Introduction
Large-area cutaneous and subcutaneous tissue defects
caused by surgeries for trauma or tumor are often refractory to
repair and induce a high disability rate, seriously impairing the
physical and sociopsychological wellbeing of patients. The repair
of these defects usually requires the microsurgical transfer of
skin flaps. In 1985 and 1987, Luo (1) and Chen (2) reported the use of a free anterolateral
thigh flap (FALTF) with a lateral femoral circumflex artery (LFCA)
pedicle in the repair of large-area soft tissue defects. This type
of flap has been frequently used in head and neck reconstruction
(3,4). The descending branch of the LFCA is the
most common blood supply of the anterolateral thigh (ALT). The
musculocutaneous or intermuscular perforator arteries of the LFCA
descending branch primarily nourish the ALT skin; however, these
perforator arteries exhibit small orifices and high
inter-individual variability, leading to challenges in the
preoperative localization of these vessels, and subsequently
complicating FALTF dissection and harvest (5). Accurate preoperative assessment of ALT
perforating vessels therefore plays a critical role in a successful
FALTF transfer, as this enables surgeons to understand the pattern,
intermuscular orientation and convergence of perforating
vessels.
Advances in medical imaging technologies allow the
visualization of LFCA perforating vessels in a three-dimensional
(3D) manner. Multiple techniques, such as digital subtraction
angiography (DSA), computed tomography angiography (CTA) and
magnetic resonance angiography (MRA) (6), have been used to localize perforating
vessels for the purpose of flap harvest and transfer. Among these
techniques, CTA is considered to be the mainstay technique for the
preoperative assessment of FALTF transfer, according to the current
literature. Previous studies have shown that the use of CTA in
assisting flap outlining decreased the risk of complications and
increased the repair success rate (6–8).
Contrast-enhanced 3D MRA (3D-CE-MRA) is a highly accurate, specific
imaging tool for the diagnosis of arterial disorders (9), although it has rarely been used in
FALTF transfer for repairing large-area soft tissue defects
(6). The present study aimed to
retrospectively investigate the effectiveness of 3D-CE-MRA for
visualizing the 3D anatomy of the LFCA and its perforating vessels
in 68 patients scheduled for elective FALTF transfer.
Patients and methods
Patients
The study protocol was approved by the Institutional
Review Board of Jinhua Municipal Central Hospital (Jinhua, China).
Sixty-eight patients scheduled for elective FALTF transfer were
consecutively referred to the imaging center for 3D-CE-MRA of the
bilateral lower limbs between September 2009 and November 2010.
This retrospective patient cohort comprised 53 males and 15
females, aged 27–91 years (mean, 65 years). A wide range of
vascular tree imaging analyses were conducted on the lower limb
extremities of 50 patients. Among these, nine cases of vascular
tree imaging were only taken from the iliac artery to the knee
arteries, due to calf vein pollution. This facilitated surgical
evaluation. The indications for 3D-CE-MRA were as follows: i)
Having no known lower limb vascular disease; and ii) being
scheduled for FALTF transfer of which 10 patients later underwent
FALTF surgery. FALTF transfer is a flap surgical procedure used to
repair large areas of skin and tissue defects. The
contraindications were i) lower limb arteriosclerosis obliterans;
ii) renal insufficiency; and iii) ineligibility for MR imaging
(MRI). Among these patients, 50 underwent large-scale angiography
of the entire bilateral lower limbs, 9 patients underwent
angiography from the common iliac artery to the superior genicular
artery only, due to calf venous contamination, and 9 patients
underwent angiography from the external iliac artery to the
superior genicular artery only. All patients gave informed written
consent prior to participation in this study.
3D-CE-MRA
A Magnetom Avanto 1.5 T MRI system (Siemens Medical
Solutions, Erlangen, Germany), equipped with a total image matrix
peripheral vascular MRI coil and an integrated flexible coil, was
used for 3D-CE-MRA at a switching rate of 200 mT/msec and a
gradient field of 45 mT/m. The bilateral lower limbs were scanned
from the common iliac artery to the superior genicular artery or
from the renal artery to the dorsalis pedis artery, using a fast
low-angle shot gradient-echo sequence. An angiocatheter (Linhua
Medical Devices Co. Ltd., Suzhou, China) was inserted into the
antecubital vein, and a 30-ml gadolinium-diethylene triamine
penta-acetic acid bolus (0.2–0.3 mmol/kg; Beilu Pharmaceutical Co.
Ltd., Beijing, China) was injected at a rate of 2.5–4.0 ml/sec
using a high-pressure syringe pump (Ulrich Medical, Ulm, Germany),
followed by rinsing with normal saline. LFCA MRA was started at a
single-sequence time of 16 sec, immediately following the peak time
of the common or internal/external iliac artery MRA on real-time
Siemens Care Bolus sequence (Siemens Medical Solutions) tracking,
at a repetition time (TR) of 32.56 msec, echo time (TE) of 1.19
msec and a slice thickness of 40 mm. Angiograms were captured in
three segments using an auto-shifting bed technique to
automatically reconstruct the entire angiogram of the vascular tree
of the bilateral lower limb. The post-dosing FLASH 3D-core
post-angiogram was subtracted from the pre-dosing FLASH 3D-core
pre-angiogram to reconstruct the LFCA angiogram using the following
parameters: TR, 2.94 msec; TE, 1 msec; flip angle, 25°; slices per
slab, 88; slice thickness, 1.40 mm; distraction factor, 20%; and
fat suppression, none.
Image processing and data
analysis
All captured image data were transmitted to a Syngo
B17.0 3D image analysis workstation (Siemens Medical Solutions),
and the arteries of the bilateral thighs were reconstructed in 3D
using the maximum intensity projection (MIP), incorporating
thin-slab MIP (TS-MIP), following the digital subtraction. TS-MIP
is a technique used to determine the thickness of an MIP layer.
Non-subtractive reconstruction was concomitantly used to visualize
muscular and subcutaneous soft tissues.
Two board-certified radiovascular surgeons and two
board-certified plastic surgeons were assigned to review the LFCA
3D-CE-MRA images in a double-blind manner to evaluate the origin
pattern of the LFCA and the distribution, orientation and
convergence of perforating vessels, as well as the length of the
vascular pedicle. Anatomical patterns of the LFCA were classified
into four types, as previously described (10,11):
Type Ia, the LFCA originates from the superolateral part of the
deep femoral artery (DFA) and trifurcates into the ascending,
transverse and descending branches constantly; type Ib, the LFCA
originates variably from the femoral artery (FA) but trifurcates
into the ascending, transverse and descending branches constantly;
type II, two trunks of the LFCA ascending, transverse and
descending branches originate from the DFA or the FA; type III, the
LFCA ascending, transverse and descending branches originate from
the DFA or the FA, respectively; and type IV, one of the three LFCA
branches is absent, most commonly the transverse branch (Table I).
 | Table I.Anatomical classification of the LFCA
(n=136). |
Table I.
Anatomical classification of the LFCA
(n=136).
| Type | n (%) | Definition |
|---|
| I | 101 (74.3) |
|
| Ia | 80
(58.8) | The LFCA originated
from the superolateral part of the DFA and trifurcated into the
ascending, transverse and descending branches constantly. |
| Ib | 21
(15.4) | The LFCA originated
variably from the FA but trifurcated into the ascending, transverse
and descending branches constantly. |
| II | 20
(14.7) | Two trunks of the
LFCA ascending, transverse and descending branches originated from
the DFA or the FA. |
| III | 3
(2.2) | The LFCA ascending,
transverse and descending branches originated from the DFA or the
FA, respectively. |
| IV | 12 (8.8) | One of the three LFCA
branches was absent, most commonly the transverse branch. |
Results
Origin pattern, trifurcation and
perforating branches of LFCAs
As shown in Table I,
the LFCAs of the bilateral lower limbs were well visualized by
3D-CE-MRA in all 68 patients. The anatomical pattern of the LFCA
was type Ia in 80 cases (58.8%), type Ib in 21 cases (15.4%), type
II in 20 cases (14.7%), type III in 3 cases (2.2%) and type IV in
12 cases (8.8%). Tertiary vascular trees (primary, main trunk of
LFCA; secondary, ascending, transverse and descending branches;
tertiary, muscular perforating vessels) were identified in 94 limbs
(69.1%). The intermuscular orientation of the perforating vessels
was also clearly shown with MRA. The length of the descending
branch vascular pedicle (the linear distance from the origin of the
branch to the distal end) ranged from 41 to 323 mm. As shown in
Fig. 1A and B, the left LFCA (type
Ia) originated from the left DFA and subsequently trifurcated into
the ascending, transverse and descending branches. As shown in
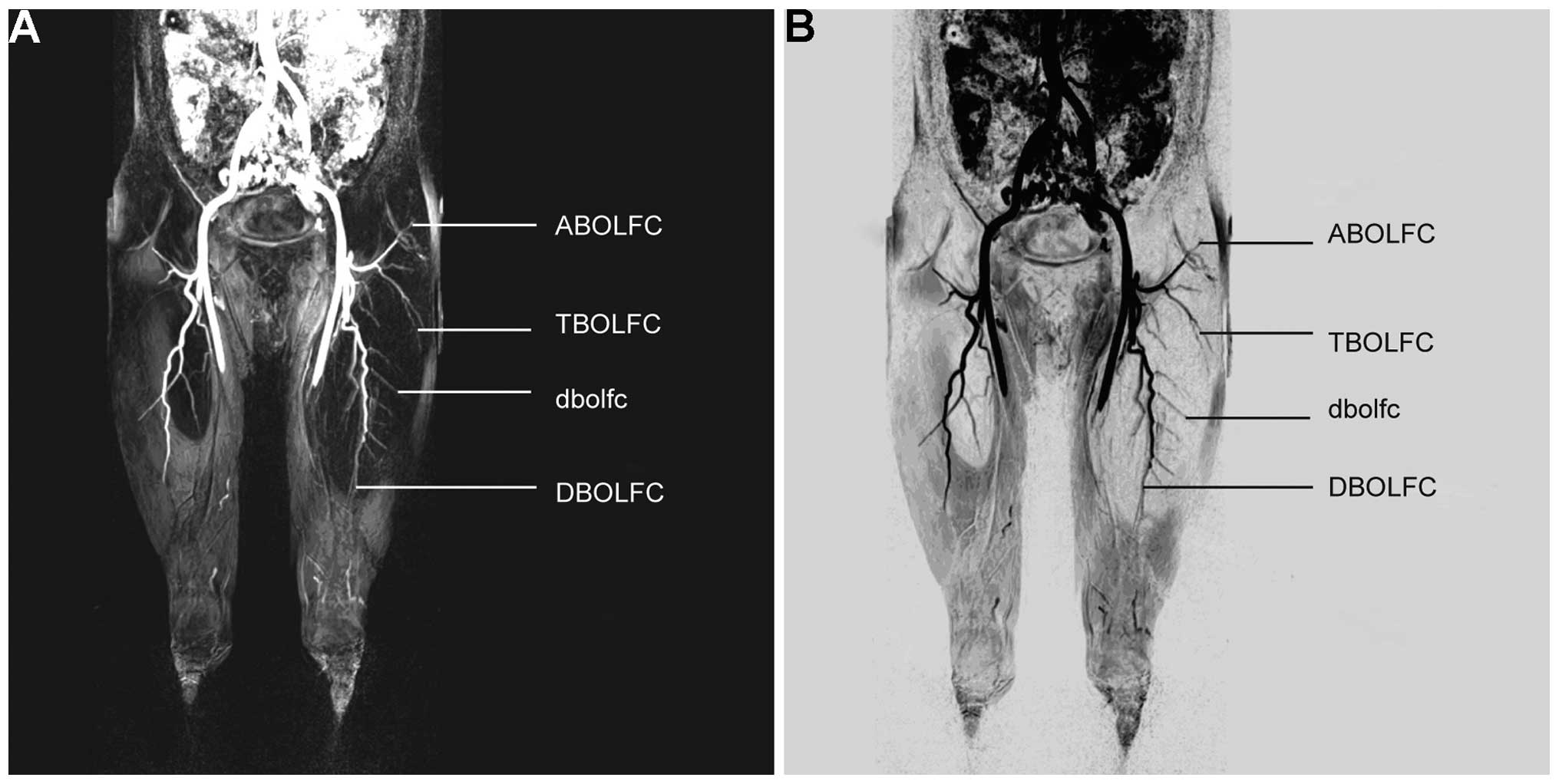
Fig. 2A and B, the right LFCA (type
Ib) originated variably from the right DFA and subsequently
trifurcated into the ascending, transverse and descending branches.
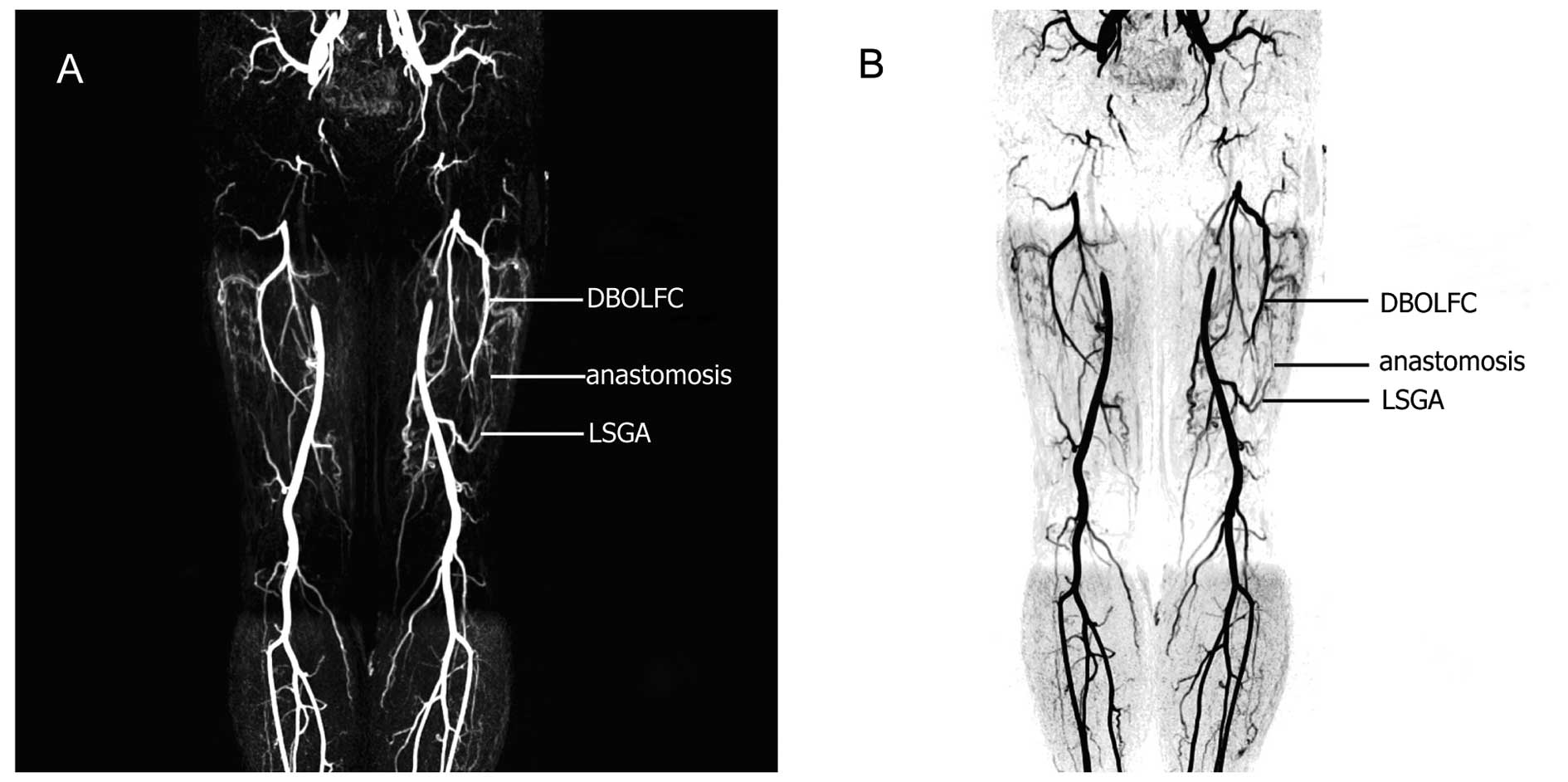
As shown in Fig. 3A and B, the
descending branches of the bilateral femoral circumflex arteries
converged with the ascending branch of the bilateral superior
genicular arteries. Type Ib LFCA, i.e. an LFCA originating variably
from the FA, was identified in 21 cases. Among these cases, TS-MIP
reconstruction of the opening portion of femoral artery revealed
the specific origin of the LFCA to be from the anterolateral part
of the FA in 10 cases and from the posterolateral part of the FA in
11 cases, and another from the inferior part of the DFA in 19
cases.
LFCA perforating branches and flap
outline
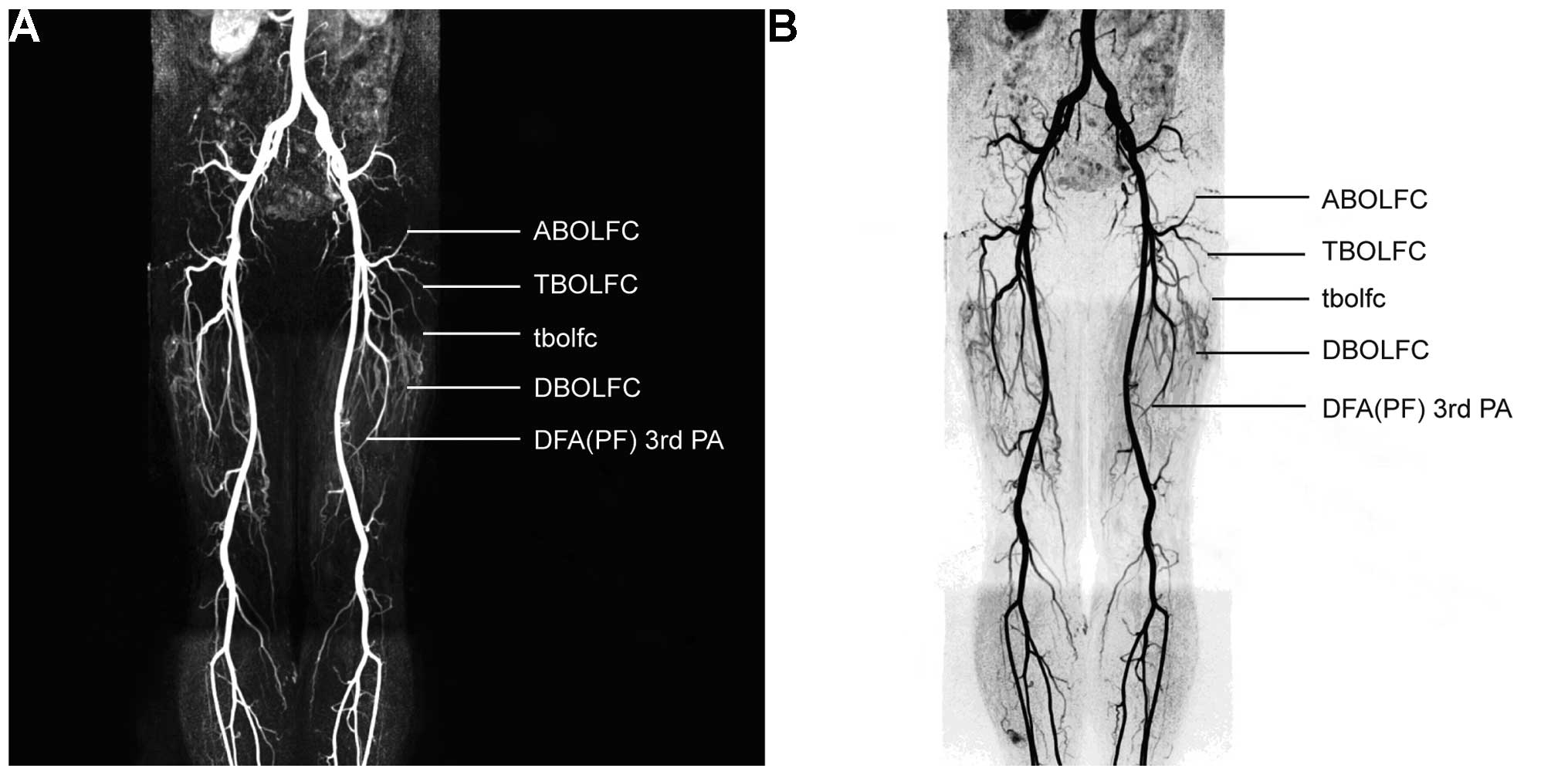
All three-tier LFCA branches were well visualized in
subtractive 3D-MRA. Thick-slab MIP revealed the distribution and
spatial pattern of the vessels, and TS-MIP ignored unrelated data
to better visualize the target anatomy, namely the LFCA and its
secondary and tertiary branches (Fig. 4A
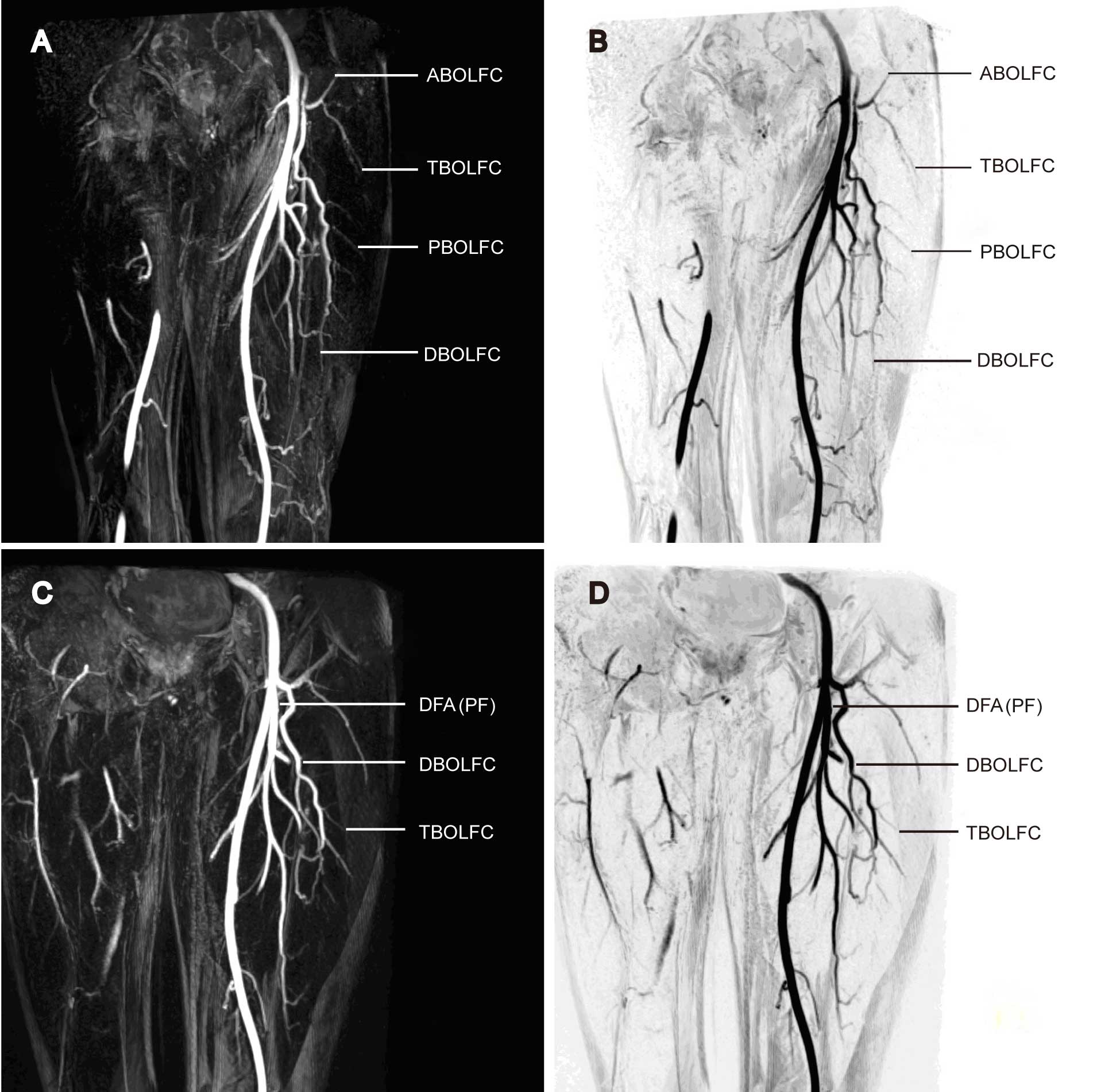
and B). The descending branch of the right LFCA was found to
trifurcate into three parallel perforating arteries (Fig. 5A and B). The space between the
terminal perforating arteries and the skin was clearly visualized
to help in the decision of the surgical approach and the evaluation
of flap outlining. The proposed flap was therefore primarily
supplied by the LFCA descending branch. By contrast, the left LFCA
descending branch extended into a single artery, without visible
perforating arteries (Fig. 5C and
D). The LFCA transverse branch was shown to be proximal to the
skin, and its caliber could be precisely evaluated using MRA. For
each patient, flap size was adjusted based on the surface area of
limb cutaneous and subcutaneous tissue defects. According to the
preoperative MRA results, the flaps were designed to encompass the
center of the blood vessel (perforating artery pedicle), and
typically 5–8 cm diameter flaps were appropriate.
Case reports
FALTF transfer was performed in 4 patients to cover
the skin and soft tissue defects in the feet/pedal dorsum (n=3) or
hand dorsum (n=1) (Fig. 6A–D). The
preoperative evaluation results of the limb soft tissue defects are
shown in Table II. CE-MRA with
TS-MIP was used to outline the flap based on the distance of the
perforating vessels to the skin, the calibers of the perforating
vessels and the flap blood supply. Preoperative CE-MRA assessment
offered an accurate localization of the perforating artery
pedicles, which originated from the LFCA descending branch in 3
patients and from the LFCA transverse branch in 1 patient. The
intraoperative measurements were identical to the preoperative MRA
results in terms of the origin, localization, caliber and
distribution pattern of the perforating vessels. The vascular
caliber was adequate for flap transfer, and the flap size was
appropriate for covering the wound. All patients underwent an
uneventful flap transfer, without missing any perforating vessels
or requiring additional flap outlining. All flaps were well
perfused and survived the transfer. All wounds healed by primary
intention, and no surgical site infection or wound dehiscence
occurred.
 | Table II.Clinical results of patients who
underwent free anterolateral thigh flap transfer (n=4). |
Table II.
Clinical results of patients who
underwent free anterolateral thigh flap transfer (n=4).
| Case | Age
(years)/gender | Wound
localization | Origin of flap
perforating artery | Flap harvested | Flap transfer
outcome |
|---|
| 1 | 62/M | Right foot skin
defect | Right anterolateral
thigh, DBOLFCA | 14×8.0-cm
musculocutaneous | Survived |
| 2 | 59/M | Right lateral
malleolus skin defect | Right anterolateral
thigh, DBOLFCA | 12×8.0-cm
cutaneous | Survived |
| 3 | 40/M | Right hand dorsum
skin | Right anterolateral
thigh, TBOLFCA | 14×8.0-cm
cutaneous | Survived |
| 4 | 57/M | Left foot dorsum skin
defect | Left anterolateral
thigh, DBOLFCA | 15×8.5-cm
cutaneous | Survived |
Discussion
Normally, the LFCA trifurcates into three branches:
Ascending, transverse and descending. The ascending branch runs in
the tensor fasciae latae muscle and the anterolateral part of the
iliac crest; the transverse branch advances into the anterolateral
part of the greater trochanter; and the descending branch supplies
the lateral femoral muscles and the ALT skin. The soft tissues
nourished by the three LFCA branches are frequently harvested as
flaps for repairing large-area soft tissue defects (3,4). The use
of a FALTF with a high-level cutaneous perforating artery pedicle
has been reported in previous studies (8,12,13).
This flap preserves the subcutaneous adipose tissue for covering
soft tissue defects; however, the revision of subcutaneous adipose
tissue converts the flap into a thin flap with a dermal vascular
network. This type of flap has been widely used, therefore, in the
repair and reconstruction of the hand, head and face (8,14,15).
Successful flap transfer requires an accurate
preoperative assessment of the perforating vessels and their
convergence. Thus, preoperative outlining of FALTF depends on the
anatomical investigation of the LFCA perforating arteries.
Currently, Doppler ultrasonography using the ABC method and CE
Doppler ultrasound angiography are typically used by orthoplastic
surgeons for preoperative body surface marking (14). These methods, however, only lead to
an empirical decision of access approaches, and the proposed flaps
are temporarily outlined in accordance with the intraoperative
finding of vascular localization. CTA is used as one of the
mainstay techniques for the preoperative assessment of ALT flaps,
and has shown favorable results in current practice (15). In the present study, 3D-CE-MRA
offered a direct, accurate visualization of the vascular anatomy
without any clinically significant adverse effects, and
consequently enabled surgeons to better outline the intended flap
based on the preoperative anatomical evaluation.
The present results showed that the LFCA arose from
the DFA in 82.3% of patients and from the FA in 15.4% of patients;
however, our results were marginally different from those reported
by Uzel et al (11) in 2008,
who found that the LFCA was branched from the DFA in 77.3% of cases
and from the FA in 19.1% of cases. Since Luo (1) and Chen (2) initially described the classification of
LFCA branching patterns, a number of modified classifications have
been reported, such as those of Kimata et al (16) and Shieh et al (12). In the present study, we proposed
another modified classification in line with the LFCA branching
patterns on MRA: Type Ia, the LFCA originates from the
superolateral part of the deep FA and trifurcates into the
ascending, transverse and descending branches constantly; type Ib,
the LFCA originates variably from the FA but trifurcates into the
ascending, transverse and descending branches constantly; type II,
two trunks of the LFCA ascending, transverse and descending
branches originate from the DFA or the FA; type III, the LFCA
ascending, transverse and descending branches originate from the
DFA or the FA, respectively; and type IV, the LFCA originates from
the DFA and bifurcates into the ascending and descending branches
only, with the transverse branch missing. In terms of hemodynamics,
this modified classification describes the LFCA origin more
accurately than the description by Uzel et al (11) in 2008. This modified classification
also enables surgeons to precisely evaluate the dynamics of the
major LFCA branches for outlining a variety of skin flaps or
composite tissue flaps (6,17).
It has been well established that the preoperative
outlining for perforating artery flaps follows four primary
principles: ‘Point’, ‘line’, ‘plane’ and ‘arch’. Perforating
vessels are ideally localized prior to surgery, with Doppler
ultrasonography frequently used for preoperative flap vascular
investigation. This technique is minimally invasive, easy to use
and less costly; however, it is unable to determine the vascular
origin and is subject to a high false-positive rate. Furthermore,
Doppler ultrasonography has low sensitivity and accuracy, and
cannot offer visual images for intraoperative use, due to the
absence of hologram and 3D anatomical reconstructions, even for
experienced operators (18,19). Multiple intrinsic disadvantages are
also associated with DSA, such as high cost, invasiveness, risk of
iatrogenic injuries and low reproducibility. In addition, DSA
imaging cannot visualize muscles, nerves, fat and other soft
tissues, resulting in an incomplete visualization of soft tissues.
DSA does, however, allow a clearer visualization of the perforating
vessels than CTA or MRA and, therefore, remains the gold standard
technique for imaging the vascular 3D anatomy. Multi-detector CTA
can reconstruct the 3D anatomy of vessels located in the flap and
surgical field but requires delicate equipment; this technique is
also subject to high costs and a risk of potential radiation injury
(20–22).
Newly emerging 3D-CE-MRA has been incorporated in
vascular interventions (23),
although it is rarely reported in the evaluation of LFCA
perforating vessels. This medical imaging modality is easy to use
and presents a high success rate (24). Furthermore, TS-MIP allows an
objective, visual assessment of vascular origin, perforating vessel
distribution and vascular pedicle measurement. This technique
offers a dynamic patterning of intermuscular perforating vessels
and neighboring vascular networks. As it is a minimally invasive,
visual and accurate imaging technique, 3D-CE-MRA is considered to
be an effective preoperative assessment tool. This technique has
numerous advantages (25). First,
CE-MRA allows simultaneous flap outlining at multiple sites.
Secondly, CE-MRA offers a 3D, full-scale, multi-angle
reconstruction of vessels, which is particularly useful in cases
requiring additional flap outlining due to an inability to identify
an appropriate perforating artery. Thirdly, CE-MRA can be used for
the postoperative evaluation of flaps and adjoining soft tissues in
case of poor perfusion. These advantages were validated by the
present results from 4 patients undergoing 3D-CE-MRA for FALTF
outlining.
The techniques of Test Bolus and Care Bolus
(26,27) may be used in 3D-CE-MRA. The first
method measures the time from zero to contrast peak concentration,
followed by MRA imaging, resulting in high quality angiography but
requiring a relatively experienced operator. The second method uses
real-time tracing and trigger acquisition and is easy to use in
general practice, although it also requires an experienced
operator. In the present study, the latter method, i.e. the
real-time tracing and trigger acquisition method, was used. The
dose of contrast media was determined to be ~30 ml in the majority
of patients due to the following two reasons: Double-dose contrast
media offers a better-visualized vasculature, and this dose is not
likely to require any additional media for calf MRA. A higher bolus
injection rate results in a more legible contrast-enhanced artery,
as well as perforating vessel convenience and lateral circulation;
however, the peak time is relatively short in case of a high
injection rate, which builds up challenges in MRA scanning and
requires the use of a magnetic resonance scanner with a high
temporal resolution. We therefore proposed an injection rate of
~3.0 ml/sec (2.5–4.0 ml/sec in the patients), which achieved a
favorable angiography peak time and also avoided potential local
injuries from contwrast medium leakage. For angiography from the
common iliac artery to the superior genicular artery, multiple
serial scanning should be repeated, if possible, to visualize
arteries and veins simultaneously. The results from the present
study validated the excellent outcome of this approach, as the LFCA
was well visualized in all patients, in terms of origin, pathway
and distribution.
The MIP technique is normally used for
post-angiography processes, and allows the visualization of the
target anatomy, either positively or negatively. Images derived
from the Care Bolus approach are similar to those from DSA, and
allow the review against the DSA finding, which is particularly
suitable for reviewers with DSA experience (28,29). The
incorporation of TS-MIP excludes the interference from a large
number of unrelated structures and preserves legible, realistic
images; therefore, this technique only visualizes the target
anatomy and better characterizes the flap vascular architecture and
distribution. In the present study, MRA with TS-MIP visualized all
three-tier LFCA branches in the majority of donor sites and aided
surgeons to outline the flap prior to surgery.
There were a number of limitations in this study.
First, only the static 3D anatomy of the LFCA was demonstrated, as
well as its major branches and perforating vessels, rather than the
dermal vascular network. Secondly, the determination of the flap
size was neither prospective nor accurate. Finally, the sample size
was relatively small. Our ongoing study aims to quantitatively
evaluate the donor site blood supply and flap size using MRA with
3D perfusion imaging.
In conclusion, 3D-CE-MRA is a clinically effective
investigational modality in evaluating the 3D anatomy of LFCA for
FALTF transfer; however, the effectiveness and safety of this
technique require validation in further large-scale, prospective,
randomized, controlled studies.
References
|
1
|
Luo LS: A new free skin flap-anterolateral
femoral flap - its anatomy and clinical application. Zhonghua Zheng
Xing Shao Shang Wai Ke Za Zhi. 1:50–52. 1985.(In Chinese).
PubMed/NCBI
|
|
2
|
Chen LF: The application of a free
anterolateral femoral cutaneous flap for the repair of chronic
ulcers of foot and ankle. Zhonghua Zheng Xing Shao Shang Wai Ke Za
Zhi. 3:118–119, 157. 1987.(In Chinese). PubMed/NCBI
|
|
3
|
Zhang B, Li DZ, Xu ZG and Tang PZ: Free
anterolateral thigh flap for reconstruction of head and neck
defects. Zhonghua Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 41:447–450.
2006.(In Chinese). PubMed/NCBI
|
|
4
|
Park CW and Miles BA: The expanding role
of the anterolateral thigh free flap in head and neck
reconstruction. Curr Opin Otolaryngol Head Neck Surg. 19:263–268.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sharabi SE, Hatef DA, Koshy JC, et al: Is
primary thinning of the anterolateral thigh flap recommended? Ann
Plast Surg. 65:555–559. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Smit JM, Klein S and Werker PM: An
overview of methods for vascular mapping in the planning of free
flaps. J Plast Reconstr Aesthet Surg. 63:e674–e682. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garvey PB, Selber JC, Madewell JE, et al:
A prospective study of preoperative computed tomographic
angiography for head and neck reconstruction with anterolateral
thigh flaps. Plast Reconstr Surg. 127:1505–1514. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu SC, Chiu WK, Chen SY, et al:
Comparison of surgical result of anterolateral thigh flap in
reconstruction of through-and-through cheek defect with/without CT
angiography guidance. J Craniomaxillofac Surg. 39:633–638. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hnatiuk B, Emery DJ and Wilman AH: Effects
of doubling and tripling the spatial resolution in standard 3D
contrast-enhanced magnetic resonance angiography of carotid artery
disease. J Magn Reson Imaging. 27:71–77. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lakhiani C, Lee MR and Saint-Cyr M:
Vascular anatomy of the anterolateral thigh flap: A systematic
review. Plast Reconstr Surg. 130:1254–1268. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Uzel M, Tanyeli E and Yildirim M: An
anatomical study of the origins of the lateral circumflex femoral
artery in the Turkish population. Folia Morphol (Warsz).
67:226–230. 2008.PubMed/NCBI
|
|
12
|
Shieh SJ, Chiu HY, Yu JC, et al: Free
anterolateral thigh flap for reconstruction of head and neck
defects following cancer ablation. Plast Reconstr Surg.
105:2349–2357. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rastogi S, Patwardhan B, Gulati A and
Thayath MN: Anterolateral thigh free flap for the reconstruction of
through and through defect of cheek following cancer ablation.
Indian J Dent Res. 23:275–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Donnelly R, Hinwood D and London NJ: ABC
of arterial and venous disease. Non-invasive methods of arterial
and venous assessment. BMJ. 320:698–701. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen SY, Lin WC, Deng SC, Chang SC, Fu JP,
Dai NT, Chen SL, Chen TM and Chen SG: Assessment of the perforators
of anterolateral thigh flaps using 64-section multidetector
computed tomographic angiography in head and neck cancer
reconstruction. Eur J Surg Oncol. 36:1004–1011. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kimata Y, Uchiyama K, Ebihara S, et al:
Anatomic variations and technical problems of the anterolateral
thigh flap: A report of 74 cases. Plast Reconstr Surg.
102:1517–1523. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu D, Zhang S, Tang M and Ouyang J:
Development and current status of perforator flaps. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 25:1025–1029. 2011.(In Chinese).
PubMed/NCBI
|
|
18
|
Masia J, Larrañaga J, Clavero JA, et al:
The value of the multidetector row computed tomography for the
preoperative planning of deep inferior epigastric artery perforator
flap: Our experience in 162 cases. Ann Plast Surg. 60:29–36. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rozen WM, Garcia-Tutor E, Alonso-Burgos A,
et al: Planning and optimising DIEP flaps with virtual surgery: The
Navarra experience. J Plast Reconstr Aesthet Surg. 63:289–297.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jia Y, Liu W, Zeng A, et al: Clinical
application of multidetector row CT angiography for preoperative
evaluation of nourished vessels of flaps. Zhonghua Zheng Xing Wai
Ke Za Zhi. 24:275–278. 2008.(In Chinese). PubMed/NCBI
|
|
21
|
Ribuffo D, Atzeni M, Saba L, et al: Angio
computed tomography preoperative evaluation for anterolateral thigh
flap harvesting. Ann Plast Surg. 62:368–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saint-Cyr M, Schaverien M, Wong C, et al:
The extended anterolateral thigh flap: Anatomical basis and
clinical experience. Plast Reconstr Surg. 123:1245–1255. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lin J, Li D and Yan F: High-resolution 3D
contrast-enhanced MRA with parallel imaging techniques before
endovascular interventional treatment of arterial stenosis. Vasc
Med. 14:305–311. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tizon X, Lin Q, Hansen T, Borgefors G,
Johansson L, Ahlström H and Frimmel H: Identification of the main
arterial branches by whole-body contrast-enhanced MRA in elderly
subjects using limited user interaction and fast marching. J Magn
Reson Imaging. 25:806–814. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schaverien MV, Ludman CN, Neil-Dwyer J and
McCulley SJ: Contrast-enhanced magnetic resonance angiography for
preoperative imaging of deep inferior epigastric artery perforator
flaps: Advantages and disadvantages compared with computed
tomography angiography: A United Kingdom perspective. Ann Plast
Surg. 67:671–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kramer H, Zenge M, Schmitt P, et al:
Peripheral magnetic resonance angiography (MRA) with continuous
table movement at 3.0 T: Initial experience compared with
step-by-step MRA. Invest Radiol. 43:627–634. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Butz B, Dorenbeck U, Borisch I, et al:
High-resolution contrast-enhanced magnetic resonance angiography of
the carotid arteries using fluoroscopic monitoring of contrast
arrival: Diagnostic accuracy and interobserver variability. Acta
Radiol. 45:164–170. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Neil-Dwyer JG, Ludman CN, Schaverien M, et
al: Magnetic resonance angiography in preoperative planning of deep
inferior epigastric artery perforator flaps. J Plast Reconstr
Aesthet Surg. 62:1661–1665. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schaverien MV, Ludman CN, Neil-Dwyer J and
McCulley SJ: Contrast-enhanced magnetic resonance angiography for
preoperative imaging of deep inferior epigastric artery perforator
flaps: advantages and disadvantages compared with computed
tomography angiography: A United Kingdom perspective. Ann Plast
Surg. 67:671–674. 2011. View Article : Google Scholar : PubMed/NCBI
|