Introduction
Skin is in continuous contact with the external
environment, and protects the human body from potentially hazardous
environmental threats, including physical, chemical and biochemical
factors (1). Furthermore, skin
prevents the entry of bacteria, fungi and viruses into the body,
and provides a protective barrier that prevents moisture loss
(2). Skin aging comprises several
intrinsic processes, which are predominately genetically
determined, and extrinsic aging that is typically associated with
sun exposure; however, other factors may be involved in extrinsic
skin aging, including ultraviolet (UV) irradiation, excessive
alcohol consumption and environmental pollution (1,2).
Chronic exposure of human skin to the sun is
characterized by epidermal hyperplasia and changes in the
biomechanical properties of the dermis (3). These lead to wrinkle formation that may
be observed histologically, and the development of deep wrinkles,
nodules, irregular hyperpigmentation, telangiectasia and skin that
has a leathery, rough texture, all of which are clinically evident
(3).
Repeated exposure to UV radiation ultimately causes
premature skin aging, or photoaging, which is characterized by the
formation of fine and coarse wrinkles, an increase in the thickness
of the skin, dryness, laxity and pigmentation (4). Exposure of the skin to UV light
initiates the generation of active oxygen species in the skin
(5), and exposure of the skin to UVB
radiation is associated with elevated risks of erythema, edema,
hyperplasia, sunburn-cell formation, photoaging, immune system
suppression and skin cancer (6).
The role of probiotics in the regulation of
intestinal health has been widely investigated for over 100 years.
Probiotics are used with increasing frequency to treat medical
conditions such as allergic diseases and atopic dermatitis, and to
prevent dental caries and respiratory infections (7). Human clinical trials have demonstrated
that probiotic supplementation may relieve atopic dermatitis and
dry skin (8). Several studies
involving Lactobacillus acidophilus have demonstrated that
probiotics are effective against atopic dermatitis (9,10).
However, the anti-wrinkle effects of tyndalized L.
acidophilus have not been investigated. Thus, the present study
examined the effects of tyndalized L. acidophilus on
hairless mice that had developed skin damage following skin
exposure to UVB radiation.
Materials and methods
Materials
Tyndalized L. acidophilus, or ID-ACT3302, was
obtained from Ildong Pharmaceutical Co., Ltd. (Seoul, South Korea).
A total of 21 6-week-old HR-1 hairless male mice were purchased
from Japan SLC, Inc. (Shizuoka, Japan). UVB irradiation was
administered using a UVM-225D Mineralight UV Display Lamp (UVP,
Inc., Upland, CA, USA). Rabbit anti-phospho-stress-activated
protein kinase/Jun-amino-terminal kinase (SAPK/JNK; cat. no. 9251),
anti-SAPK/JNK (cat. no. 9252), anti-phospho-p44/42
mitogen-activated protein kinase (MAPK) extracellular
signal-regulated protein kinases 1 and 2 (ERK1/2; cat. no. 9101),
anti-p44/42 MAPK (ERK1/2; cat. no. 9102), anti-phospho-p38 MAPK
(cat. no. 9211) and anti-p38 MAPK (cat. no. 9212) polyclonal
antibodies were purchased from Cell Signaling Technology (Danvers,
MA, USA). Goat anti-matrix metalloproteinase (MMP)-1 polyclonal
antibody (cat. no. sc12348), mouse anti-MMP-9 monoclonal antibody
(cat. no. sc-21733), goat anti-β-actin polyclonal antibody (cat.
no. sc-1616) and horseradish peroxidase (HRP)-conjugated
anti-rabbit (cat. no. sc-2030) and anti-goat (cat. no. sc-2020)
secondary antibodies were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA).
Experimental animals and oral
administration
After purchase, the HR-1 hairless male mice were
stabilized for 1 week prior to the commencement of the study. The
animals were housed in a climate-controlled facility at a
temperature of 24°C, at 50% humidity with a 12-h dark:light cycle
and ad libitum access to food and water. All experimental
protocols were approved by the Korea Institute of Oriental Medicine
Institutional Animal Care and Use Committee (Daejeon, South Korea).
The mice were divided into 3 groups as follows: Control (n=5),
UVB-treated vehicle (n=5), and UVB-treated tyndalized L.
acidophilus (n=5) groups. Mice from the UVB-treated tyndalized
L. acidophilus group were orally administered 0.1 ml water
containing 100 mg of tyndalized L. acidophilus/kg body
weight/day. The control group did not receive irradiation with UVB,
or any other form of treatment. The vehicle group mice were orally
administered 0.1 ml water and underwent irradiation with UVB.
UVB irradiation
UVB irradiation was administered using a UVM-225D
Mineralight UV Display Lamp (UVP) that emitted at a wavelength of
302 nm. The strength of the UV radiation was measured using a
HD2102-2 UV meter (Delta OHM Srl, Padova, Italy). UVB radiation was
applied to the backs of the mice 3 times/week for 12 weeks. The
amount of irradiation was progressively increased from 60
mJ/cm2/exposure during week 1 (1 minimal erythematous
dose = 60 mJ/cm2) to 90 mJ/cm2/exposure
during week 7.
Skin hydration and transepidermal
water loss (TEWL)
A corneometer (Courage + Khazaka electronic GmbH,
Cologne, Germany) was used to determine the hydration levels of the
skin, and a Tewameter (Courage + Khazaka electronic GmbH) was used
to measure TEWL, which is an indicator of the barrier function of
the skin's epidermis.
Histological investigation
The dorsal skin was removed from each hairless mouse
and fixed in 10% neutral-buffered formalin. Using a conventional
method, the fixed tissue samples were washed with distilled water,
dehydrated using an ethanol gradient, cleared with xylene and
embedded in paraffin wax, after which 5 µm sections were cut using
a microtome. The tissue sections were stained with hematoxylin and
eosin (H&E) and Masson's trichrome stain for collagen fiber
analysis. The thickness of the epidermis was measured under light
microscopy using an eyepiece micrometer (Olympus Corporation,
Tokyo, Japan).
Western blotting
Protein was extracted from the skin tissue samples
using radioimmunoprecipitation assay lysis buffer (Thermo Fisher
Scientific, Inc., Rockford, IL, USA), following tissue
homogenization (Precellys® 24; Bertin Technologies,
Montigny-le-Bretonneux, France). Total protein concentration was
determined using the DC Protein Assay (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). Protein lysates (20 µg) from each sample were
electrophoresed on a 10% sodium dodecyl sulfate-polyacrylamide gel
and then transferred to polyvinylidene fluoride membranes. The
membranes were blocked with a 5% skimmed milk solution for 1 h at
room temperature. Following blocking, the blots were incubated
overnight at 4°C with a the primary antibodies at a dilution of
1:1,000. The blots were washed 3 times for 10 min each in
Tris-buffered saline (Bio-Rad Laboratories, Inc.). The membranes
were then incubated for 2 h with HRP-conjugated anti-rabbit and
anti-goat secondary antibodies at a dilution of 1:5,000. The
proteins were detected using an enhanced chemiluminescence
solution. Images were captured by ImageQuant LAS 4000 digital
imaging system (GE Healthcare Bio-Sciences, Pittsburgh, PA, USA)
and were analyzed using Multi Gauge software, version 3.0
(Fujifilm, Tokyo, Japan).
Statistical analysis
All measurements were undertaken in triplicate, and
all values are presented as the mean ± standard error. An analysis
of variance and Tukey's test was used to determine differences in
the results among the study groups. Statistical analyses were
performed using GraphPad Prism 5.0 software (GraphPad Software,
Inc., La Jolla, CA, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
Evaluation of TEWL and skin
hydration
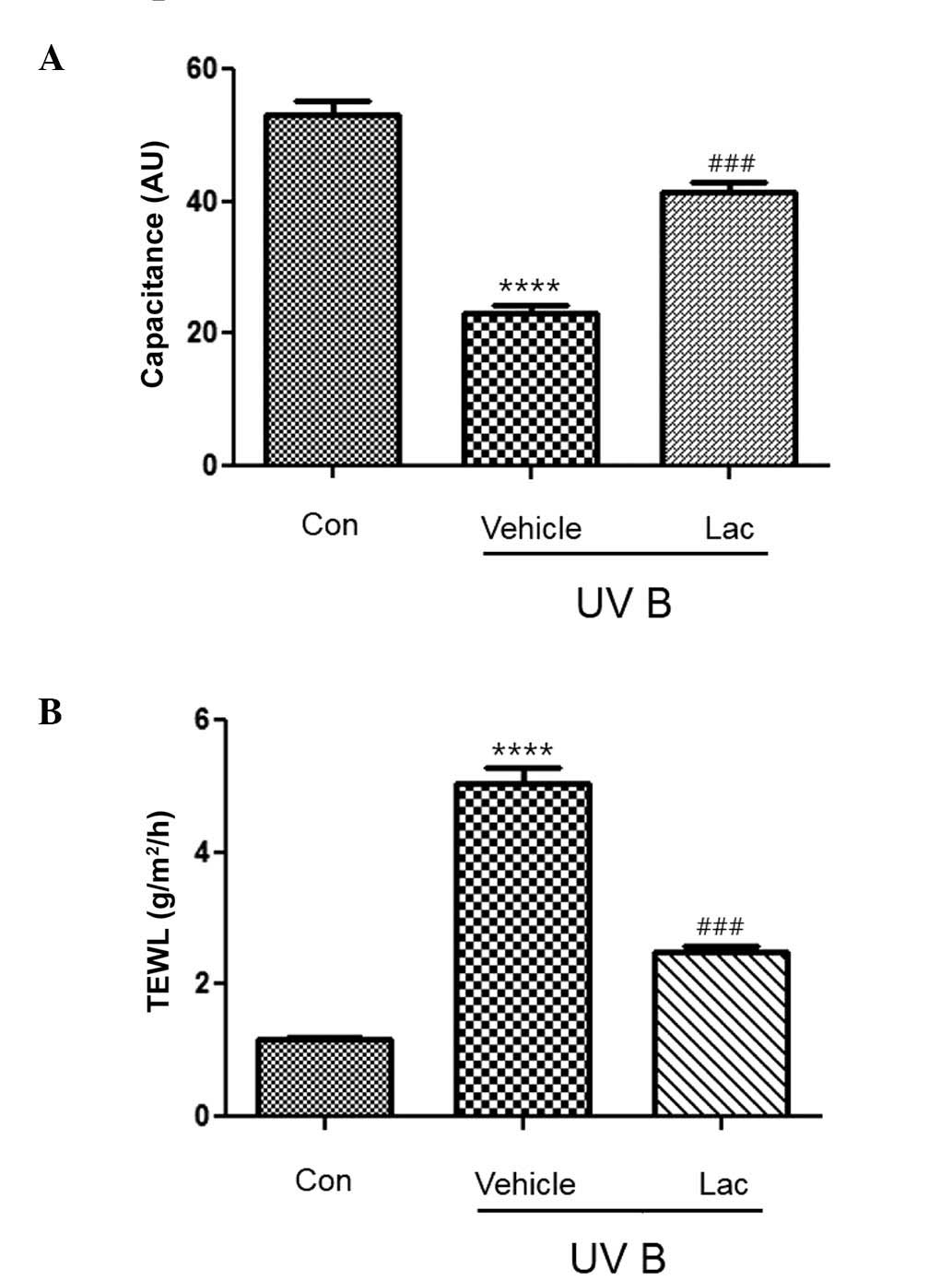
The hydration of the stratum corneum was
significantly reduced in the UVB-treated vehicle group, as compared
with the control group (P<0.0001; Fig. 1A). The hydration of the skin from the
UVB-treated tyndalized L. acidophilus group mice was not
reduced to the same extent as that observed in the UVB-treated
vehicle group and it was significantly different, as compared with
the UVB-treated vehicle group (P<0.001; Fig. 1A).
TEWL was significantly higher in the UVB-treated
vehicle group (Fig. 1B;
P<0.0001), as compared with the control group. However, TEWL was
significantly reduced in the UVB-treated tyndalized L.
acidophilus group, as compared with the UVB-treated vehicle
group (P<0.001; Fig. 1B).
Histological evaluation of the
anti-wrinkle effect of L. acidophilus in UVB-irradiated hairless
mice
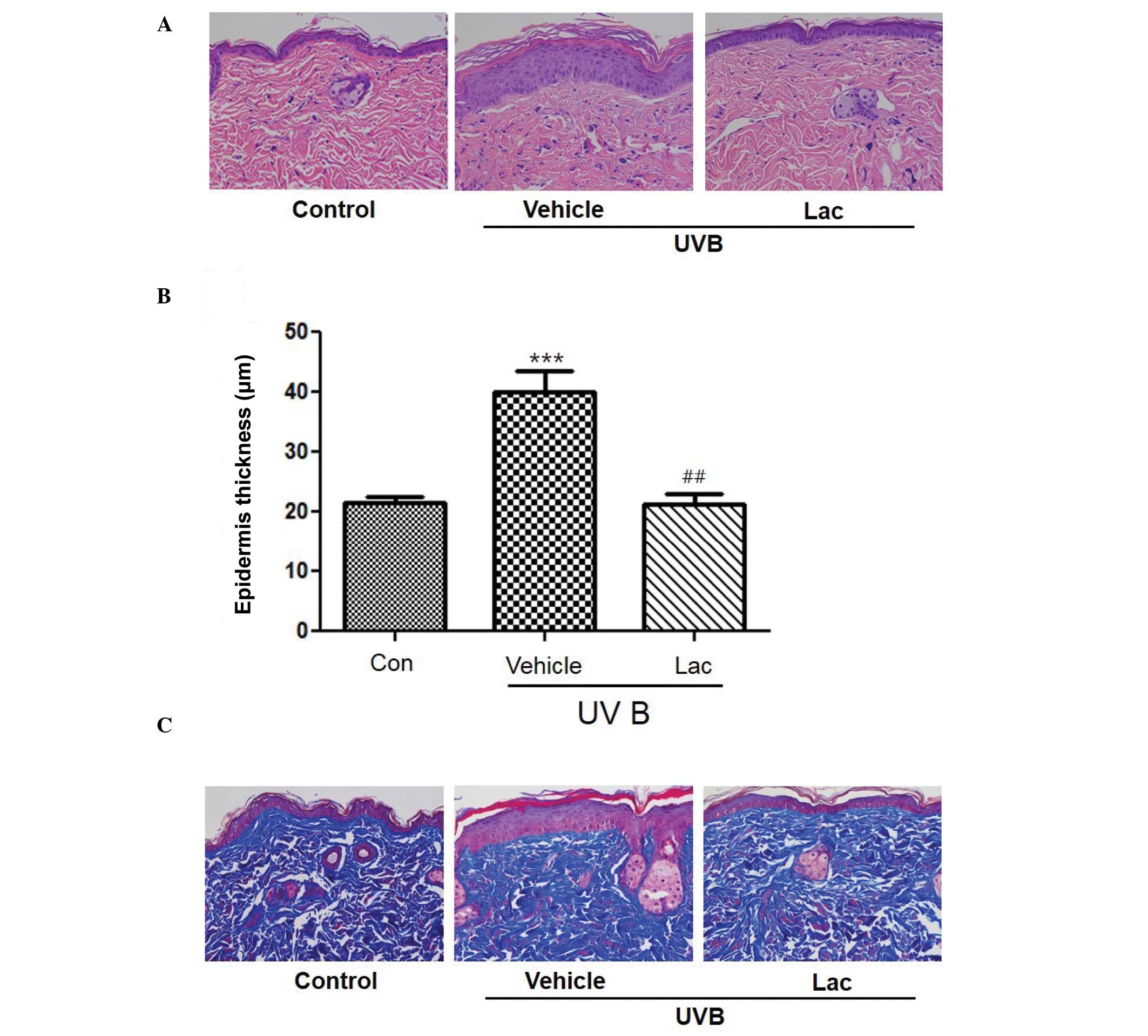
To determine the wrinkle-reducing effects of
tyndalized L. acidophilus, skin was removed from the
hairless mice and skin tissue sections were stained. H&E
staining of the skin confirmed that the thickness of the stratum
corneum and the epidermis had increased in the UVB-treated vehicle
group compared with the skin from the control group (Fig. 2A and B; P<0.001). Masson's
trichrome staining revealed that the collagen was uniformly
distributed in the dermal layer (Fig.
2C). Collagen fibers were increased in the UVB-treated
tyndalized L. acidophilus group compared with the
UVB-treated vehicle group (Fig. 2C).
These results suggest that tyndalized L. acidophilus reduced
the level of skin-wrinkling resulting from UV irradiation.
Evaluation of the anti-wrinkle effect
of tyndalized L. acidophilus based on changes in epidermal
thickness
To evaluate the anti-wrinkle effect of tyndalized
L. acidophilus, changes in the thickness of the epidermis
were investigated by measuring the distance from the keratin layer
to the epidermal basement membrane in skin sections stained with
H&E using a microscope. The skin epidermal thickness was
significantly increased in the UVB-treated vehicle group, as
compared with the control group (P<0.001; Fig. 2B). The skin epidermal thickness of
the the UVB-treated tyndalized L. acidophilus group was
decreased by 54.1%, as compared with the UVB-treated vehicle group,
and this difference was statistically significant (P<0.01).
These results suggest that tyndalized L. acidophilus is able
to reduce the thickness of the epidermis and may be an effective
anti-wrinkle agent.
Western blotting
UVB irradiation increased the expression of the
MMPs, MMP-1 and MMP-9. Orally administered 100 mg tyndalized L.
acidophilus reduced the expression levels of MMP-1 and MMP-9
(Fig. 3A). Furthermore, tyndalized
L. acidophilus treatment suppressed the UVB-induced
upregulation of MMP-1 and MMP-9. The effect of tyndalized L.
acidophilus on UVB-induced MAPK phosphorylation was examined in
mouse skin, as MMP-9 is primarily regulated by MAPK activation. As
revealed in Fig. 3B, UVB irradiation
induced the phosphorylation of p38, ERK 1/2, and JNK. Pretreatment
with tyndalized L. acidophilus attenuated the
phosphorylation of p38, ERK1/2, JNK, and MAPK in UVB-irradiated
hairless mouse skin. In addition, tyndalized L. acidophilus
inhibited the UVB-induced phosphorylation of MAPK/ERK kinase (MEK;
Fig. 3C). The aforementioned data
suggest that tyndalized L. acidophilus protects mouse skin
from UVB-induced damage.
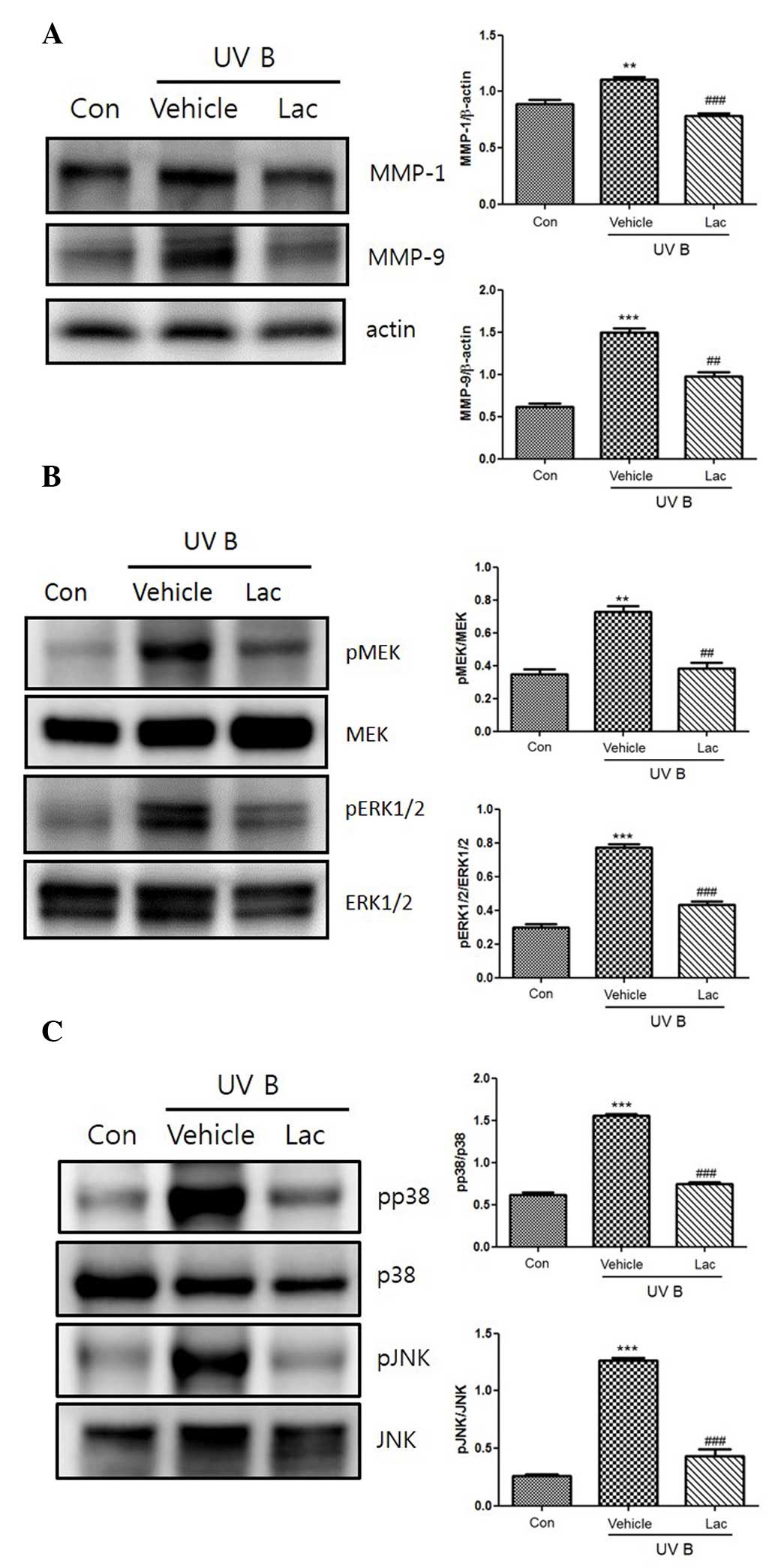 | Figure 3.(A) The inhibitory effect of
tyndalized Lactobacillus acidophilus on the expression of
MMP-1 and MMP-9 in UVB-irradiated skin. The expression levels of
the MMPs were detected by western blot analysis. (B) The inhibitory
effects of tyndalized L. acidophilus on the UVB-induced
expression of MAPKs. Tyndalized L. acidophilus inhibited the
phosphorylation of JNK 1/2, and p38. (C) Tyndalized L.
acidophilus inhibited the phosphorylation of ERK or MEK, and
ERK 1/2. Lac, tyndalized L. acidophilus; con, control; UV,
ultraviolet; MMP, matrix metalloproteinase; JNK, Jun-amino-terminal
kinase; MEK, MAPK/ERK kinase; ERK1/2, extracellular
signal-regulated protein kinase 1/2. **P<0.01, ***P<0.001 vs.
the vehicle group. ##P<0.01, ###P<0.001
vs. the control group. |
Discussion
Photoaging refers to premature skin aging caused by
chronic exposure to UV radiation, particularly the UVB component,
which is regarded as the primary cause of skin damage (11). Acute or chronic exposure to UVB
radiation is a major cause of dermatologic disorders. UVB
irradiation of the skin induces acute inflammation, which is
characterized by erythema, edema and immunosuppression (12). The changes that result from UV
irradiation that are perceived to be associated with aging include
a loss of elasticity, pigmentary changes and deep wrinkling
(13). The majority of these changes
arise as a result of damage to the dermis, which is visible
histologically as elastotic material.
An increase in TEWL impairs the functions of the
enzymes within the skin and this results in visibly dry and aged
skin (14). UVB irradiation causes
skin damage, which leads to skin dehydration and increases in TEWL.
High TEWL values, which are indicative of disturbances in the
skin's barrier function, are frequently correlated with low levels
of hydration of the stratum corneum (15). In the current study, tyndalized L.
acidophilus effectively reduced skin damage, and this was
associated with increases in skin hydration and decreases in
TEWL.
Wrinkle formation is associated with the regulation
of collagen synthesis and degradation. As marked degenerative
changes are observed in the extracellular matrix (ECM) of the
dermis during wrinkle formation, ECM-degrading enzymes are
considered to be involved in wrinkle formation (16). The effects of tyndalized L.
acidophilus on wrinkle formation caused by UVB irradiation were
investigated in replicas of the dorsal skin of the mice.
Photoaging is characterized by the degradation of
collagen and the accumulation of abnormal elastin in the
superficial dermis (17). Epidermal
thickness is used to quantify skin photoaging as epidermal
hypertrophy has been hypothesized to cause wrinkle formation
(18). In the present study, H&E
staining and Masson's trichrome staining demonstrated the effects
of tyndalized L. acidophilus on changes within the dorsal
skin histologically. H&E staining revealed that UVB irradiation
increased the thickness of the epidermis, and that the
administration of tyndalized L. acidophilus alleviated this
effect.
MMPs form a family of enzymes that possess
ECM-degrading properties. UVB irradiation is known to MMP synthesis
(19). UVB irradiation induces MMP
production by activating cellular signaling transduction pathways
(19). Each member of the MMP family
has a unique function in response to the skin undergoing
irradiation by UVB, and MMP-1 serves an important role in the
degradation of the ECM that is caused by photoaging (20). Previous research has demonstrated
that decursin inhibits the UVB-induced expression levels of MMP in
human dermal fibroblasts by regulating nuclear factor-κB (21). In the current study, it was revealed
that UVB irradiation led to increases in the expression levels of
MMP-1 and MMP-9, and that the administration of tyndalized L.
acidophilus reduced the expression of MMP-1 and MMP-9
proteins.
p38 MAPK is a member of a highly conserved family of
serine/threonine protein kinases that includes ERK and c-JNK.
Various stressors, such as UV irradiation, oxidative injury, heat
shock, cytokines and other pro-inflammatory stimuli, lead to the
induction of the p38 MAPK-dependent signaling cascade (22). Major signaling pathways known to
mediate UVB-induced biological responses involve MAPKs (23). The present study investigated whether
the inhibitory effect of tyndalized L. acidophilus was
associated with the downregulation of the MAPK family of protein
kinases and MEK. It was determined that the expression levels of
JNK, p38, and ERK increased in UVB-irradiated skin, and that
tyndalized L. acidophilus attenuated the elevations in the
expression levels of the aforementioned proteins.
In conclusion, tyndalized L. acidophilus
effectively inhibited wrinkle formation induced by UVB irradiation,
and this inhibition was attributed to the downregulation of MMPs.
Therefore, tyndalized L. acidophilus may serve as a
potential agent for preventing skin photoaging and wrinkle
formation.
Acknowledgements
The present study was supported by a grant from the
Korea Institute of Oriental Medicine (grant no. K14101).
Glossary
Abbreviations
Abbreviations:
|
ECM
|
extracellular matrix;
|
|
ERK
|
extracellular signal-regulated protein
kinases;
|
|
H&E
|
hematoxylin and eosin;
|
|
MAPK
|
mitogen-activated protein kinase;
|
|
MEK
|
mitogen-activated protein
kinase/extracellular signal-regulated protein kinase;
|
|
MMP
|
matrix metalloproteinase;
|
|
JNK
|
Jun-amino-terminal kinase;
|
|
TEWL
|
transepidermal water loss;
|
|
UV
|
ultraviolet
|
References
|
1
|
Uitto J and Bernstein EF: Molecular
mechanisms of cutaneous aging: Connective tissue alterations in the
dermis. J Investig Dermatol Symp Proc. 3:41–44. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pontius AT and Smith PW: An antiaging and
regenerative medicine approach to optimal skin health. Facial Plast
Surg. 27:29–34. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yano K, Kajiya K, Ishiwata M, Hong YK,
Miyakawa T and Detmar M: Ultraviolet B-induced skin angiogenesis is
associated with a switch in the balance of vascular endothelial
growth factor and thrombospondin-1 expression. J Invest Dermatol.
122:201–208. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Moriwaki S and Takahashi Y: Photoaging and
DNA repair. J Dermatol Sci. 50:169–176. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Masaki H, Atsumi T and Sakurai H:
Protective activity of hamamelitannin on cell damage of murine skin
fibroblasts induced by UVB irradiation. J Dermatol Sci. 10:25–34.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rabe JH, Mamelak AJ, McElgunn PJ, Morison
WL and Sauder DN: Photoaging: Mechanisms and repair. J Am Acad
Dermatol. 55:1–19. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tanriover MD, Aksoy DY and Unal S: Use of
probiotics in various diseases: Evidence and promises. Pol Arch Med
Wewn. 122(Suppl 1): S72–S77. 2012.
|
|
8
|
Meneghin F, Fabiano V, Mameli C and
Zuccotti GV: Probiotics and atopic dermatitis in children.
Pharmaceuticals (Basel). 5:727–744. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Inoue Y, Kambara T, Murata N,
Komori-Yamaguchi J, Matsukura S, Takahashi Y, Ikezawa Z and Aihara
M: Effects of oral administration of Lactobacillus
acidophilus L-92 on the symptoms and serum cytokines of atopic
dermatitis in Japanese adults: A double-blind, randomized, clinical
trial. Int Arch Allergy Immunol. 165:247–254. 2014.PubMed/NCBI
|
|
10
|
Sunada Y, Nakamura S and Kamei C: Effect
of Lactobacillus acidophilus strain L-55 on the development
of atopic dermatitis-like skin lesions in NC/Nga mice. Int
Immunopharmacol. 8:1761–1766. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pyun HB, Kim M, Park J, Sakai Y, Numata N,
Shin JY, Shin HJ, Kim DU and Hwang JK: Effects of collagen
tripeptide supplement on photoaging and epidermal skin barrier in
UVB-exposed hairless mice. Prev Nutr Food Sci. 17:245–253. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee CH, Wu SB, Hong CH, Yu HS and Wei YH:
Molecular mechanisms of UV-induced apoptosis and its effects on
skin residential cells: The implication in UV-based phototherapy.
Int J Mol Sci. 14:6414–6435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Inomata S, Matsunaga Y, Amano S, Takada K,
Kobayashi K, Tsunenaga M, Nishiyama T, Kohno Y and Fukuda M:
Possible involvement of gelatinases in basement membrane damage and
wrinkle formation in chronically ultraviolet B-exposed hairless
mouse. J Invest Dermatol. 120:128–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verdier-Sévrain S and Bonté F: Skin
hydration: A review on its molecular mechanisms. J Cosmet Dermatol.
6:75–82. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Proksch E, Brandner JM and Jensen JM: The
skin: An indispensable barrier. Exp Dermatol. 17:1063–1072. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kligman LH, Gebre M, Alper R and Kefalides
NA: Collagen metabolism in ultraviolet irradiated hairless mouse
skin and its correlation to histochemical observations. J Invest
Dermatol. 93:210–214. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Vayalil PK, Mittal A, Hara Y, Elmets CA
and Katiyar SK: Green tea polyphenols prevent ultraviolet
light-induced oxidative damage and matrix metalloproteinases
expression in mouse skin. J Invest Dermatol. 122:1480–1487. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Urikura I, Sugawara T and Hirata T:
Protective effect of fucoxanthin against UVB-induced skin
photoaging in hairless mice. Biosci Biotechnol Biochem. 75:757–760.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim SR, Jung YR, An HJ, Kim DH, Jang EJ,
Choi YJ, Moon KM, Park MH, Park CH, Chung KW, et al: Anti-wrinkle
and anti-inflammatory effects of active garlic components and the
inhibition of MMPs via NF-κB signaling. PLoS One. 8:e738772013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ho JN, Lee YH, Park JS, Jun WJ, Kim HK,
Hong BS, Shin DH and Cho HY: Protective effects of aucubin isolated
from eucommia ulmoides against UVB-induced oxidative stress in
human skin fibroblasts. Biol Pharm Bull. 28:1244–1248. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hwang BM, Noh EM, Kim JS, Kim JM, Hwang
JK, Kim HK, Kang JS, Kim DS, Chae HJ, You YO, et al: Decursin
inhibits UVB-induced MMP expression in human dermal fibroblasts via
regulation of nuclear factor-κB. Int J Mol Med. 31:477–483.
2013.PubMed/NCBI
|
|
22
|
Kim AL, Labasi JM, Zhu Y, Tang X, McClure
K, Gabel CA, Athar M and Bickers DR: Role of p38 MAPK in
UVB-induced inflammatory responses in the skin of SKH-1 hairless
mice. J Invest Dermatol. 124:1318–1325. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bode AM and Dong Z: Mitogen-activated
protein kinase activation in UV-induced signal transduction. Sci
STKE. 2003:RE22003.PubMed/NCBI
|