Introduction
Sodium-glucose co-transporter 2 (SLC5A2) belongs to
the Na+-glucose cotransporter family and acts as a
critical molecule in the process of glucose re-absorption from
urine in the proximal convoluted tubule (1). The SLC5A2 gene is mapped to
16p11.2 (2) with a 7.7-kb nucleic
acid, containing 14 exons, and encoding 672 amino acids. The SLC5A2
protein is located in the early proximal convoluted tubule, segment
S1, and has a Na+-to-glucose coupling ratio of 1:1
(3). Mutations in SLC5A2
(OMIM: 182381) have been confirmed as being responsible for the
vast majority of cases of familial renal glucosuria (4–6).
However, an increased glucose excretion was not observed in all
individuals heterozygous for a specific mutation, and even among
family members with identical SLC5A2 mutations, only some
had mild glucosuria (4–6). In previous studies, a homozygous
mutation (a G to A transition at position 1320) of p.W440X within
exon 11 was found in two patients (4,7). The
parents and relatives of the patients in those studies were found
to be heterozygous for the mutation and exhibited almost no renal
glucosuria. The present study is notable in that a patient with
evident renal glucosuria (8.3 g/day) was found to have a
heterozygous mutation of c.1319G>A:p.W440X. As direct evidence
for SLC5A2 heterozygous mutation of p.W440X in renal tissues
has not yet been obtained, the present study reports the
identification of a novel heterozygous mutation in SLC5A2
and further investigated the effect of the mutant SLC5A2
gene on protein expression in renal tissues.
Materials and methods
Patient and control individuals
The patient was a 36-year-old woman who had
persistent glucosuria with normal serum glucose and no other
evidence of renal disease. Glucosuria was quantified by 24 h urine
collection. A total of 100 healthy Chinese individuals (200
chromosomes) were included as controls. In the immunofluorescence
analysis of renal biopsy specimens, normal renal tissue from tumor
patients following partial nephrectomy and biopsy tissue from
patients with minimal change disease and diabetic nephropathy were
included as normal and disease controls, respectively.
The study protocol was approved by the Medical
Ethics Committee of Inner Mongolia People's Hospital (Hohhot,
China). Informed written consent was obtained from all participants
prior to participation in the study.
Mutation screening
Genomic DNA was extracted by salting out from
peripheral white blood cells. The entire coding region and adjacent
intronic segments of the SLC5A2 gene were screened for
mutation by direct sequencing of polymerase chain reaction (PCR)
products, generated with primers described previously (8). The genomic DNA reference sequences of
SLC5A2 (NC_000016.10, OMIM: 182381, Gene ID: 6524) were
obtained from the Entrez gene database. The 200 control chromosomes
were tested by direct sequencing of PCR products to ensure that
these mutations do not represent common polymorphisms.
Immunofluorescence studies on renal
biopsy specimen
Renal specimens were evaluated by routine methods
using indirect immunofluorescence, and light and electron
microscopy. SLC5A2 expression in the renal biopsy specimens was
detected by immunofluorescence staining as described in our
previous study (9). In brief, renal
biopsy specimens were incubated overnight at 4°C with a goat
anti-human polyclonal antibody targeting SLC5A2 (1:50; sc-47404;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), and then a
fluorescein isothiocyanate-labeled donkey anti-goat IgG antibody
(1:200; sc-2024; Santa Cruz Biotechnology, Inc.) for 30 min at
37°C. Normal renal tissue from tumor patients following partial
nephrectomy was included as a normal control. Biopsy tissue from
patients with minimal change disease and diabetic nephropathy was
included as a disease control. Specific binding was defined by
replacing primary antibody with 1% bovine serum albumin
(Sigma-Aldrich, St. Louis, MO, USA). Sections were observed using a
confocal microscope (Olympus FV1000; Olympus Corporation, Tokyo,
Japan).
Results
Clinical characterization of the
patient
The patient was a 36-year-old woman who was referred
to the renal division because of repeated glucosuria. The patient
had no polyuria, polydipsia or weight loss. A review of systems and
the physical examination were completely normal. The patient's
blood pressure was 110/80 mmHg and body weight was 50 kg. Fasting
plasma glucose (4.84 mmol/l), albumin (46.7 g/l), creatinine (71.00
µmol/l), sodium (142.00 mmol/l), chloride (106.0 mmol/l), potassium
(4.40 mmol/l), calcium (2.34 mmol/l), phosphate (0.96 mmol/l),
magnesium (0.82 mmol/l), bicarbonate (26.30 mmol/l), uric acid (225
µmol/l) and glycated hemoglobin (5.7%) levels were all within the
normal range. Routine urinary analysis revealed 2+ to
4+ results for glucose with no other abnormalities. A
quantitative test for urine glucose provided a result of 8.3 g/24
h. Urinary glucose excretion in family members was confirmed by
qualitative test. The father and a uncle of patient had increased
urinary glucose excretions of 1–2+. No other marked
abnormality was detected in the urine and blood biochemistry of
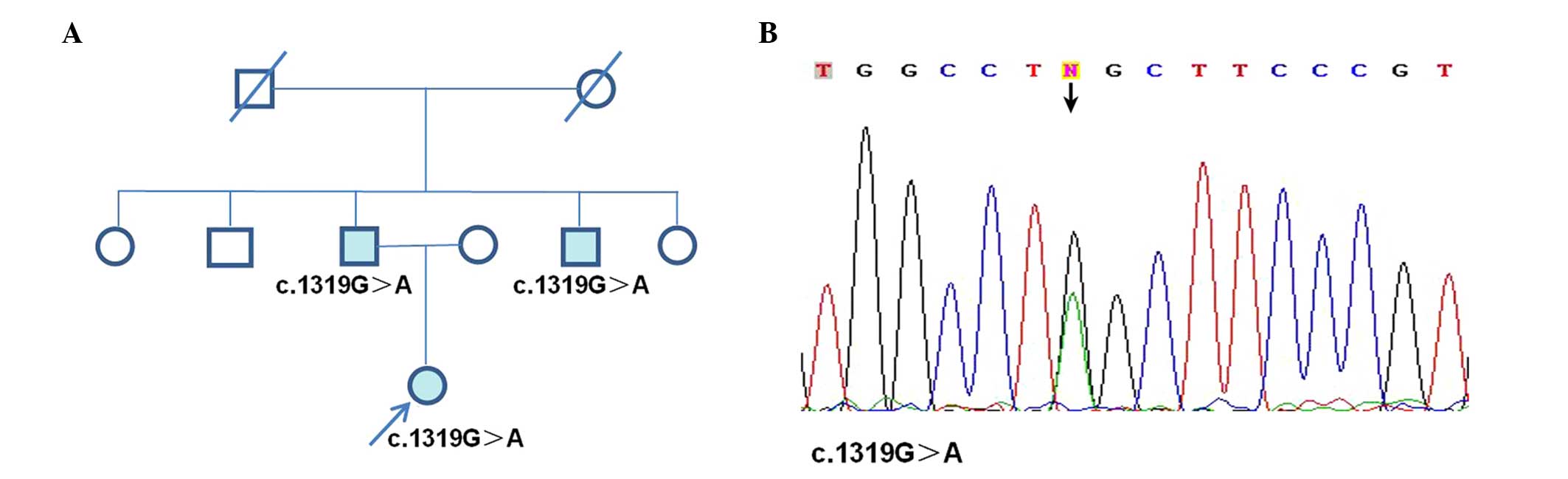
family members (Fig. 1A). Secondly,
the patient was characterized by persistent glucosuria despite
normal serum glucose and the absence of overt tubular dysfunction.
Routine analysis of renal specimens using indirect
immunofluorescence, and light and electron microscopy revealed no
apparent glomerular or tubulointerstitial lesions. Furthermore, a
novel mutation was identified in the patient. From above all, the
patient could be diagnosis of familial renal glucosuria (FRG)
securely.
Identification of a novel mutation in
the patient
Using mutation screening of genomic DNA, a novel
missense mutation was identified in the patient (c.1319G>A:
p.W440X, Fig. 1). This identified
mutation was not detected in any of the 200 chromosomes derived
from 100 healthy, unrelated individuals, indicating that the
mutation does not represent a common polymorphism.
Identified mutation alters SLC5A2
expression in the kidney
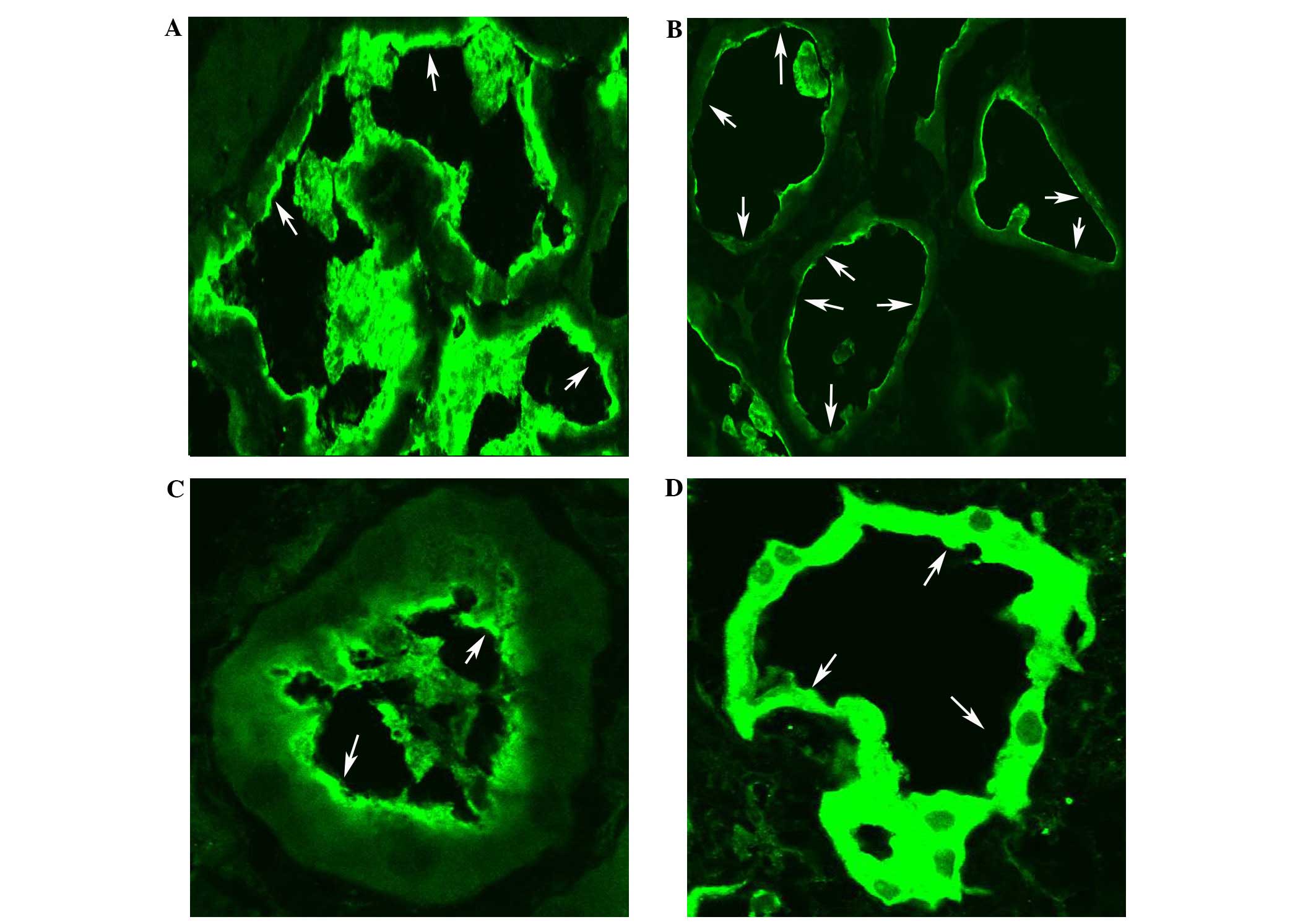
Renal biopsy in the patient with familial renal
glucosuria (FRG) revealed no apparent glomerular or
tubulointerstitial lesions when examined by immunofluorescence,
light and electron microscopy. However, the expression of SLC5A2 in
the apical side of the proximal convoluted tubule was
discontinuously decreased in comparison with that in both normal
and disease controls (patients with minimal change disease or
diabetic nephropathy; Fig. 2).
Discussion
Glucose is the fuel that provides energy for normal
activity in humans, and the major source of glucose is
carbohydrates in food. The kidneys help to keep blood glucose
levels normal by reabsorbing ~180 g glucose per day through
filtration in the proximal tubules (1). SLC5A2 is responsible for the active
transport of glucose across the brush border membrane, and is
expressed almost exclusively in the kidney, accounting for the bulk
of glucose reabsorption (1). Studies
report that SLC5A2 mutations are involved in FRG (9–12). The
long-term outcome of patients with FRG is very good, and so SLC5A2
inhibitors have been the subject of particular attention in the
search for potential new drug targets for the treatment of diabetes
(13,14). However, safety issues have hindered
the development of SLC5A2 inhibitors (15). Research on patients with FRG may
bring about a breakthrough in the study of the treatment of
diabetes. In previous studies, although a homozygous mutation (G to
A transition at position 1320) within exon 11 was found in two
patients, the effect of the mutation, especially the heterozygous
mutation of p.W440X, on the expression of SLC5A2 in the kidney is
not yet fully known (4,7). In the present study, to exclude tubular
impairments or other renal diseases that could cause glucosuria,
the patient agreed to undergo a renal biopsy. This revealed no
apparent glomerular or tubulointerstitial lesions under
immunofluorescence, light and electron microscopy.
A novel SLC5A2 mutation of
c.1319G>A:p.W440X was found in the present patient with FRG. The
data reveal that a patient with a heterozygous mutation had
clinical manifestations (urinary glucose excretion rate, 8.3
g/day), which implies that the mutation causes clinically relevant
SLC5A2 dysfunction. In the immunofluorescence examination of SLC5A2
in the renal biopsy specimen, SLC5A2 was detected in the apical
side of the proximal convoluted tubule, discontinuously decreased
in comparison with both normal and disease controls. Therefore, the
wild-type SLC5A2 exhibits normal expression and the mutation
appears to decrease the expression of SLC5A2 in the apical side of
the proximal convoluted tubule. Therefore, the mutation may affect
transport activity by altering protein processing and impairing
protein insertion into the plasma membrane. Furthermore, it implies
that abnormal distribution of mutant SLC5A2 might play a key role
in affecting transport activity. From the results, the inheritance
of renal glucosuria can be described as a codominant trait as a
whole, and it may be inferred that variable penetrance may be
associated with the compensatory capacity of wild-type SLC5A2. To
the best of our knowledge, the altered SLC5A2 expression caused by
heterozygous mutation of p.W440X has not been reported previously
in human subjects. This observation may shed light on the function
of SLC5A2; however, further analysis is required.
In summary, a novel SLC5A2 mutation was
identified in a Chinese patient with FRG, and SLC5A2 was observed
to be discontinuously decreased in the apical side of the proximal
convoluted tubule in the patient on renal biopsy. The mutant SLC5A2
proteins may have significantly lowered SLC5A2 expression in the
apical side of the proximal convoluted tubule. This study provides
valuable information concerning the role of the SLC5A2 molecule
from genotype to phenotype.
Acknowledgements
This study was funded by the National Natural
Science Foundation of China (grant no. 81160087), the Natural
Science Fund of Inner Mongolia Autonomous Region (grant no.
2010BS1102), 2015 Science and Technology Planning Project of Inner
Mongolia Autonomous Region (grant no. 201502107) and the Fund
Program of Inner Mongolia People's Hospital (grant no. 201510).
Glossary
Abbreviations
Abbreviations:
|
SLC5A2
|
sodium-glucose co-transporter 2
|
|
FRG
|
familial renal glucosuria
|
References
|
1
|
Wright EM, Hirayama BA and Loo DF: Active
sugar transport in health and disease. J Intern Med. 261:32–43.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wells RG, Mohandas TK and Hediger MA:
Localization of the Na+/glucose cotransporter gene SGLT2
to human chromosome 16 close to the centromere. Genomics.
17:787–789. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kanai Y, Lee WS, You G, Brown D and
Hediger MA: The human kidney low affinity Na (+)/glucose
cotransporter SGLT2: Delineation of the major renal reabsorptive
mechanism for D-glucose. J Clin Invest. 93:397–404. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Santer R, Kinner M, Lassen CL,
Schneppenheim R, Eggert P, Bald M, Brodehl J, Daschner M, Ehrich
JH, Kemper M, et al: Molecular analysis of the SGLT2 gene in
patients with renal glucosuria. J Am Soc Nephrol. 14:2873–2882.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Calado J, Sznajer Y, Metzger D, Rita A,
Hogan MC, Kattamis A, Scharf M, Tasic V, Greil J, Brinkert F, et
al: Twenty-one additional cases of familial renal glucosuria:
Absence of genetic heterogeneity, high prevalence of private
mutations and further evidence of volume depletion. Nephrol Dial
Transplant. 23:3874–3879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee H, Han KH, Park HW, Shin JI, Kim CJ,
Namgung MK, Kim KH, Koo JW, Chung WY, Lee DY, et al: Familial renal
glucosuria: A clinicogenetic study of 23 additional cases. Pediatr
Nephrol. 27:1091–1095. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Van den Heuvel LP, Assink K, Willemsen M
and Monnens L: Autosomal recessive renal glucosuria attributable to
a mutation in the sodium glucose cotransporter (SGLT2). Hum Genet.
111:544–547. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magen D, Sprecher E, Zelikovic I and
Skorecki K: A novel missense mutation in SLC5A2 encoding SGLT2
underlies autosomal-recessive renal glucosuria and aminoaciduria.
Kidney Int. 67:34–41. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yu L, Hou P, Lv JC, Liu GP and Zhang H: A
novel sodium-glucose co-transporter 2 gene (SGLT2) mutation
contributes to the abnormal expression of SGLT2 in renal tissues in
familial renal glucosuria. Int Urol Nephrol. 46:2237–2238. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yu L, Lv JC, Zhou XJ, Zhu L, Hou P and
Zhang H: Abnormal expression and dysfunction of novel SGLT2
mutations identified in familial renal glucosuria patients. Hum
Genet. 129:335–344. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lee YW: Clinical and genetic analysis in a
patient with primary renal glucosuria: Identification of a novel
mutation in the SLC5A2 gene. Exp Ther Med. 6:1532–1534.
2013.PubMed/NCBI
|
|
12
|
Yu L, Hou P, Lv JC, Liu GP and Zhang H:
Novel SLC5A2 variants contribute to renal glucosuria in chinese
families: Abnormal expression and dysfunction of variant SLC5A2.
Hum Mutat. 36:79–86. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Scholl-Bürgi S, Santer R and Ehrich JH:
Long-term outcome of renal glucosuria type 0: The original patient
and his natural history. Nephrol Dial Transplant. 19:2394–2396.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Isaji M: Sodium-glucose cotransporter
inhibitors for diabetes. Curr Opin Investig Drugs. 8:285–292.
2007.PubMed/NCBI
|
|
15
|
Ledford H: Diabetes drugs ride a bumpy
road. Nature. 504:1982013. View
Article : Google Scholar : PubMed/NCBI
|