Introduction
Frontotemporal lobar degeneration (FTLD) is
increasingly recognized as a prevalent cause of dementia,
particularly in individuals <65 years old at the time of
diagnosis (1). FTLD encompasses a
variety of clinical syndromes with marked behavioral, frontal
executive and language symptoms that can be differentiated into the
following three clinical syndromes: Behavioral variant
frontotemporal dementia (bvFTD), semantic dementia (SD) and
progressive non-fluent aphasia (PNFA); these three phenotypes have
characteristic clinical manifestations and different patterns of
brain atrophy, and are histopathologically heterogeneous (2,3). This
creates challenges for the establishment of an optimal
pharmacological disease management model. To date, the U.S. Food
and Drug Administration (FDA) has not approved any pharmacological
treatments that specifically target FTLD and alter the course of
the disease (4).
Memantine is a moderate-affinity, non-competitive
antagonist of the N-methyl-D-aspartate (NMDA) receptor and is an
FDA-approved treatment for moderate-to-severe Alzheimer's disease
(AD) (5). However, a growing body of
clinical evidence suggests that the beneficial effect of memantine
is not limited to AD. For example, it has beneficial effects in
Parkinson's disease-related forms of dementia and other mixed
causes of dementia (6). In addition,
memantine has been demonstrated to ease or improve behavioral and
cognitive manifestations of these forms of dementia, in particular
various behavioral disturbances (irritability, agitation,
aggression and difficulty eating) (7–9).
Previously reviewed studies have observed that NMDA
receptors are upregulated in FTLD and suggest that aberrant
glutamatergic neurotransmission and glutamate excitotoxicity
mediating cellular dysfunction may be implicated in the
pathogenesis of FTLD (10).
Therefore, it is hypothesized that memantine may be useful for
treating the symptoms of FTLD.
Data from a number of clinical trials using
memantine to treat mild-to-moderate FTLD [Mini-Mental State
Examination (MMSE) score, ≥15; average Clinical Dementia Rating
(CDR), <2] have been published. In a small case series,
memantine was observed to have beneficial effects on the total
Neuropsychiatric Inventory (NPI) score, in particular improving the
apathy subscale scores, and the level of agitation and anxiety
(11). In an open-label study, a
transient improvement of the total NPI score was observed in
patients with FTLD undergoing memantine treatment (12). In addition, it was reported in one
case of FTLD that symptoms of apathy improved in response to
treatment with memantine (13).
Furthermore, memantine was demonstrated to elevate cortical
metabolic activities in the frontal and temporal regions, and in
the salience network hubs in FTLD (14,15),
thereby improving the quality of life of patients with FTLD and
their families (12). These studies
highlight the potential clinical applications of memantine in FTLD;
however, a randomized controlled trial for memantine demonstrated
little efficacy in the treatment of FTLD, and the results suggested
that it may even hasten cognitive decline (16,17).
This suggests that the drug should not be prescribed to patients
with FTLD.
FTLD can be misdiagnosed as a mental illness, as a
number of patients with FTLD may display significant personality
changes, behavioral abnormalities and language impairments in the
early stages of the disease (18).
It was demonstrated that patients with FTLD had significantly
shorter survival and faster rates of cognitive and functional
decline compared to those with Alzheimer disease (AD). The years
from initial evaluation to death in FTLD patients is 4.2 years, but
6.0 years in AD patients (log-rank test=5.17, P< 0.05)(19). According to the survey of
epidemiology, from the time of symptom onset, the mean survival in
all FTLD is estimated to range from 6.6 to 11.0 years. The mean
survival from time of clinical diagnosis of FTLD is estimated to
range between 3 and 4 years, which means that patients with FTLD
admitted to hospital will progress to a moderate or severe stage in
no more than 3 and 4 years (20,21). The
majority of studies have focused on the effects of memantine on
mild-to-moderate (MMSE score, >15) FTLD and have neglected its
possible benefits for the treatment of patients in
moderate-to-severe stages of FTLD (16).
One study concluded that memantine demonstrates a
lack of efficacy in treating patients with FTLD and therefore
suggested that the drug may not improve a patient's overall
behavioral and cognitive function (17); however, the study did not closely
analyze the effects of memantine on specific subsets of behavioral
and neuropsychiatric symptoms or cognitive impairment. Therefore,
in the present study, the efficacy, safety and tolerability of a
6-month treatment regimen of memantine was evaluated in patients
with FTLD. The difference between moderate-to-severe (MMSE scores,
4–20) and mild (MMSE scores, 21–26) behavioral variant
frontotemporal degeneration outpatients was evaluated, and specific
subscales of behavioral and cognitive functions were analyzed.
Materials and methods
Patient selection and exclusion
criteria
In this 6-month open-label clinical study, 60
outpatients from October 2008 to October 2014 were diagnosed with
bvFTD according to consensus diagnostic criteria (22,23).
These patients were enrolled in the Cognitive Impairment Clinic
(CIC) at the Neurology Department of Tianjin Huanhu Hospital
(Tianjin, China). Memantine drug-naive patients were enrolled in
the final trial. Patients that were prescribed oxiracetam (10
patients, 0.8 g thrice daily), a memory-improving agent widely used
in senile dementia patients, or a cholinesterase inhibitor (8
patients, 5 mg once daily) were excluded from the present study.
Other exclusion criteria were as follows: i) Aphasia, severe
illness and difficulty completing procedures of the study; ii) the
pattern of deficits was better accounted for by other
non-degenerative neurological or psychiatric disorders; iii)
serious chronic conditions within the previous year; iv) magnetic
resonance imaging (MRI) or computed tomography (CT) findings
inconsistent with bvFTD diagnosis criteria; v) biomarker results
that strongly indicated a diagnosis of AD or other forms of
dementia; vi) acetylcholinesterase inhibitor treatment and other
nootropic or antipsychotic medications taken within 4 weeks of
baseline measurement recording; vii) metabolic or inflammatory
brain disorders.
Patients were 53–84 years old with Montreal
Cognitive Assessment (MoCA) scores between 4 and 29 at the initial
screening during enrollment. All participants were required to have
an MRI or brain CT scan within 1 year to confirm the bvFTD
diagnosis, and a number of patients received
18F-fluorodeoxyglucose (18F-FDG) positron
emission (PET)/CT in order to further confirm the diagnosis.
Study design and diagnostic
procedures
A total of 42 drug-naive patients were treated with
10 mg memantine (Lundbeck, Valby, Denmark), which is the maximal
FDA-approved dose for the treatment of AD, twice daily over a
6-month period. Patients were divided into the following groups
according to their MMSE score: Mild bvFTD (MMSE score, 21–26); and
moderate-to-severe bvFTD (MMSE score, 4–20).
The following five parameters, including subscale
scores, were evaluated as primary and secondary endpoints: General
condition (CDR); cognitive function [MMSE/Montreal Cognitive
Assessment (MoCA)]; neuropsychiatric behavior (NPI); emotional
state [Hamilton Depression Rating Scale (HAMD)]; and activities of
daily living (ADL). Diagnostic procedures included the evaluation
of medical history, psychiatric and neurological examination, and
laboratory screening. A confirmed diagnosis from two experienced
neurologists was required to conclude the diagnosis. Physical and
neurological examinations, in addition to routine laboratory
screening, were performed on the first hospital visit and every 4
weeks thereafter. Primary endpoint parameters included the
baseline-to-endpoint changes in the NPI Questionnaire (NPI-Q)
(24) and CDR; high scores indicate
greater impairment. Secondary endpoint assessments included the
following: Chinese version of the MoCA (25,26) and
MMSE (27,28) scores (range, 0–30 for both; high
scores indicate better cognitive function); ADL scale including two
major categories, Basic Activity of Daily Life (BADL) and
Instrumental Activity of Daily Life (IADL; high scores indicate
greater impairment) (29,30); and the HAMD (high scores indicate
greater impairment) (31).
Adverse effects
Patient safety was monitored at each hospital visit
by physical and neurological examination, the recording of adverse
events and performing routine laboratory analysis on the blood of
the patients. Adverse events were recorded by spontaneous reporting
from patients or their caregivers.
Ethical considerations
Each patient that participated in the present study
provided informed consent. At each study visit, a reliable
caregiver was present and supervised the administration of the
patients' medication. The present study was approved by the local
institutional ethical standards committee of Tianjin Huanhu
Hospital (Tianjin, China) on human experimentation.
Statistical analysis
Baseline demographic qualitative variables were
analyzed using the χ2 test, while normally distributed
quantitative variables were analyzed using independent two-sample
t-tests. The changes in outcome parameters between the baseline and
the six-month endpoints were analyzed using the
Wilcoxon-Mann-Whitney test. Statistical analyses were performed
using SPSS version 21.0 (IBM SPSS, Armonk, NY, USA). Values are
recorded as the mean ± standard error or mean ± standard deviation.
All tests were two-tailed, and P<0.05 was considered to indicate
a statistically significant difference.
Results
Patient enrollment and baseline
demographic data
A total of 42 patients were included in the study
and 41 completed the trial. The mild and moderate-to-severe bvFTD
groups comprised 20 and 22 patients, respectively. The two groups
were comparable with regard to age, gender, marital status, body
mass index, level of education, disease duration, accompanying
diseases and family history of dementia (Table I).
 | Table I.Baseline demographics and clinical
characteristics of mild, moderate-to-severe and combined behavioral
variant frontotemporal degeneration groups. |
Table I.
Baseline demographics and clinical
characteristics of mild, moderate-to-severe and combined behavioral
variant frontotemporal degeneration groups.
| Variable | Mild |
Moderate-to-severe | Total | P-value |
|---|
| Age,
yearsa | 67.40±1.88 | 68.05±1.83 | 67.74±1.30 | 0.807 |
| Gender,
male/female | 8/12 | 10/12 | 18/24 | 0.721 |
| Marriage, n
(%) | 16 3650.00) | 20 (90.91) | 36 (85.71) | 0.570 |
| Body mass index,
kg/m2 a | 23.45±1.04 | 21.82±0.78 | 22.60±0.65 | 0.212 |
| Education,
yearsa | 10.60±0.93 | 8.05±0.96 | 9.26±0.70 | 0.064 |
| Hypertension, n
(%) | 3 (15.00) | 9 (40.91) | 12 (28.57) | 0.130 |
| Diabetes mellitus,
n (%) | 2 (10.00) | 3 (13.64) | 5 (11.90) | 1.000 |
| Coronary heart
disease, n (%) | 2 (10.00) | 3 (13.64) | 5 (11.90) | 1.000 |
| Cerebral trauma, n
(%) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
|
| Smoking, n (%) | 5 (25.00) | 6 (27.27) | 11 (26.19) | 0.867 |
| Drinking, n
(%) | 3 (15.00) | 4 (18.18) | 7 (16.67) | 1.000 |
| Dementia family
history, n (%) | 0 (0) | 5 (22.73) | 5 (11.90) | 0.073 |
| Disease duration,
yearsa | 2.13±0.31 | 1.77±0.29 | 1.94±0.21 | 0.408 |
| Patient number
(completed 26 weeks) | 20 (20) | 22 (21b) | 42 (41b) |
|
Adverse events
No medically significant changes were observed
during the study with regard to routine laboratory parameters or
vital signs. In patients treated with memantine, the adverse events
with the highest frequency were constipation (6/42), somnolence
(6/42), mild headache (4/42) and dizziness (3/42). None of the
patients withdrew from the study due to the experiencing of side
effects.
Memantine treatment
Efficacy was determined by primary and secondary
outcome measurements of the patients with bvFTD. Primary endpoints
included the evaluation of CDR and NPI-Q between the baseline and
final hospital visit of the memantine-treated patients with bvFTD.
The mean CDR results at the final visit, in comparison with
baseline values at the initial visit, were 1.77±0.12 and
1.68±0.125, respectively; there was no significant difference
between the scores of the full cohort (Table II).
 | Table II.Analysis of primary and secondary
outcome measures between the baseline and final visit of patients
with behavioral variant frontotemporal degeneration receiving
memantine treatment. |
Table II.
Analysis of primary and secondary
outcome measures between the baseline and final visit of patients
with behavioral variant frontotemporal degeneration receiving
memantine treatment.
| Variable | Baseline | 6 Months | Z-value | P-value |
|---|
| Primary
endpoints |
|
| Clinic
dementia rating |
1.68±0.125 |
1.77±0.12 | −0.836 | 0.403 |
|
Neuropsychiatric Inventory
Questionnaire |
15.76±2.250 |
11.95±1.79 | −0.856 | 0.392 |
| Secondary
endpoints |
|
|
Neuropsychiatric Inventory
Caregiver Distress |
8.49±1.420 |
7.00±1.05 | −0.458 | 0.647 |
|
Mini-Mental State
Examination |
18.10±1.080 |
16.43±1.03 | −3.126 | 0.002a |
|
Montreal Cognitive Assessment,
Chinese version |
12.02±1.030 |
10.33±1.08 | −3.026 | 0.002a |
|
Activity of Daily Life |
30.79±1.680 |
35.07±1.96 | −3.878 | 0.001a |
| Basic
Activity of Daily Life |
13.55±0.640 |
14.86±0.83 | −3.181 | 0.001a |
|
Instrumental Activity of Daily
Living |
17.17±1.140 |
20.26±1.23 | −3.937 | 0.001a |
|
Hamilton Depression Rating
Scale |
9.05±0.890 |
8.88±0.82 | −0.197 | 0.844 |
Longitudinal NPI-Q data displayed an overall change
from baseline to 6 months of 3.81±2.18 points (Z=−0.856, P=0.392,
Table III). A detailed analysis of
the NPI-Q subscale scores revealed no significant changes in the
majority of subcategories such as delusions, hallucinations,
depression, anxiety, elation, apathy, disinhibition, irritability,
aberrant motor behavior (AMB), nighttime behavior and
appetite/eating. The subscale for agitation was an exception, which
was significantly different between the baseline and the final
visit (Z=−2.127, P=0.033; Table
III).
 | Table III.Detailed analysis of Neuropsychiatric
Inventory Questionnaire subscale scores between baseline and final
visit after 6 months for all patients with bvFTD receiving
memantine treatment. |
Table III.
Detailed analysis of Neuropsychiatric
Inventory Questionnaire subscale scores between baseline and final
visit after 6 months for all patients with bvFTD receiving
memantine treatment.
| Variable | Baseline | 6 Months | Z-value | P-value |
|---|
| Delusions |
0.63±0.190 |
0.59±0.19 | −0.419 | 0.675 |
| Hallucinations |
0.63±0.180 |
0.44±0.18 | −1.279 | 0.201 |
| Agitation |
1.98±0.390 |
1.34±0.29 | −2.127 | 0.033a |
| Depression |
1.24±0.380 |
0.71±0.22 | −1.638 | 0.101 |
| Anxiety |
0.68±0.190 |
0.73±0.20 | −0.241 | 0.809 |
| Elation |
0.24±0.110 |
0.34±0.17 | −0.447 | 0.655 |
| Apathy |
2.83±0.580 |
1.90±0.43 | −1.432 | 0.152 |
| Disinhibition |
1.24±0.450 |
0.71±0.20 | −0.717 | 0.473 |
| Irritability |
2.02±0.380 |
1.46±0.28 | −1.726 | 0.084 |
| Aberrant motor
behavior |
1.49±0.370 |
1.51±0.38 | 0.000 | 1.000 |
| Nighttime
behavior |
1.54±0.410 |
1.32±0.36 | −0.460 | 0.645 |
|
Appetite/eating |
1.22±0.370 |
0.85±0.26 | −1.011 | 0.312 |
| Total scores |
15.76±2.250 |
11.95±1.79 | −0.856 | 0.392 |
Of the secondary endpoints analyzed in patients with
bvFTD treated with memantine, no statistically significant
differences were detected between the total NPI Caregiver Distress
(NPI-D) and HAMD scores between the baseline and final visit
(Table II). By contrast,
significant reductions in the total score of the MMSE (Z=−3.126,
P=0.002) and MoCA, Chinese version (Z=−3.026, P=0.002) were
observed, and a significant increase was observed in ADL (Z=−3.878,
P=0.001), BADL (Z=−3.181, P=0.001) and IADL (Z=−3.937, P=0.001)
between the baseline and final visit. This indicates the presence
of cognitive and functional deterioration in patients with bvFTD
treated with memantine.
Subgroup analysis of patients with
mild (n=20) and moderate-to-severe (n=22) bvFTD
In order to determine whether the primary endpoints
were affected by disease severity, patients with mild (n=20) and
moderate-to-severe (n=22) bvFTD were analyzed separately (Table IV). Following 6 months of memantine
treatment, the subgroup of patients with moderate-to-severe bvFTD
exhibited significantly improved total NPI-Q scores (Z=−2.488,
P=0.013), and significant improvements in the subscales of
agitation (Z=−2.058, P=0.04), depression (Z=−2.511, P=0.012),
apathy (Z=−2.586, P=0.01) and disinhibition (Z=−2.047, P=0.041)
compared with those at baseline. By contrast, memantine caused no
significant changes in patients with mild bvFTD with regard to the
total NPI-Q score (14.50±2.82 baseline vs. 15.70±3.00 after 6
months, P=0.192) or individual subscale scores. After 6 months, the
comparison of NPI-Q scores between groups demonstrated that 5
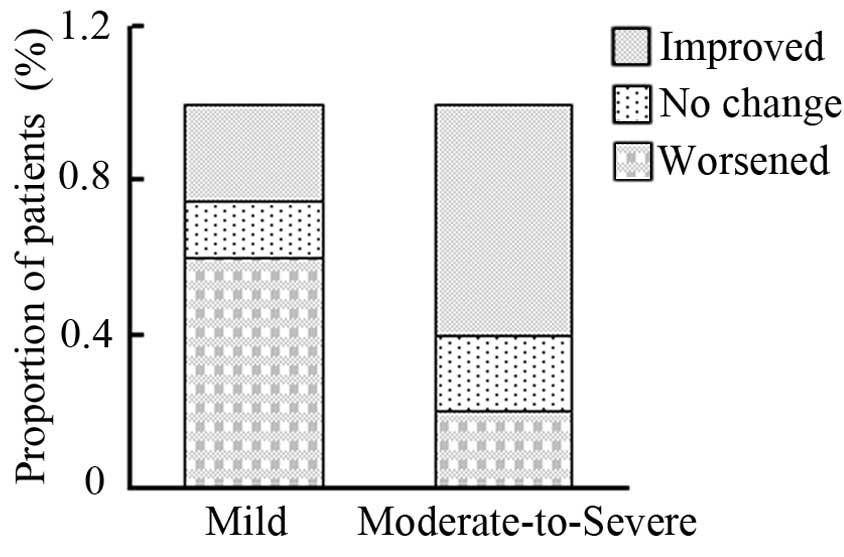
patients improved, 3 patients remained stable and 12 patients
worsened in the mild bvFTD group; whereas 12 patients improved, 4
patients remained stable and 4 patients worsened in the
moderate-to-severe bvFTD group (Table
V). The outcome proportions of the patients, on the basis of
improved, unchanged or worsened NPI-Q scores, was found to differ
significantly between the mild and moderate-to-severe bvFTD groups
(P=0.028; Fig. 1).
 | Table IV.Detailed analysis of Neuropsychiatric
Inventory Questionnaire neuropsychiatric subdomains between mild
and moderate-to-severe behavioral variant frontotemporal dementia
in patients receiving memantine treatment. |
Table IV.
Detailed analysis of Neuropsychiatric
Inventory Questionnaire neuropsychiatric subdomains between mild
and moderate-to-severe behavioral variant frontotemporal dementia
in patients receiving memantine treatment.
|
| Mild (n=20) | Moderate-to-severe
(n=20) |
|---|
|
|
|
|
|---|
| Variable | Baseline | 6 Months | P-value | Baseline | 6 Months | P-value |
|---|
| Delusions |
0.35±0.20 |
0.65±0.33 | 0.109 |
0.90±0.32 |
0.52±0.20 | 0.113 |
| Hallucinations |
0.45±0.25 |
0.55±0.34 | 0.414 |
0.81±0.27 |
0.33±0.17 | 0.068 |
| Agitation |
2.10±0.57 |
1.40±0.34 | 0.233 |
1.86±0.54 |
1.29±0.48 | 0.040a |
| Depression |
1.35±0.70 |
1.00±0.41 | 0.595 |
1.14±0.37 |
0.43±0.16 | 0.012a |
| Anxiety |
0.65±0.25 |
1.20±0.37 | 0.077 |
0.71±0.29 |
0.29±0.12 | 0.071 |
| Elation |
0.10±0.10 |
0.40±0.31 | 0.317 |
0.38±0.19 |
0.29±0.17 | 0.317 |
| Apathy |
2.50±0.69 |
3.15±0.77 | 0.305 |
3.14±0.93 |
0.71±0.23 | 0.010a |
| Disinhibition |
1.15±0.70 |
0.90±0.26 | 0.382 |
1.33±0.57 |
0.52±0.30 | 0.041a |
| Irritability |
2.00±0.55 |
1.95±0.49 | 0.862 |
2.05±0.55 |
1.00±0.24 | 0.073 |
| Aberrant motor
behavior |
1.50±0.61 |
1.80±0.63 | 0.180 |
1.48±0.43 |
1.24±0.44 | 0.354 |
| Nighttime
behavior |
1.60±0.52 |
1.80±0.65 | 0.684 |
1.48±0.64 |
0.86±0.33 | 0.498 |
|
Appetite/Eating |
0.75±0.33 |
0.90±0.39 | 0.655 |
1.67±0.65 |
0.81±0.34 | 0.173 |
| Total scores |
14.50±2.82 |
15.70±3.00 | 0.192 |
16.95±3.52 |
8.38±1.76 | 0.013a |
 | Table V.Changes in Neuropsychiatric Inventory
Questionnaire scores in memantine-treated patients with mild and
moderate-to-severe behavioral variant frontotemporal dementia. |
Table V.
Changes in Neuropsychiatric Inventory
Questionnaire scores in memantine-treated patients with mild and
moderate-to-severe behavioral variant frontotemporal dementia.
| Observation | Milda,b |
Moderate-to-Severea,b |
|---|
| Improved | 5 | 12 |
| No change | 3 | 4 |
| Worsened | 12 | 4 |
Discussion
The present study compared the changes in cognitive
function, and neuropsychiatric and behavioral symptoms, in patients
with mild and moderate-to-severe bvFTD using standardized tests
after 6 months of memantine treatment. Analysis of the combined
cohort of 42 patients with bvFTD demonstrated that cognitive
performance worsened, indicated by changes in MoCA and MMSE scores,
and ability to perform activities in daily living deteriorated,
indicated by changes in ADL, during the 6-month period of memantine
treatment. Although memantine had no beneficial effects on the
total or subscale NPI-Q scores of the combined cohort of 42
patients with bvFTD, the moderate-to-severe patient subgroup
performed significantly better than the mild patient group in their
response to memantine treatment. This was demonstrated by changes
to the total NPI-Q score, and subscale scores for agitation,
depression, apathy and disinhibition. Memantine appeared to be safe
and well-tolerated, and no patient withdrew from the trial as a
result of adverse reactions.
FTLD is a clinically, anatomically and
histopathologically heterogeneous disorder (3), making it difficult to design a
therapeutic trial that could accommodate all possible therapeutic
outcome measures. This, therefore, creates particular challenges
for determining an optimal management model with a single
pharmacological agent. Although memantine is an FDA-approved AD
medication, it is not indicated for FTLD treatment. Furthermore, a
recent randomized controlled trial discouraged the prescription of
memantine to FTLD patients (16,17).
Despite this, the evidence-based clinical practices guidelines,
clinical experience indicates that a high percentage of patients
with FTLD are treated with AChEI, memantine or both. A
cross-sectional design using 1,092 cases with AD and 64 cases with
FTLD were registered by the Registry of Dementias of Girona.
Memantine was used by 17.2% and 10.9% of patients with AD and FTLD,
respectively. There is a discrepancy regarding clinical practice
and the recommendations based upon clinical evidence (32).
The aggregate analysis of neuropsychological,
cognitive and functional performance across a cohort of patients
with bvFTD in the present study indicated that treatment with
memantine worsened a patient's cognitive function and ability to
perform activities in daily living, and this is consistent with a
previously reported randomized controlled clinical trial (16). Notably, each enrolled subject in the
present study had significant neuropsychiatric disturbances, as
assessed by NPI-Q and NPI-D scores. The high prevalence of
neuropsychiatric symptoms in patients with bvFTD has been
previously reported in other community-based studies (33,34).
Patients with moderate-to-severe bvFTD had a higher NPI-Q total
score than patients with mild bvFTD. In the current study, it was
observed that behavioral disturbances improved markedly in patients
with moderate-to-severe bvFTD, in comparison with patients with
mild bvFTD, after 6 months of memantine treatment. Detailed
analysis of behavioral subscales demonstrated that the most notable
improvements were observed in agitation, depression, apathy and
disinhibition. Despite the relatively small sample size included in
the present study, this is a potentially important finding.
Different responses to memantine treatment in
patients with mild and moderate-to-severe bvFTD may be attributed
to the heterogeneous profile of preserved and/or impaired brain
regions, or selective functional systems in different stages of the
disease. Neuropathological and imaging studies of early FTLD
suggest that the disease begins in the anterior cingulate cortex
(ACC) and frontoinsula (FI) before spreading throughout a
circumscribed network of brain regions (35,36). ACC
and FI are the exclusive location of the von Economo neuron (VEN)
that is specifically and selectively attacked in the early stages
of bvFTD; these are implicated in several neuropsychiatric
illnesses, in particular in disturbances of empathy, social
awareness and self-control (37,38). In
addition, the VEN somatodendritic compartment appears to primarily
express dopamine (D3), serotonin (5HT-1b, 2b) and vasopressin (1a)
receptors, but does not respond to glutamic acid (39). VEN damage in early-stage bvFTD may
explain why selective serotonin reuptake inhibitors, such as
paroxetine (40) or
5-hydroxytryptamine 2A (5-HT2A) antagonists (41), but not NMDA receptor antagonists,
have modest benefits in improving the behavioral symptoms of
patients with mild FTLD.
In more advanced stages of bvFTD, the loss of
frontal and temporal neurons becomes more severe with greater deep
layer involvement as the disease spreads throughout the white
matter to more posterior regions and the subcortex (42). Moreover, presynaptic proteins such as
α-synuclein and neurofilament proteins would be lost during severe
cases of bvFTD; these two proteins are relatively preserved in a
number of mild cases of bvFTD (43).
A previous study demonstrated that memantine treatment can
stimulate dendritic spine maturation and promote excitatory synapse
formation, restoring excitatory synapses to a normal range in mice
with fragile X syndrome (44). Based
on these findings, it can be suggested that memantine exerts its
therapeutic capacity by alleviating behavioral disturbances through
promoting synapse formation and modulating glutamate neuronal
excitability in later stages of bvFTD.
Chow et al demonstrated using
18F-FDG PET analysis that patients with mild FTLD (mean
CDR score, 1.35) who were treated with memantine displayed
increased cortical metabolic activities in the frontal and temporal
brain regions, and in the salience network hubs, although no
improvements in clinical symptoms were detected (14,15).
This suggests that the curative effect of memantine is too weak to
be detected at a heterogeneous clinical symptom level; however, the
results demonstrated that memantine induces metabolic changes in
patients with early stage FTLD. It remains to be tested whether
pharmaceutical effects on cortical metabolic activities would lead
to clinical improvements as the disease progresses from mild to
moderate-to-severe stage bvFTD.
In conclusion, to the best of our knowledge, the
present study is the first to evaluate the effect of memantine on
detailed longitudinal changes assessed by behavioral, cognitive and
functional scales in patients with mild and moderate-to-severe
bvFTD. The results demonstrate that memantine improves
neuropsychiatric symptoms observed in patients with
moderate-to-severe bvFTD, in particular the symptoms of agitation,
depression, apathy and disinhibition. However, the current data
must be interpreted carefully due to the relatively small number of
patients with FTLD involved in the study. In the future,
larger-scale, randomized, controlled studies with hierarchical
design for subtype and severity of dementia are required in order
to further evaluate the efficacy of memantine in patients with
moderate-to-severe bvFTD.
Acknowledgements
The present study was supported by grants from the
Tianjin Health Bureau of Science and Technology Research Foundation
(no. 2013KG121), the Tianjin Science and Technology Plan Foundation
(no. 13ZCZDSY01600), the Special Fund of National Clinical and
Medical Research (no. L2014071) and the Youth Fund of National
Nature Science Foundation of China (no. 81301629).
References
|
1
|
Baborie A, Griffiths TD, Jaros E, McKeith
IG, Burn DJ, Richardson A, Ferrari R, Moreno J, Momeni P, Duplessis
D, et al: Pathological correlates of frontotemporal lobar
degeneration in the elderly. Acta Neuropathol. 121:365–371. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Seilhean D, Bielle F, Plu I and Duyckaerts
C: Frontotemporal lobar degeneration: Diversity of FTLD lesions.
Rev Neurol (Paris). 169:786–792. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shinagawa S: Phenotypic variety in the
presentation of frontotemporal lobar degeneration. Int Rev
Psychiatry. 25:138–144. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kaye ED, Petrovic-Poljak A, Verhoeff NP
and Freedman M: Frontotemporal dementia and pharmacologic
interventions. J Neuropsychiatry Clin Neurosci. 22:19–29. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Danysz W and Parsons CG: Alzheimer's
disease, β-amyloid, glutamate, NMDA receptors and memantine -
searching for the connections. Br J Pharmacol. 167:324–352. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kalia LV, Kalia SK and Salter MW: NMDA
receptors in clinical neurology: Excitatory times ahead. Lancet
Neurol. 7:742–755. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cummings JL, Schneider E, Tariot PN and
Graham SM: Memantine MEM-MD-SG-02 Study group: Behavioral effects
of memantine in Alzheimer disease patients receiving donepezil
treatment. Neurology. 67:57–63. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peng D, Yuan X and Zhu R: Memantine
hydrochloride in the treatment of dementia subtypes. J Clin
Neurosci. 20:1482–1485. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Stubendorff K, Larsson V, Ballard C,
Minthon L, Aarsland D and Londos E: Treatment effect of memantine
on survival in dementia with Lewy bodies and Parkinson's disease
with dementia: A prospective study. BMJ Open. 4:e0051582014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lipton SA: Paradigm shift in
neuroprotection by NMDA receptor blockade: Memantine and beyond.
Nat Rev Drug Discov. 5:160–170. 2006. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Swanberg MM: Memantine for behavioral
disturbances in frontotemporal dementia: A case series. Alzheimer
Dis Assoc Disord. 21:164–166. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Boxer AL, Lipton AM, Womack K, Merrilees
J, Neuhaus J, Pavlic D, Gandhi A, Red D, Martin-Cook K, Svetlik D
and Miller BL: An open-label study of memantine treatment in 3
subtypes of frontotemporal lobar degeneration. Alzheimer Dis Assoc
Disord. 23:211–217. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Links KA, Black SE, Graff-Guerrero A,
Wilson AA, Houle S, Pollock BG and Chow TW: A case of apathy due to
frontotemporal dementia responsive to memantine. Neurocase.
19:256–261. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chow TW, Graff-Guerrero A, Verhoeff NP,
Binns MA, Tang-Wai DF, Freedman M, Masellis M, Black SE, Wilson AA,
Houle S and Pollock BG: Open-label study of the short-term effects
of memantine on FDG-PET in frontotemporal dementia. Neuropsychiatr
Dis Treat. 7:415–424. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chow TW, Fam D, Graff-Guerrero A, Verhoeff
NP, Tang-Wai DF, Masellis M, Black SE, Wilson AA, Houle S and
Pollock BG: Fluorodeoxyglucose positron emission tomography in
semantic dementia after 6 months of memantine: An open-label pilot
study. Int J Geriatr Psychiatry. 28:319–325. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Boxer AL, Knopman DS, Kaufer DI, Grossman
M, Onyike C, Graf-Radford N, Mendez M, Kerwin D, Lerner A, Wu CK,
et al: Memantine in patients with frontotemporal lobar
degeneration: A multicentre, randomised, double-blind,
placebo-controlled trial. Lancet Neurol. 12:149–156. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodges JR: Hope abandoned: Memantine
therapy in frontotemporal dementia. Lancet Neurol. 12:121–123.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bird T, Knopman D, VanSwieten J, Rosso S,
Feldman H, Tanabe H, Graff-Raford N, Geschwind D, Verpillat P and
Hutton M: Epidemiology and genetics of frontotemporal
dementia/Pick's disease. Ann Neurol. 54(Suppl 5): S29–S31. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rascovsky K, Salmon DP, Lipton AM,
Leverenz JB, DeCarli C, Jagust WJ, Clark CM, Mendez MF, Tang-Wai
DF, Graff-Radford NR and Galasko D: Rate of progression differs in
frontotemporal dementia and Alzheimer disease. Neurology.
65:397–403. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boxer AL, Gold M, Huey E, Hu WT, Rosen H,
Kramer J, Gao FB, Burton EA, Chow T, Kao A, Leavitt BR, et al: The
advantages of frontotemporal degeneration drug development (part 2
of frontotemporal degeneration: the next therapeutic frontier).
Alzheimers Dement. Oct 10–2012.(Epub ahead of print)
doi:10.1016/j.jalz.2012.03.003. PubMed/NCBI
|
|
21
|
Riedl L, Mackenzie IR, Förstl H, Kurz A
and Diehl-Schmid J: Frontotemporal lobar degeneration: current
perspectives. Neuropsychiatr Dis Treat. Feb 13–2014.(Epub ahead of
print) doi:10.2147/NDT.S38706. PubMed/NCBI
|
|
22
|
Neary D, Snowden JS, Gustafson L, Passant
U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M, et
al: Frontotemporal lobar degeneration: A consensus on clinical
diagnostic criteria. Neurology. 51:1546–1554. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rascovsky K, Hodges JR, Knopman D, Mendez
MF, Kramer JH, Neuhaus J, van Swieten JC, Seelaar H, Dopper EG,
Onyike CU, et al: Sensitivity of revised diagnostic criteria for
the behavioural variant of frontotemporal dementia. Brain. 134(Pt
9): 2456–2477. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang T, Xiao S, Li X, Wang H, Liu Y, Su N
and Fang Y: Reliability and validity of the Chinese version of the
neuropsychiatric inventory in mainland China. Int J Geriatr
Psychiatry. 27:539–544. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Freitas S, Simões MR, Alves L, Duro D and
Santana I: Montreal Cognitive Assessment (MoCA): Validation study
for frontotemporal dementia. J Geriatr Psychiatry Neurol.
25:146–154. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hu JB, Zhou WH, Hu SH, Huang ML, Wei N, Qi
HL, Huang JW and Xu Y: Cross-cultural difference and validation of
the Chinese version of Montreal Cognitive Assessment in older
adults residing in Eastern China: Preliminary findings. Arch
Gerontol Geriatr. 56:38–43. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Dong Y, Yean Lee W, Hilal S, Saini M, Wong
TY, Chen CL, Venketasubramanian N and Ikram MK: Comparison of the
Montreal Cognitive Assessment and the Mini-Mental State Examination
in detecting multi-domain mild cognitive impairment in a Chinese
sub-sample drawn from a population-based study. Int Psychogeriatr.
25:1831–1838. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Katzman R, Zhang MY, Ouang-Ya-Qu, Wang ZY,
Liu WT, Yu E, Wong SC, Salmon DP and Grant I: A Chinese version of
the Mini-Mental State Examination; impact of illiteracy in a
Shanghai dementia survey. J Clin Epidemiol. 41:971–978. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang D, Zheng J, Kurosawa M, Inaba Y and
Kato N: Changes in activities of daily living (ADL) among elderly
Chinese by marital status, living arrangement, and availability of
healthcare over a 3-year period. Environ Health Prev Med.
14:128–141. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhang M, Zhu Z and Chen P: Community
investigation of the activities of daily living (ADL) and medical
conditions of the elderly in Shanghai. Zhonghua Yi Xue Za Zhi.
78:124–127. 1998.(In Chinese). PubMed/NCBI
|
|
31
|
Zheng YP, Zhao JP, Phillips M, Liu JB, Cai
MF, Sun SQ and Huang MF: Validity and reliability of the Chinese
Hamilton Depression Rating Scale. Br J Psychiatry. 152:660–664.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López-Pousa S, Calvó-Perxas L, Lejarreta
S, Cullell M, Meléndez R, Hernández E, Bisbe J, Perkal H, Manzano
A, Roig AM, Turró-Garriga O, Vilalta-Franch J and Garre-Olmo J:
Registry of Dementias of Girona Study Group (ReDeGi Study Group):
Use of antidementia drugs in frontotemporal lobar degeneration. Am
J Alzheimers Dis Other Demen. 27:260–266. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Srikanth S, Nagaraja AV and Ratnavalli E:
Neuropsychiatric symptoms in dementia-frequency, relationship to
dementia severity and comparison in Alzheimer's disease, vascular
dementia and frontotemporal dementia. J Neurol Sci. 236:43–48.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Quaranta D, Marra C, Rossi C, Gainotti G
and Masullo C: Different apathy profile in behavioral variant of
frontotemporal dementia and Alzheimer's disease: A preliminary
investigation. Curr Gerontol Geriatr Res. 2012:7192502012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Broe M, Hodges JR, Schofield E, Shepherd
CE, Kril JJ and Halliday GM: Staging disease severity in
pathologically confirmed cases of frontotemporal dementia.
Neurology. 60:1005–1011. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Seeley WW: Selective functional, regional,
and neuronal vulnerability in frontotemporal dementia. Curr Opin
Neurol. 21:701–707. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Allman JM, Tetreault NA, Hakeem AY, Manaye
KF, Semendeferi K, Erwin JM, Park S, Goubert V and Hof PR: The von
Economo neurons in frontoinsular and anterior cingulate cortex in
great apes and humans. Brain Struct Funct. 214:495–517. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim EJ, Sidhu M, Gaus SE, Huang EJ, Hof
PR, Miller BL, DeArmond SJ and Seeley WW: Selective frontoinsular
von Economo neuron and fork cell loss in early behavioral variant
frontotemporal dementia. Cereb Cortex. 22:251–259. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Allman JM, Watson KK, Tetreault NA and
Hakeem AY: Intuition and autism: A possible role for Von Economo
neurons. Trends Cogn Sci. 9:367–373. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Moretti R, Torre P, Antonello RM, Cazzato
G and Bava A: Frontotemporal dementia: Paroxetine as a possible
treatment of behavior symptoms. A randomized, controlled, open
14-month study. Eur Neurol. 49:13–19. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lebert F, Stekke W, Hasenbroekx C and
Pasquier F: Frontotemporal dementia: A randomised, controlled trial
with trazodone. Dement Geriatr Cogn Disord. 17:355–359. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kril JJ, Macdonald V, Patel S, Png F and
Halliday GM: Distribution of brain atrophy in behavioral variant
frontotemporal dementia. J Neurol Sci. 232:83–90. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Taniguchi S, McDonagh AM, Pickering-Brown
SM, Umeda Y, Iwatsubo T, Hasegawa M and Mann DM: The neuropathology
of frontotemporal lobar degeneration with respect to the
cytological and biochemical characteristics of tau protein.
Neuropathol Appl Neurobiol. 30:1–18. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wei H, Dobkin C, Sheikh AM, Malik M, Brown
WT and Li X: The therapeutic effect of memantine through the
stimulation of synapse formation and dendritic spine maturation in
autism and fragile X syndrome. PLoS One. 7:e369812012. View Article : Google Scholar : PubMed/NCBI
|