the same
time points. The survival rate was observed on days 1, 2, 3, 4, 5,
6, 7 and 14 after NS or ZY injection. In addition, arterial blood
gas evaluation was conducted at 1.5 h after the onset of
H2 inhalation (2.5 h after NS or ZY injection) in all
groups.
Experiment two: Effects of H2 and/or
hyperoxia treatment on organ injury in ZY-challenged mice
Additional 36 animals were used in this experiment
and were assigned to six groups (n=6 per group). The grouping
method and experimental protocols were the same as Experiment One.
At 24 h after NS or ZY injection, all the animals were sacrificed
with sodium pentobarbital (50 mg/kg, intraperitoneally), and the
blood samples and organs were collected for detecting serum
biochemical parameters and organ histopathology.
In addition, at 24 h after NS or ZY injection, the
serum levels of the proinflammatory cytokines high-mobility group
box 1 (HMGB1) and tumor necrosis factor-α (TNF-α), the
antioxidative enzyme superoxide dismutase (SOD) and the oxidative
product 8-iso-prostaglandin F2α (8-iso-PGF2α) were evaluated.
Arterial blood gas analysis
The arterial blood gas analysis (for pH,
PaO2 and PaCO2) was conducted using a GEM
Premier 3000 gas analyzer (Instrumentation Laboratory Spa, Milan,
Italy).
Serum biochemical parameters assay
The serum was separated from whole blood samples by
centrifugation at 1,100 × g for 10 min, aliquoted and stored at
−80°C until assayed (8,20–22). The
samples were evaluated using a biochemistry autoanalyzer (7150;
Hitachi, Ltd., Tokyo, Japan) to measure serum levels of alanine
aminotransferase (ALT, IU/l), aspartate aminotransferase (AST,
IU/l), blood urea nitrogen (BUN, mmol/l) and creatinine (Cr,
µmol/l).
Organ histological examination
The lung, liver and kidneys were removed
immediately, fixed in 4% paraformaldehyde (Sigma-Aldrich), embedded
in paraffin, and sectioned at 4–6 µm. After deparaffinization and
rehydration, the sections were stained with hematoxylin and eosin
(Sigma-Aldrich). Based on the scoring standard in our previous
studies (8,22), the histological slides were blindly
examined and scored by two experienced pathologists.
Detection of SOD activity
The activities of SOD were measured using commercial
kits purchased from Cayman Chemical Company (Ann Arbor, MI, USA).
Total SOD activity was assayed according to the manufacturer's
instructions and the protocol described in our previous studies
(8,20–22). All
spectrophotometric readings were performed using a
spectrophotometer (DU-640B; Beckman Coulter, Inc.). All assays were
conducted in triplicates.
Detection of 8-iso-PGF2α
Measurement of 8-iso-PGF2α, a free radical-catalyzed
product of arachidonic acid, can offer a reliable approach for
quantitative measurement of oxidative stress status in vivo
(23). The levels of serum
8-iso-PGF2α were detected using specific enzyme-linked
immunosorbent assay (ELISA) kits (cat. no. 516351; Cayman Chemical
Company) and a Spectramax M5 microplate reader (Molecular Devices,
LLC, Sunnyvale, CA, USA) (8,20–22). All
standards and samples were run in duplicate.
Detection of inflammatory cytokines
The levels of serum TNF-α (R&D Systems, Inc.,
Minneapolis, MI, USA) and HMGB1 (IBL International GmbH, Hamburg,
Germany) were detected using specific ELISA kits and the Molecular
Devices microplate reader (8,20–22).
All standards and samples were run in duplicate.
Statistical analysis
Survival rates are expressed as percentages and
measurement data are expressed as the mean ± standard error of the
mean. The analysis of survival rates were tested using Fisher's
exact probability method. The inter-group differences of the rest
data were tested by one-way analysis of variance followed by Least
Significant Difference t-test for multiple comparisons. Statistical
analyses were performed using SPSS software, version 16.0 (SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Differences in arterial blood gas
during treatment
In the present study, the effects of H2
inhalation on arterial pH, PaO2 and PaCO2
were evaluated in all groups at 1.5 h after the onset of
H2 and/or O2 inhalation (2.5 h after NS or ZY
injection). The levels of PaO2 in the ZY +
O2, ZY + H2 + O2 and NS +
H2 + O2 groups were 405.1±19.2, 410.3±18.7
and 408.4±21.5 mmHg, respectively. The PaO2
levels in the NS, ZY and ZY + H2 groups were 97.18±8.29,
96.72±10.31 and 95.46±8.46 mmHg, respectively. There were no
differences in the levels of arterial pH and
PaCO2 among all groups (data not
shown). The results demonstrate that hyperoxia exposure can
significantly increase the PaO2 level, and 2%
H2 has no marked effects on the
PaO2 level in ZY-challenged mice during the
treatment.
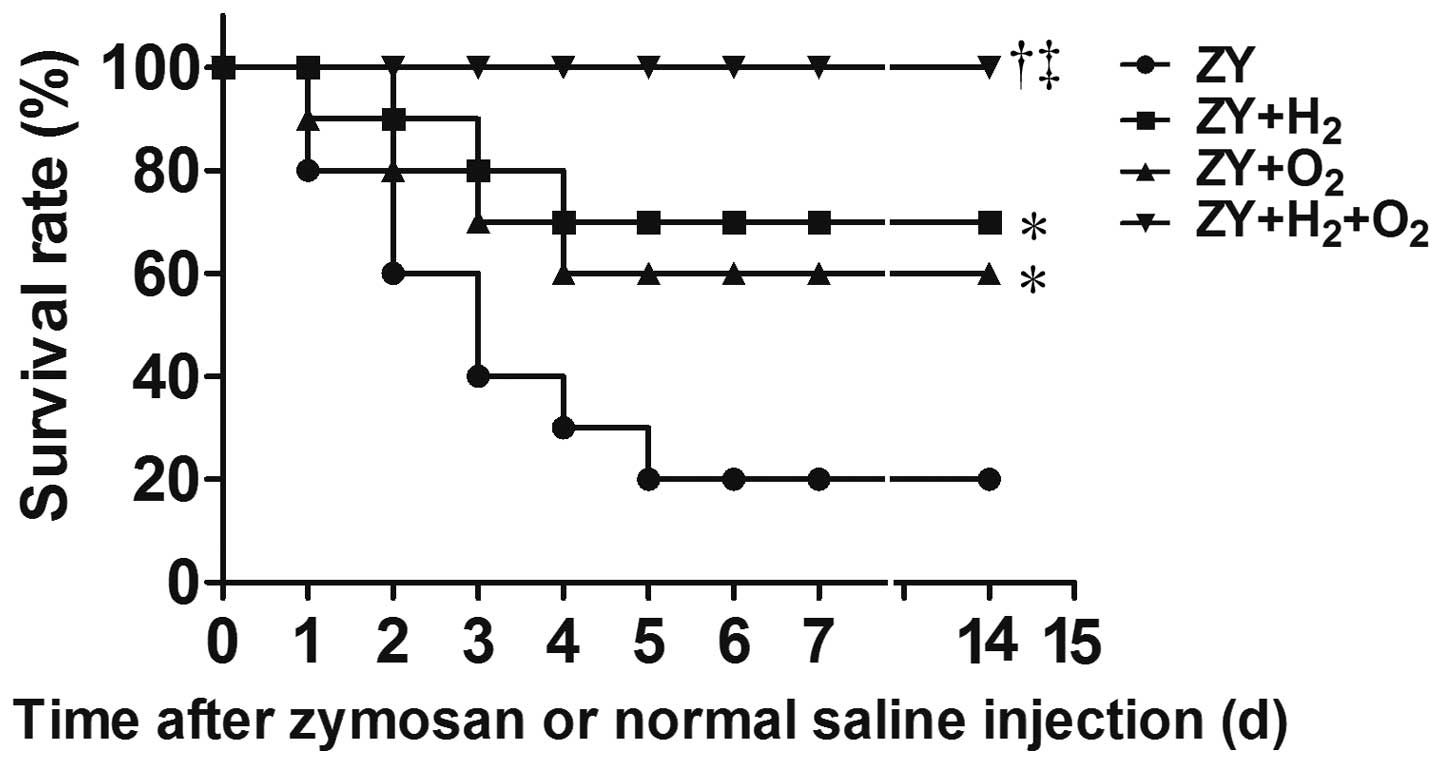
Combination therapy with H2
and hyperoxia improves the survival rate in ZY-challenged mice
The 14-day survival rate of ZY-challenged mice was
20% (P<0.05 vs. NS group; Fig.
1). The results showed that either 98% O2 or 2%
H2 exposure for 3 h starting at 1 and 6 h after ZY
injection, respectively, improved the 14-day survival rate of
ZY-challenged mice to 60 or 70% (P<0.05 vs. ZY group; Fig. 1). Furthermore, combination therapy
with 2% H2 and 98% O2 increased the 14-day
survival rate of ZY-challenged mice to 100% (P<0.05 vs. ZY
group; Fig. 1). In addition, all
mice in the NS and NS + H2 + O2 groups
survived during the experiment. These results suggest that
combination therapy with H2 and hyperoxia can improve
the survival rate of ZY-challenged mice in a synergistic
manner.
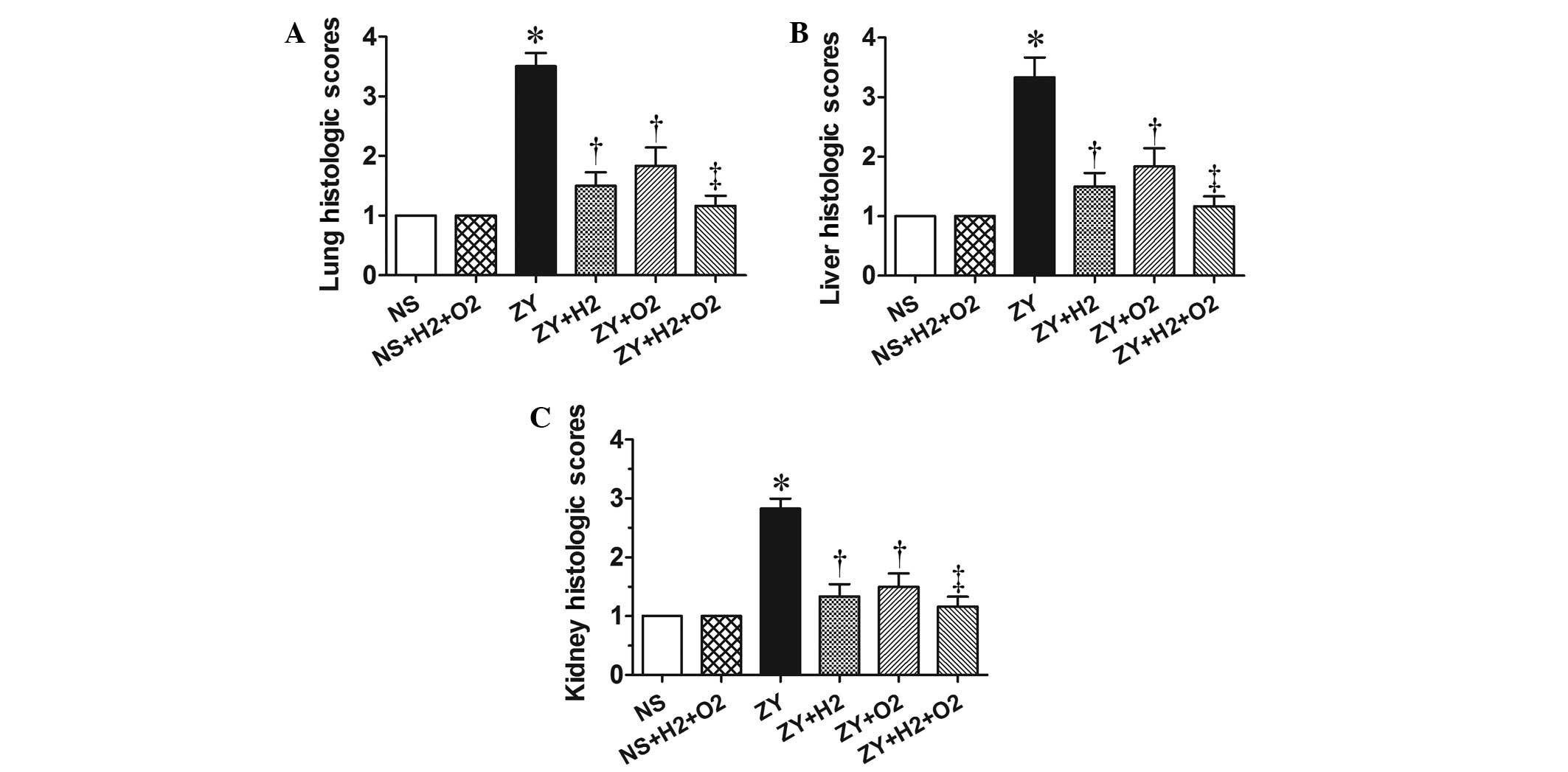
Combination therapy with H2
and hyperoxia improves organ histopathological scores in
ZY-challenged mice
At 24 h after ZY or NS injection, the animals in all
groups were killed for histopathological analysis. According to the
scoring standard in our previous studies (8,22), the
histopathological changes in lung, liver and kidney were scored
using a scale of 1 to 4. As shown in the Fig. 2, the histopathological scores for
lung, liver and kidney in the ZY group were 3–3.5, significantly
increased compared with the NS group (P<0.05). These abnormal
changes in ZY-challenged mice were attenuated by either 98%
O2 or 2% H2 treatment alone (P<0.05 vs. ZY
group; Fig. 2). Furthermore, these
abnormal changes in ZY-challenged mice were more markedly
ameliorated by combination therapy with 98% O2 and 2%
H2 compared with either treatment alone (Fig. 2). These data indicate that
combination therapy with H2 and hyperoxia has an
enhanced efficacy against multiple organ damage in ZY-challenged
mice.
Combination therapy with H2
and hyperoxia improves serum biochemical parameters in
ZY-challenged mice
As shown in Fig. 3,
the ZY-challenged mice appeared significantly impaired liver and
kidney function at 24 h, which was assessed by serum biochemical
parameters for liver and kidney function (ALT, AST, Cr and BUN).
The ZY-challenged mice showed a significant increase in the levels
of serum ALT, AST, Cr and BUN (P<0.05 vs. NS group), which were
significantly attenuated by 2% H2 or 98% O2
treatment alone (Fig. 3).
Furthermore, these abnormal changes of biochemical parameters in
ZY-challenged mice were more notably ameliorated by combination
therapy with 98% O2 and 2% H2 (Fig. 3). These results demonstrate that
combination therapy with H2 and hyperoxia has a more
substantially beneficial effect on liver and kidney dysfunction in
ZY-challenged mice.
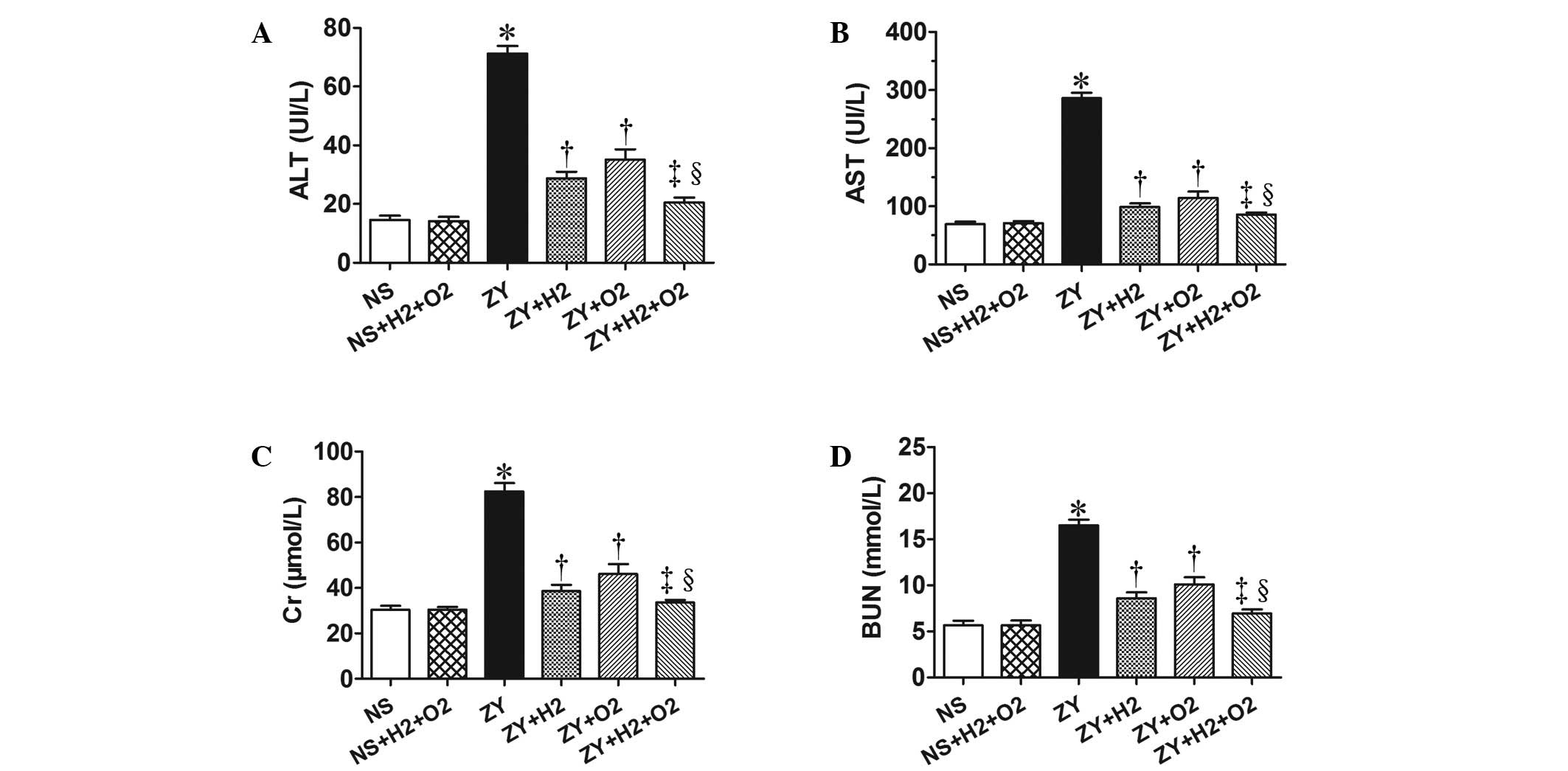 | Figure 3.Effects of H2 and/or
hyperoxia on the serum biochemical parameters in zymosan-challenged
mice. Serum levels of (A) ALT, (B) AST, (C) Cr and (D) BUN. Mice
were treated with or without 2% H2 and/or 98%
O2 inhalation for 3 h starting at 1 and 6 h after NS or
zymosan injection, respectively. At 24 h after NS or zymosan
injection, all the animals were anesthetized, and the blood samples
were collected for detection of the serum biochemical parameters.
Values represent the mean ± standard error of the mean (n=6 per
group). *P<0.05 vs. NS group; †P<0.05 vs. ZY
group; ‡P<0.05 vs. ZY + H2 group;
§P<0.05 vs. ZY + O2 group. ALT, alanine
aminotransferase; AST, aspartate aminotransferase; BUN, blood urea
nitrogen; Cr, creatinine; UI/L, international units per liter; NS,
normal saline; ZY, zymosan. |
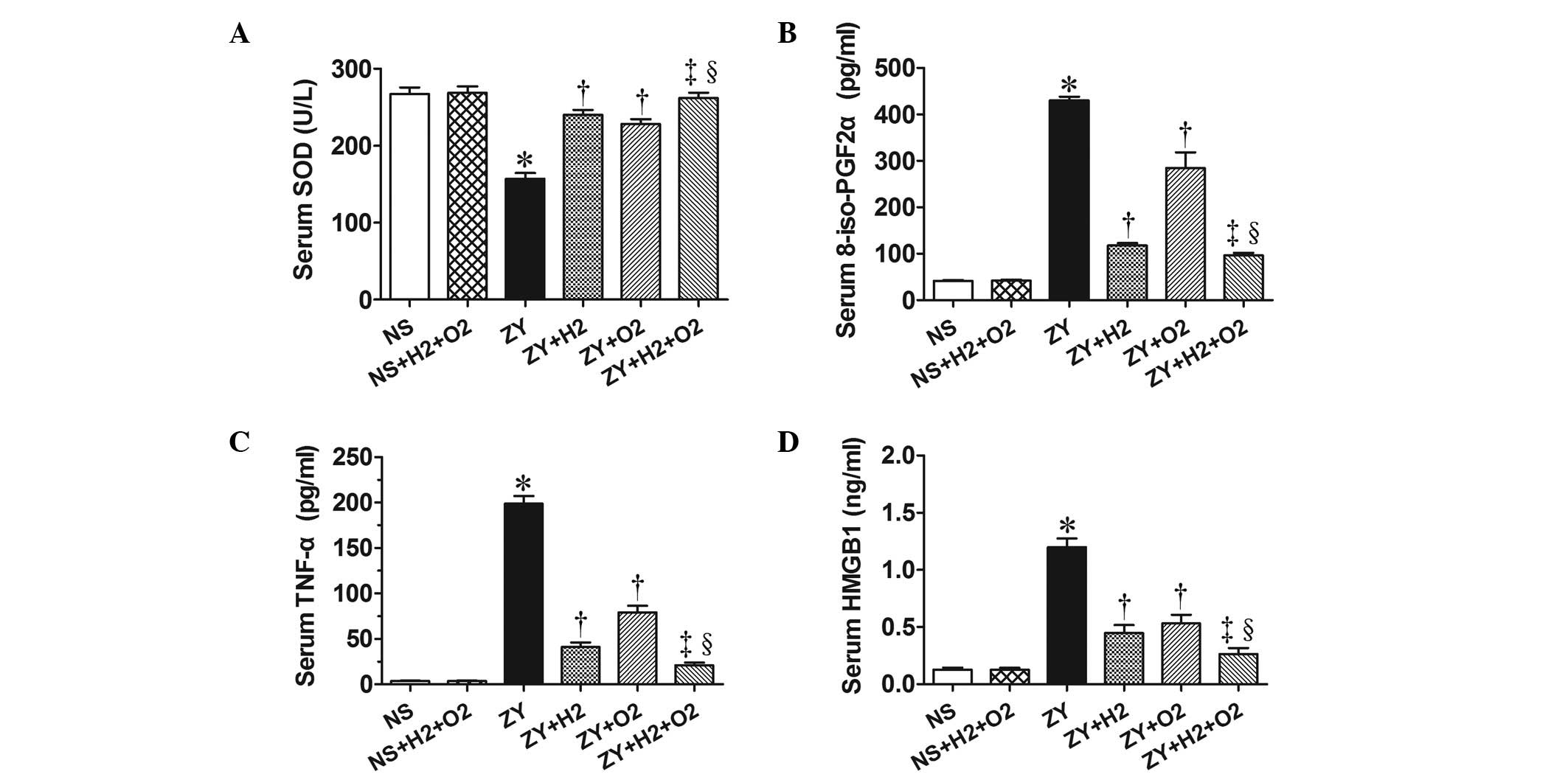
Combination therapy with H2
and hyperoxia prevents the abnormal changes of antioxidant
enzymatic activities, oxidative product and inflammatory cytokines
in ZY-challenged mice
The activity of the antioxidative enzyme SOD, the
levels of the oxidative product 8-iso-PGF2α and the levels of
proinflammatory cytokines (HMGB1 and TNF-α) in serum of all animals
were observed at 24 h after NS or ZY injection. The results showed
decreased SOD activity and increased levels of 8-iso-PGF2α, HMGB1
and TNF-α in the serum of the ZY-challenged mice (P<0.05 vs. NS
group; Fig. 4). Furthermore, the
results showed that 2% H2 or 98% O2 alone
significantly increased the SOD activity and decreased the
8-iso-PGF2α level in serum of ZY-challenged mice (P<0.05;
Fig. 4). In addition, 2%
H2 or 98% O2 alone significantly decreased
the serum HMGB1 and TNF-α levels of the ZY-challenged mice
(P<0.05; Fig. 4). These abnormal
changes in oxidative stress and inflammatory cytokines were more
markedly improved by combination therapy with 98% O2 and
2% H2 (Fig. 4).
These data suggest that combination therapy with
H2 and hyperoxia produces a more beneficial effect on
survival rate and organ damage in ZY-challenged mice, which is
associated with decreased levels of oxidative product and
proinflammatory cytokines and increased levels of antioxidative
enzymes in the serum.
Discussion
The present study demonstrated that either
H2 or hyperoxia treatment alone improved the survival
rate of ZY-challenged mice, while combination therapy with
H2 and hyperoxia could synergistically increase the
survival rate of ZY-challenged mice, which was greater than
treatment with either gas alone. Furthermore, H2 or
hyperoxia treatment alone protected against lung, liver and kidney
damage in ZY-challenged mice, while combination therapy with
H2 and hyperoxia provided cumulative protection against
these organ damage of ZY-challenged mice. In addition, the
beneficial effects of combination therapy with H2 and
hyperoxia on ZY-induced organ injury were associated with decreased
levels of oxidative product 8-iso-PGF2α, increased SOD activity and
reduced levels of inflammatory cytokines TNF-α and HMGB1 in
serum.
ZY is a substance derived from the cell wall of the
yeast Saccharomyces cerevisiae, and can induce systemic
inflammation by inducing a wide range of inflammatory mediators
(18). Based on previous studies by
the present authors and others, intraperitoneal injection of a high
dose of ZY (0.8–1.0 g/kg) can induce a generalized inflammation in
rats or mice, which is accompanied by multiple organ damage
(8,17,18,22). ZY
(1.0 g/kg, intraperitoneal injection) successfully induced sterile
inflammation model in mice, characterized by the decrease of
survival rates, histopathological injury, organ dysfunction and
abnormally decreased tissue oxygenation (8,22). In
the present study, similar changes were observed in the
ZY-challenged mice.
Oxygen therapy is widely used in clinical practice
as a mainstay of supportive treatment for patients with hypoxemia
and critical illness. It is well known that early goal-directed
therapy for MODS aims to balance oxygen delivery and demand
(24,25). Previous animal studies have shown
that hyperoxia exposure can improve organ function and survival
rate in several models of shock or sepsis (4–8,26,27). In
addition, 100% oxygen exposure for 2 and 3 h starting at 4 and 12
h, respectively, after ZY injection benefits the outcome of mice
with sterile sepsis (8). It is
speculated that improved tissue oxygenation and decreased systemic
inflammatory response are crucially involved in the protective
effects of hyperoxia treatment (4–8).
However, hyperoxia treatment can induce the production of ROS,
which are considered to be associated with oxygen toxicity
(9,10). Therefore, the use of hyperoxia is
limited in critically ill patients due to concerns that it may
exacerbate organ damage by increasing free radical formation.
H2 has been used medically to prevent
decompression sickness in deep divers for safety profiles (28). Prior studies have shown that
H2 exerts a therapeutic antioxidative effect by
selectively reducing ROS toxicity, and effectively protecting
against a number of diseases, suggesting that H2 has
potential as an antioxidant for therapeutic applications (11–15). Our
previous studies have shown that H2 treatment has a
beneficial effect on sepsis and sepsis-induced organ injury
(16,20,21). In
the present study, combination therapy with H2 and
hyperoxia appeared to produce a synergistic protective effect
against sepsis and sepsis-associated multiple organ damage
(16). The present results showed
that combination therapy with H2 and hyperoxia had a
more beneficial effect on multiple organ dysfunction/failure in the
ZY-induced generalized inflammation model.
To further investigate the possible underlying
mechanisms, the effects of H2 and/or hyperoxia treatment
on oxidant and antioxidant system in ZY-challenged mice were
investigated. In our previous studies, we found that the activities
of SOD, CAT and GSH-Px in serum and tissues are significantly
decreased during the early and late stages of ZY-induced organ
damage, indicating that ZY sets up an environment favorable for
oxidative stress (8,22). In the present study, decreased SOD
activity and increased 8-iso-PGF2α levels were detected in the
serum at 24 h after ZY injection. Furthermore, 2% H2
and/or 98% O2 treatment significantly improved SOD
activity and decreased the 8-iso-PGF2α levels in the serum. These
results suggest that the reduction of oxidative damage and the
increase of endogenous antioxidant enzymatic activities in the
serum may associated with the protective effects of H2
and/or O2 treatment, which is consistent with our
previous study (20). It has been
hypothesized that the uncontrolled and exaggerated inflammatory
response plays a major role in the pathogenesis of sepsis/MODS
(3). Inflammatory cytokines include
early inflammatory cytokines such as proinflammatory cytokines
TNF-α, IL-6 and anti-inflammatory cytokine IL-10, as well as the
late inflammatory cytokine HMGB1 (29,30).
Early and late inflammatory cytokines can interact and facilitate
the organ dysfunction and injury in sepsis/MODS. Our previous
studies have demonstrated that HMGB1 contributed to organ damage in
the ZY-induced generalized inflammation model (8,22). In
the present study, we found that ZY-challenged mice showed the
significant increase of TNF-α and HMGB1 in serum, which was
significantly attenuated by 2% H2 and/or O2
treatment. These data suggest that the protective effects of
combination therapy with H2 and hyperoxia treatment on
ZY-challenged mice are associated with a reduction in the serum
levels of early and late proinflammatory cytokines, which is
consistent with our previous study (20,21).
H2 is neither explosive nor dangerous at
a low concentration (<4.1% in oxygen). The present results
suggest that a mixed gas therapy with H2 and hyperoxia
may be a novel, safe and effective approach for preventing organ
damage and mortality in MODS.
Acknowledgements
This study was supported by research grants from the
National Natural Science Foundation of China (grant nos. 81101409
and 81471842) and Natural Science Foundation of Tianjin, China (no.
13JCQNJC11400), the Foundation of Tianjin Bureau of Public Health
(no. 2011KZ108).
Glossary
Abbreviations
Abbreviations:
|
8-iso-PGF2α
|
8-iso-prostaglandin F2α
|
|
ALT
|
alanine aminotransferase
|
|
AST
|
aspartate aminotransferase
|
|
BUN
|
blood urea nitrogen
|
|
CAT
|
catalase
|
|
CLP
|
cecal ligation and puncture
|
|
Cr
|
creatinine
|
|
GSH-Px
|
glutathione peroxidase
|
|
H2
|
hydrogen
|
|
H2O2
|
hydrogen peroxide
|
|
HMGB1
|
high-mobility group box 1
|
|
ICU
|
intensive care units
|
|
MODS
|
multiple organ dysfunction
syndrome
|
|
NS
|
normal saline
|
|
•OH
|
hydroxyl radicals
|
|
ROS
|
reactive oxygen species
|
|
SOD
|
superoxide dismutase
|
|
ZY
|
zymosan
|
References
|
1
|
Martin GS, Mannino DM, Eaton S and Moss M:
The epidemiology of sepsis in the United States from 1979 through
2000. N Engl J Med. 348:1546–1554. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shayevitz JR, Miller C, Johnson KJ and
Rodriguez JL: Multiple organ dysfunction syndrome: End organ and
systemic inflammatory response in a mouse model of nonseptic
origin. Shock. 4:389–396. 1995.PubMed/NCBI
|
|
3
|
Hotchkiss RS and Karl IE: The
pathophysiology and treatment of sepsis. N Engl J Med. 348:138–150.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asfar P, Calzia E, Huber-Lang M, Ignatius
A and Radermacher P: Hyperoxia during septic shock-Dr. Jekyll or
Mr. Hyde? Shock. 37:122–123. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Waisman D, Brod V, Rahat MA, Amit-Cohen
BC, Lahat N, Rimar D, Menn-Josephy H, David M, Lavon O, Cavari Y
and Bitterman H: Dose-related effects of hyperoxia on the lung
inflammatory response in septic rats. Shock. 37:35–102. 2012.
View Article : Google Scholar
|
|
6
|
Hauser B, Barth E, Bassi G, Simon F,
Gröger M, Oter S, Speit G, Ploner F, Möller P, Wachter U, et al:
Hemodynamic, metabolic and organ function effects of pure oxygen
ventilation during established fecal peritonitis-induced septic
shock. Crit Care Med. 37:2465–2469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Barth E, Bassi G, Maybauer DM, Simon F,
Gröger M, Oter S, Speit G, Nguyen CD, Hasel C, Möller P, et al:
Effects of ventilation with 100% oxygen during early hyperdynamic
porcine fecal peritonitis. Crit Care Med. 36:495–503. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hou L, Xie K, Li N, Qin M, Lu Y, Ma S, Ji
G and Xiong L: 100% oxygen inhalation protects against
zymosan-induced sterile sepsis in mice: The roles of inflammatory
cytokines and antioxidant enzymes. Shock. 32:451–461. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Folz RJ, Abushamaa AM and Suliman HB:
Extracellular superoxide dismutase in the airways of transgenic
mice reduces inflammation and attenuates lung toxicity following
hyperoxia. J Clin Invest. 103:1055–1066. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Altemeier WA and Sinclair SE: Hyperoxia in
the intensive care unit: Why more is not always better. Curr Opin
Crit Care. 13:73–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ohta S: Hydrogen gas and hydrogen water
act as a therapeutic and preventive antioxidant with a novel
concept. Nippon Ronen Igakkai Zasshi. 45:355–362. 2008.(In
Japanese). PubMed/NCBI
|
|
13
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cai J, Kang Z, Liu WW, Luo X, Qiang S,
Zhang JH, Ohta S, Sun X, Xu W, Tao H and Li R: Hydrogen therapy
reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci
Lett. 441:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang CS, Kawamura T, Toyoda Y and Nakao
A: Recent advances in hydrogen research as a therapeutic medical
gas. Free Radic Res. 44:971–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xie K, Fu W, Xing W, Li A, Chen H, Han H,
Yu Y and Wang G: Combination therapy with molecular hydrogen and
hyperoxia in a murine model of polymicrobial sepsis. Shock.
38:656–663. 2012.PubMed/NCBI
|
|
17
|
Cuzzocrea S, Costantino G, Mazzon E and
Caputi AP: Protective effect of N-acetylcysteine on multiple organ
failure induced by zymosan in the rat. Crit Care Med. 27:1524–1532.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Volman TJ, Hendriks T and Goris RJ:
Zymosan-induced generalized inflammation: Experimental studies into
mechanisms leading to multiple organ dysfunction syndrome. Shock.
23:291–297. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Humphreys K, Weingardt KR and Harris AH:
Influence of subject eligibility criteria on compliance with
National Institutes of Health guidelines for inclusion of women,
minorities, and children in treatment research. Alcohol Clin Exp
Res. 31:988–995. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L
and Wang G: Protective effects of hydrogen gas on murine
polymicrobial sepsis via reducing oxidative stress and HMGB1
release. Shock. 34:90–97. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie K, Yu Y, Zhang Z, Liu W, Pei Y, Xiong
L, Hou L and Wang G: Hydrogen gas improves survival rate and organ
damage in zymosan-induced generalized inflammation model. Shock.
34:495–501. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hou L, Xie K, Qin M, Peng D, Ma S, Shang
L, Li N, Li S, Ji G, Lu Y and Xiong L: Effects of reactive oxygen
species scavenger on the protective action of 100% oxygen treatment
against sterile inflammation in mice. Shock. 33:646–654. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dworski R, Roberts LJ II, Murray JJ,
Morrow JD, Hartert TV and Sheller JR: Assessment of oxidant stress
in allergic asthma by measurement of the major urinary metabolite
of F2-isoprostane, 15-F2t-IsoP (8-iso-PGF2alpha). Clin Exp Allergy.
31:387–390. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Martin DS and Grocott MP: Oxygen therapy
in critical illness: Precise control of arterial oxygenation and
permissive hypoxemia. Crit Care Med. 41:423–432. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rivers E, Nguyen B, Havstad S, Ressler J,
Muzzin A, Knoblich B, Peterson E and Tomlanovich M: Early
Goal-Directed Therapy Collaborative Group: Early goal-directed
therapy in the treatment of severe sepsis and septic shock. N Engl
J Med. 345:1368–1377. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meier J, Kemming GI, Kisch-Wedel H, Blum
J, Pape A and Habler OP: Hyperoxic ventilation reduces six-hour
mortality after partial fluid resuscitation from hemorrhagic shock.
Shock. 22:240–247. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sukhotnik I, Krausz MM, Brod V, Balan M,
Turkieh A, Siplovich L and Bitterman H: Divergent effects of oxygen
therapy in four models of uncontrolled hemorrhagic shock. Shock.
18:277–284. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kayar SR and Fahlman A: Decompression
sickness risk reduced by native intestinal flora in pigs after H2
dives. Undersea Hyperb Med. 28:89–97. 2001.PubMed/NCBI
|
|
29
|
Wang H, Bloom O, Zhang M, Vishnubhakat JM,
Ombrellino M, Che J, Frazier A, Yang H, Ivanova S, Borovikova L, et
al: HMG-1 as a late mediator of endotoxin lethality in mice.
Science. 285:248–251. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hou LC, Qin MZ, Zheng LN, Lu Y, Wang Q,
Peng DR, Yu XP, Xin YC, Ji GL and Xiong LZ: Severity of sepsis
correlated with the elevation of serum high-mobility group box 1 in
rats. Chin Med J (Engl). 122:449–454. 2009.PubMed/NCBI
|