Introduction
Pulmonary hypertension (PH) is a pathophysiological
disorder that may involve multiple clinical conditions and can
complicate numerous cardiovascular and respiratory diseases
(1). The incidence of PH is 97 cases
per million with a female-to-male ratio of 1.8 in the UK (1). Left-sided heart failure is considered
to be the most common cause of PH. Furthermore, PH may have
clinical consequences on the right-side heart function; however,
severe PH in these cases is relatively uncommon (1).
Postcapillary PH in patients with left ventricular
(LV) dysfunction is a well-defined risk factor for right
ventricular (RV) heart failure and subsequent increased morbidity
and mortality (2,3). Compensatory mechanisms to overcome RV
dysfunction are not well defined for these patients, and only
limited data are available in the literature about dealing with the
effect of postcapillary PH on right atrial (RA) dynamics and
contractile features (4,5).
For precapillary PH, RA compensatory mechanisms have
been described in animal models with inconclusive results (6,7). In
general, precapillary PH RV pressure overload leads to myocardial
hypertrophy (7), with the
detrimental effect of RV diastolic dysfunction (6,8,9). Thus, RA function is the critical factor
for RV filling, and any RA dysfunction may initiate right heart
failure in patients with PH (6,8). The
compensatory mechanism for RV diastolic dysfunction involves
increased RA contractility, thus maintaining the RV filling
(6). The importance of RA function
is underlined in a study by Shiina et al (10), which described a strong correlation
between right heart failure and reduced RA contractility in
patients with chronic precapillary PH.
The aim of the present study was to evaluate RA
function in postcapillary PH in patients with LV dysfunction due to
left heart valve pathology. Since RA calcium-dependent skinned
fiber dynamics are a surrogate for RA contractile properties, the
calcium-dependent RA contractile dynamics were investigated in
patients scheduled to undergo elective left heart valve surgery who
presented with or without postcapillary PH.
Patients and methods
Ethics
The study was approved by the Ethics Committee of
the Medical Association Rheinhessen. All patients provided written
informed consent for the use of intraoperative resected tissue in
further research examination. The study was performed according to
the Declaration of Helsinki (11).
Patients
A retrospective study was performed using
prospectively collected data from patients scheduled to undergo
elective left heart valve surgery due to aortic and mitral valve
disease at the Department of Cardiothoracic and Vascular Surgery of
the University of Mainz (Mainz, Germany) between January 2011 and
December 2014. In total, 25 patients undergoing cardiac surgery
were included in the present study. The patients were divided into
two groups, as follows: The postcapillary PH group (PH group),
consisting of 15 patients, and the non-postcapillary PH group
(non-PH group), consisting of 10 patients. The patients were
classified according to the New York Heart Association (NYHA)
classification system (12). Patient
demographics and characteristics are presented in Table I.
 | Table I.Data presented as mean ± standard
deviation, or as % (n) unless otherwise indicated. |
Table I.
Data presented as mean ± standard
deviation, or as % (n) unless otherwise indicated.
| Variable | PH group
(n=15) | Non-PH group
(n=10) | P-value |
|---|
| Age, years | 70.70±7.20 | 55.70±11.80 | 0.008 |
| Female, n (%) | 9 (60) | 4
(6) | 0.900 |
| Height, cm | 168.00±9.60 | 171.00±13.00 | 0.900 |
| Weight, kg | 68.00±12.00 | 76.00±17.00 | 0.700 |
| Body surface area,
m2 | 1.78±0.90 | 1.90±0.70 | 0.900 |
| Body-mass
index | 23.00±3.80 | 25.40±3.80 | 0.800 |
| COPD, n (%) | 0 (0) | 0 (0) |
|
| Diabetes mellitus,
n (%) | 3 (20) | 0 (0) | 0.050 |
| Creatinine
(mg/dl) | 1.01±0.80 | 0.67±0.40 | 0.700 |
| Chronic atrial
fibrillation, n (%) | 8 (53) | 2
(20) | 0.020 |
| PAVD, n (%) | 1 (6.6) | 0 (0) | 0.800 |
| Stroke incident, n
(%) | 0 (0) | 0 (0) |
|
| Arterial
hypertension, n (%) | 8 (53) | 5
(50) | 0.900 |
| NYHA class III–IV,
n (%) | 5 (38) | 0 (0) | 0.030 |
| LVEF, % | 47.00±0.14 | 60.00±0.50 | 0.007 |
Inclusion and exclusion criteria
Postcapillary PH was defined by a mean pulmonary
artery pressure (mPAP) of ≥25 mmHg (iE33 xMATRIX Echocardiography
System; Philips Healthcare, Amsterdam, The Netherlands) in the
presence of left-sided valvular heart disease and the absence of
any primary pulmonary artery hypertension (PAH), according to
previously reported guidelines (13). Preserved RV function was defined when
the tricuspid annular plane systolic excursion (TAPSE) was ≥16 mm
(14).
Patients with precapillary arterial hypertension,
idiopathic, heritable forms of PAH, PAH associated with infectious
disease, connective tissue diseases, congenital diseases, pulmonary
veno-occlusive diseases, other pulmonary diseases and chronic
thromboembolic PH were excluded from the current study.
Furthermore, patients presenting as emergency cases, patients
requiring cardiac procedures with the exception of aortic and
mitral valve surgery, endocarditis cases, and patients with
clinical and echocardiographic signs of chronic, acute right or
acute left heart failure were also excluded.
Clinical setting
Hemodynamic data from all patients were obtained
preoperatively by means of standard right heart catheterization, as
described previously (1). The mean
RA pressure (mRAP), mPAP and pulmonary artery wedge pressure (PAWP)
were recorded (iE33 xMATRIX echocardiography system).
Preoperative echocardiography was performed in all
patients (iE33 xMATRIX echocardiography system). Standard
echocardiographic measurements were performed in expiration. The
following parameters were measured: The LV ejection fraction (LVEF)
was determined according to the biplane Simpson's method (14). In addition, dilatation of the right
and left atria and ventricles was assessed by transthoracic
echocardiography (2D and M-mode; iE33 xMATRIX echocardiography
system). LV dilatation was defined when the LV end-diastolic
diameter (LVEDD) was >55 mm and RV dilation was determined when
the RV end-diastolic diameter was >30 mm. Similarly, RA
dilatation was defined when the minor RA axis was >4.4 cm and
the major axis was >5.3 cm, whereas left atrial dilatation was
defined when the end-systolic diameter was >40 mm (M-mode).
TAPSE was measured with two-dimensional M-mode
echocardiography. However, the RV diastolic function in presence of
tricuspid regurgitation (TR) and atrial fibrillation was not
assessed. Echocardiographic views, measurements, as well as
calculations, were performed according to recent guidelines
(14,15).
Skinned fiber preparation
Skinned fiber preparation was performed as
previously described (16,17). Briefly, the fibers were collected
during surgery following resection of the right auricle. Using a
non-touch-technique, the auricle was transferred in an ice-cooled
vial (4°C) containing a modified cardioplegic solution
(Krebs-Henseleit solution, which included: 118.07 mmol/l NaCl, 11.1
mmol/l C6H12O6.H2O, 4.7
mmol/l KCl, 25 mmol/l NaHCO3, 1.2 mmol/l
KH2PO4, 1.2 mmol/l
MgSO4.7H2O, 1.8 mmol/l
CaCl2.2H2O) and 30 mmol/l 2,3-butanedione
monoxime (C4H7NO2) as an
ATP-sensitive potassium channel inhibitor. For examination, the
tissue was transferred to a dish containing an ice-cooled (4°C)
preparation solution, with the following contents: 68.08 mM
C3H4N2, 65.01 mM NaN3,
380.4 mM C14H24N2O10,
154.3 mM C4H10O2S2,
203.3 mM MgCl2.6H2O, and 605.2 mM
C10H14N5O13P3Na2.
The muscle bundles were then resected out of the auricle and
transferred to a test tube containing the modified preparation
solution with 1% Triton X-100 in order to permeabilize the membrane
of the fibers, with incubation for 24 h at 4°C on a shaking device.
The purpose of these preparatory steps was to remove all
membrane-dependent properties (also known as ‘skinning’). Following
this skinning process, the RA muscle bundles were transferred to a
separate ice-cooled (4°C) dish containing preparation solution, in
order to prepare single muscle stripes (size, 2–2,5×0,3 mm) under
the microscope (magnification, ×10; Leica DM1000; Leica
Microsystems GmbH, Wetzlar, Germany).
Log10 calcium concentration (pCa)
against force measurements in RA tissue
A muscle investigation system (Gradient Program;
Scientific Instruments, Heidelberg, Germany) was used to expose the
RA fibers to gradual increase of pCa for force measurements. The
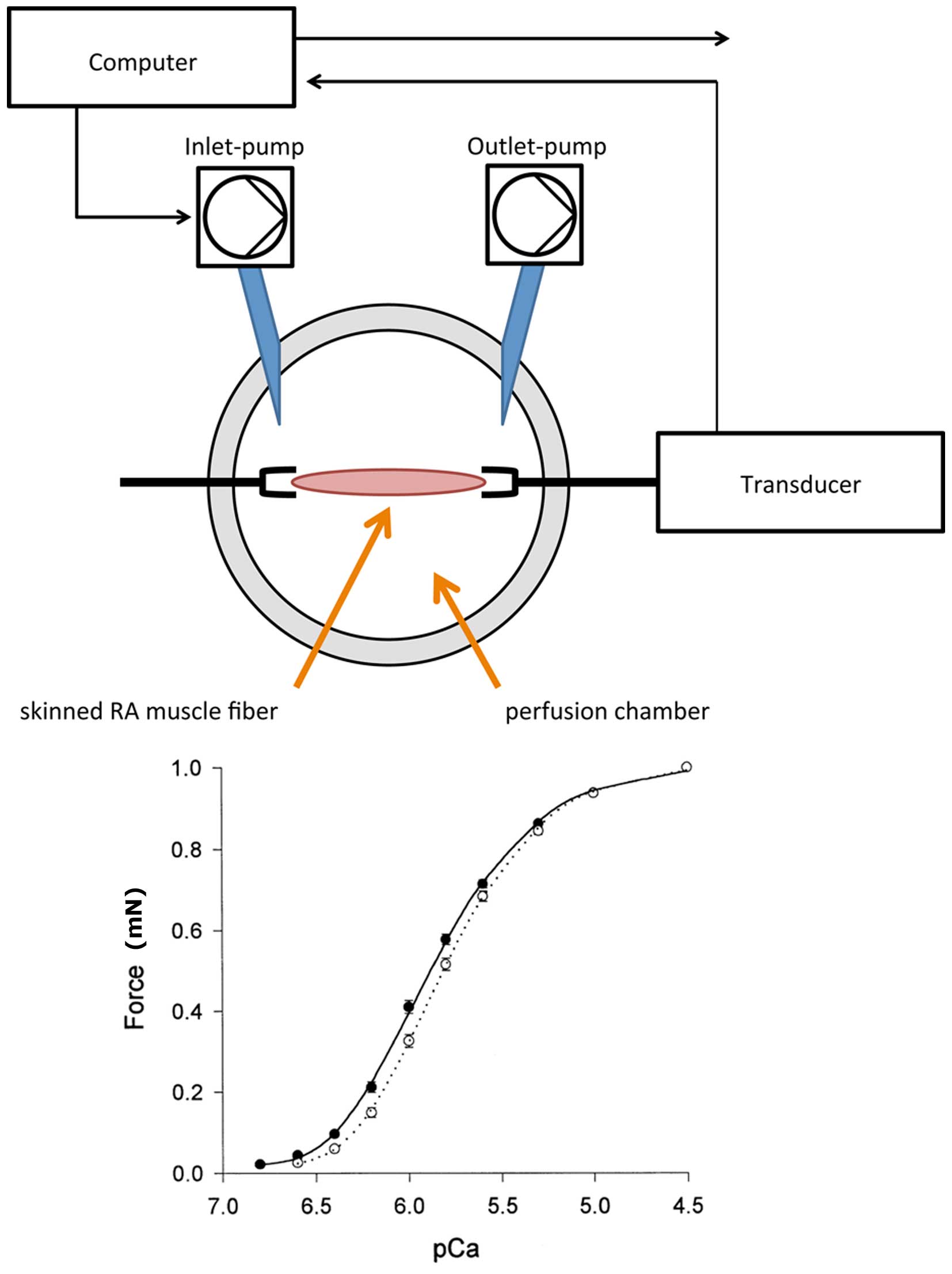
experimental set-up is depicted in Fig.
1. The RA fibers were fixed in the perfusion chamber and
incubated with a relaxation solution, containing the following:
68.08 mM C3H4N2, 327.2 mM
C4H8N3O5PNa2.4H2O,
65.01 mM NaN3, 380.4 mM
C14H24N2O10, 203.3 mM
MgCl2, 154.2 mM
C4H10O2S2, 605.2 mM
C10H14N5O13P3Na2,
and 400 U/ml creatine kinase. By adding 147.02 mM CaCl2
to the relaxation solution, a pCa-force curve was created. pCa was
shown as the negative log10 of the calcium
concentration. A specifically designed software (Gradient Program;
Scientific Instruments) was used to calculate the amount of calcium
required to achieve a stepwise increase in pCa according to the
equation described by Morano et al (16) and Fabiato and Fabiato (17). The pCa included concentrations
between 6.5 and 4.0, in 0.5 increments. For each patient, a set of
three RA fibers underwent calcium-induced force measurements. Thus,
a total of 45 samples were evaluated in the PH group and 30 samples
in the non-PH group.
Statistical analysis
Statistical analysis was performed using SPSS
software (version 23; IBM Corp., Armonk, NY, USA). Patient
continuous demographics are presented as the mean ± standard
deviation, while categorical data are presented as percentages.
Normal distribution was tested with Shapiro-Wilk test. Continuous
variables were statistically analyzed with Welch's t-test, and
categorical data were compared with Wilcoxon signed-rank test and
χ2 test. Two-sided P-values of <0.05 were considered
to indicate statistically significant differences.
Results
Demographics
The demographic information and clinical data of the
patients are depicted in Table I.
When compared with the non-PH group, patients in the PH group had a
significantly higher age (P=0.008) and higher prevalence of atrial
fibrillation (P=0.02). In addition, a higher proportion of PH
patients presented class III–IV disease (according to the NYHA
classification; P=0.03) and a reduced LVEF (P=0.007).
Indication for left heart surgery differed between
the two groups, with mitral valve regurgitation surgery required in
significantly more PH group patients, as the compared with the
non-PH group (100% vs. 30%, respectively; P 0.001).
Echocardiography
The hemodynamic and echocardiographic data of the
patients are shown in Table II. In
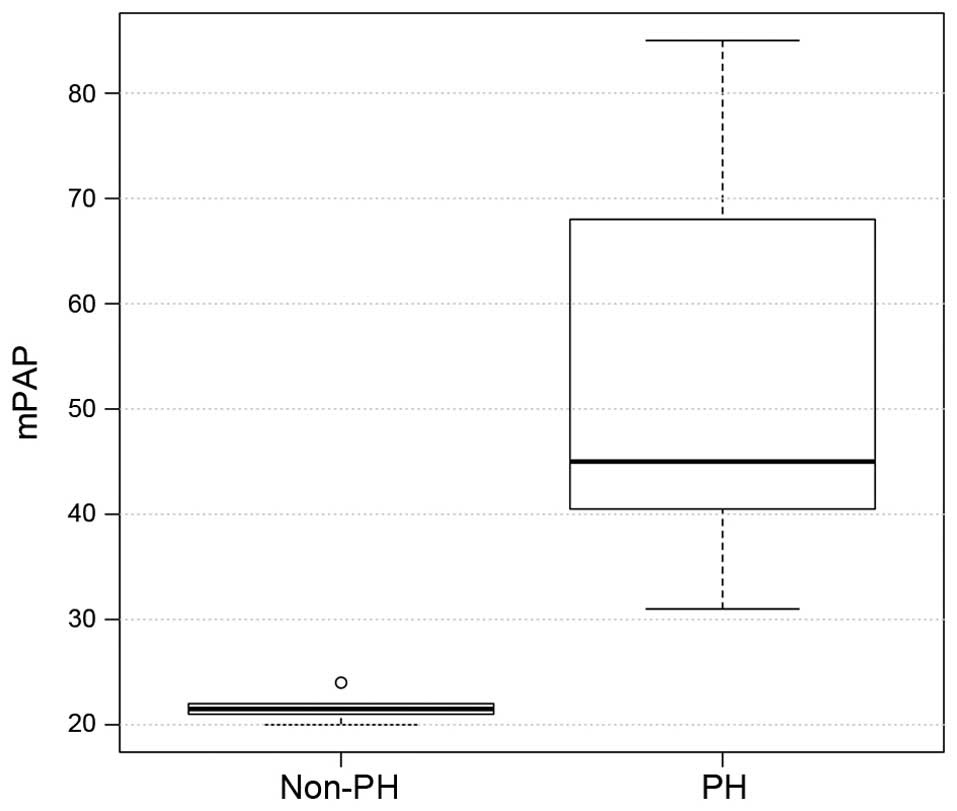
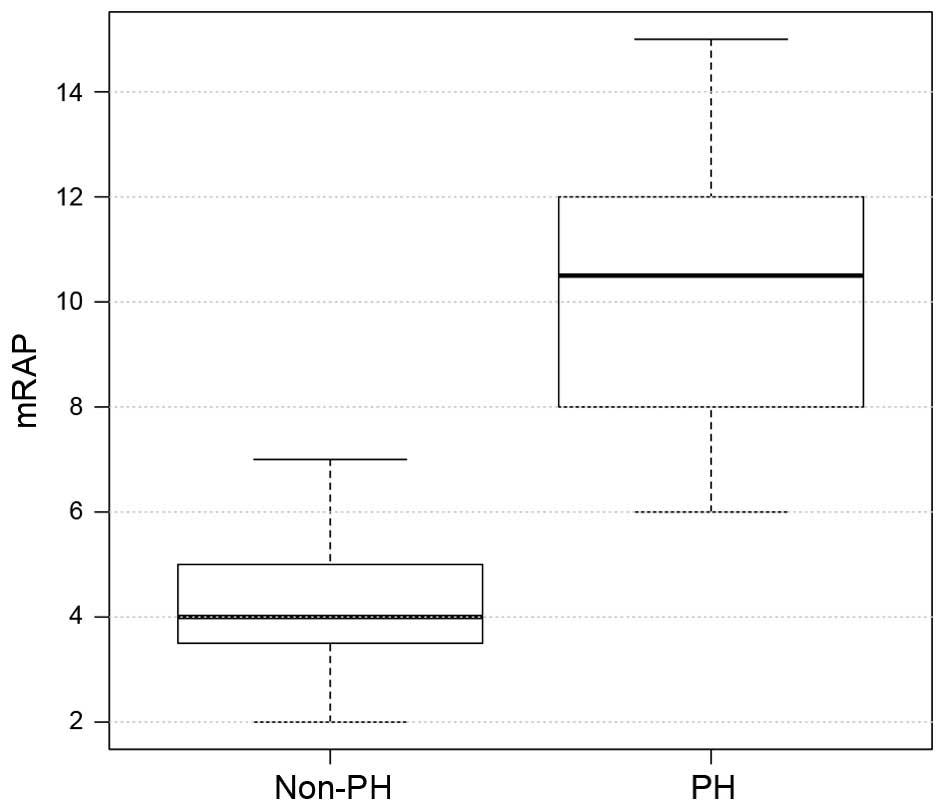
the PH group, mean PAWP, mPAP and mRAP were significantly higher,
as compared with the values in the non-PH group (all P<0.001;
Figs. 2–4), whereas the LVEF was significantly
reduced (P=0.007; Table II) as an
expression of post capillary PH with impaired LV function. Although
LV dilatation was more frequently observed in the PH group (P=0.03;
Table II), the LVEDD revealed no
differences between the two groups (60±11 mm in the PH group vs.
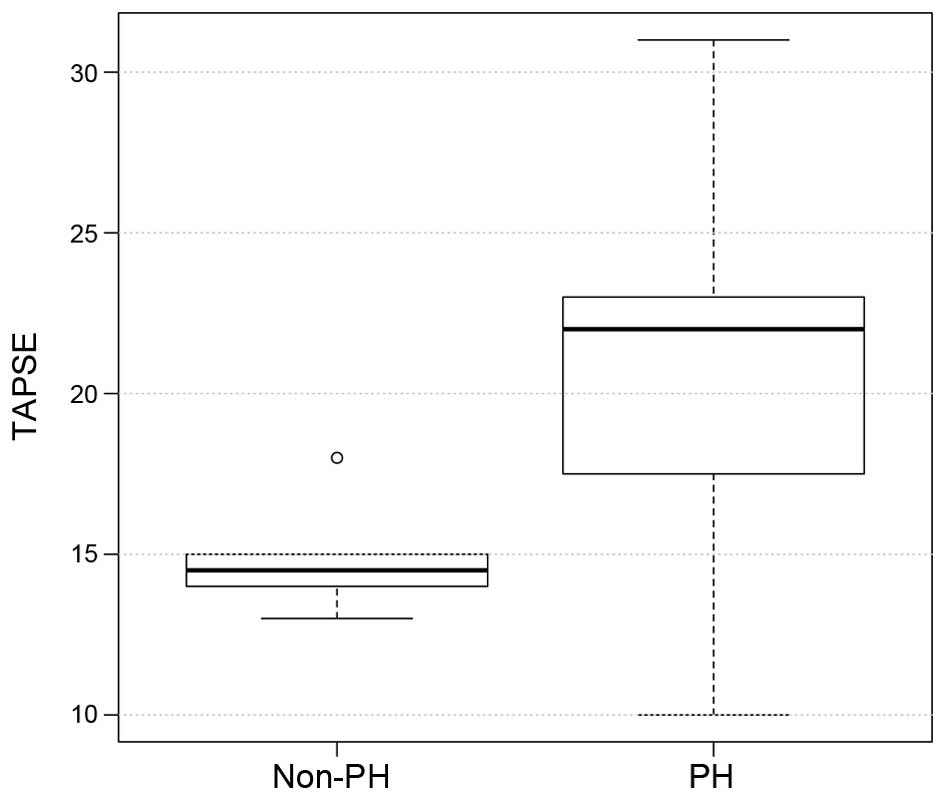
48±14 mm in the non-PH group; P=0.8; Table II). Similarly, TAPSE did not differ
significantly between the two groups (18±4.4 mm in the PH group vs.
21.5±2.2 mm in the non-PH group; P=0.8; Fig. 5), although the presence of RA
dilation (80 vs. 20%; P 0.001) and RV dilatation (20 vs. 10%; P
0.02) was significantly increased in the PH group compared with the
non-PH group (Table II).
Furthermore, significantly more patients in the PH group had a TR
≥II°, while TR was more frequently observed in the PH group (86 vs.
40%; P=0.04), when compared with the non-PH group.
 | Table II.Patient hemodynamic parameters. |
Table II.
Patient hemodynamic parameters.
| Variable | PH group
(n=15) | Non-PH group
(n=10) | P-value |
|---|
| LV ejection
fraction, % | 47.00±0.14 | 60.00±0.05 |
0.007 |
| Mean LVEDD, mm | 60.00±11.00 | 48.00±14.00 |
0.800 |
| TAPSE, mm | 18.00±4.40 | 21.50±2.20 |
0.800 |
| Tricuspid
regurgitation ≥II°, n (%) | 10 (67) | 0 (0) |
0.030 |
| Mitral valve
regurgitation, n (%) | 15
(100) | 5
(50) |
0.030 |
| Mitral valve
regurgitation ≥II°, n (%) | 15
(100) | 3
(30) |
0.001 |
| Aortic valve
stenosis, n (%) | 6
(40) | 3
(30) |
0.700 |
| Aortic
regurgitation, n (%) | 0 (0) | 2
(20) |
0.050 |
| mRAP, mmHg | 10.00±2.70 | 4±2.4 | <0.001 |
| mPAP, mmHg | 52.6±17 | 21.30±1.30 | <0.001 |
| Mean PAWP,
mmHg | 23.00±1.00 | 8.26±3.00 | <0.001 |
| RA dilatation, n
(%) | 12 (80) | 2 (20) |
0.001 |
| RV dilatation, n
(%) | 3
(20) | 1 (10) |
0.020 |
| LA dilatation, n
(%) | 11 (73) | 2 (20) |
0.040 |
| LV dilatation, n
(%) | 7
(47) | 1 (10) |
0.030 |
pCa-force measurements
The pCa-force values of the two groups are
demonstrated in Table III. Higher
force values were observed at the three highest concentrations of
calcium, with force values in the non-PH group being significantly
increased when compared with the PH group (at pCa 4.0: 4.1±0.5 vs.
2.9±0.3 mN, P=0.001; at pCa 4.5: 2.9±0.2 vs. 2.2±0.3 mN, P=0.01; at
pCa 5.0: 2.2±0.3 vs. 1.7±0.2 mN, P=0.01). In addition, calcium
sensitivity, which was defined as the pCa at half maximal force
(shown as pCa2+50) was different among
groups. The PH group achieved half maximal force at a pCa of 5.5,
whereas the non-PH group reached half maximal force at a pCa of
5.0. These findings suggest that patients with PH develop half
maximal force at a lower concentration of calcium, when compared
with non-PH patients; therefore, the affinity to calcium is higher
in the PH group.
 | Table III.pCa force values (mean ± standard
deviation). |
Table III.
pCa force values (mean ± standard
deviation).
| Variable | PH group
(n=15) | Non-PH group
(n=10) | P-value |
|---|
| pCa, mN |
|
|
|
|
4.0 | 2.90±0.30 | 4.1±0.50 | 0.001 |
|
4.5 | 2.20±0.30 | 2.9±0.20 | 0.010 |
|
5.0 | 1.70±0.20 | 2.2±0.30 | 0.010 |
|
5.5 | 1.40±0.04 | 1.2±0.03 | 0.800 |
|
6.0 | 1.00±0.04 | 0.8±0.03 | 0.900 |
|
6.5 | 0.60±0.05 | 0.4±0.03 | 0.800 |
|
pCa2+50 | 5.5 | 5.0 | 0.010 |
Discussion
The present study investigated an RA skinned fiber
model in patients with postcapillary PH due to valvular LV
dysfunction, and the results showed significantly reduced
contractile forces when compared with those in patients without PH.
These data may be interpreted as signs of impaired RA compensatory
mechanism in postcapillary PH. Notably, patients in the PH group
had absence of clinical and echocardiographic signs of overt RV
impairment, as indicated by the TAPSE values. According to previous
studies (18–20), TAPSE is a reliable echocardiographic
parameter for the assessment of RV function in the presence of
postcapillary PH. TAPSE has been recently reported to be equal or
even superior to other RV-echo-Doppler indices (18,19).
Guazzi et al (21) showed
that TAPSE and systolic PAP reflect the contractile state of the RV
in clinical settings; in this echocardiographic study about RV
contractile function, TAPSE and systolic PAP used as in vivo
indices for RV length and developed force were found to better
reflect the contractile state of RV. However, it was not possible
to exclude latent RV dysfunction with the methods used in the
present study, since TAPSE was at the lower limit of the
established values, RA and RV dilatation was present, and a higher
incidence of TR was detected. Furthermore, diastolic dysfunction
was not evaluated in the present study population due to the high
incidence of atrial fibrillation and TR, factors that make
determination of RV diastolic impairment difficult. Thus, based on
the observations, RV dysfunction can be suspected.
In precapillary PH, impaired RA contractility is
associated with signs of right heart failure (10). Since no clinical signs of right heart
failure were observed in the present study, but signs of chronic LV
dysfunction were detected, it was hypothesized that the patients
had chronic LV dysfunction involving the right ventricle at the
level of the contractile apparatus albeit with the RV function
clinically preserved. Due to the interventricular interaction
between LV and RV function, the RV function can be impaired in
patients with LV dysfunction. This physiological interaction has to
be considered, since up to one third of right-sided stroke
occurrence is due to LV septal contraction (22). This interaction has been proven in
the clinical setting of chronic volume overload in mitral valve
regurgitation. For instance, Le Tourneau et al (23) showed that, in patients with impaired
LV systolic function, the RV function is mainly dependent upon LV
remodeling and septal function, but only weakly dependent on
pulmonary systolic pressure. In precapillary PH, a compensatory
mechanism for RV functional impairment involves increased RA
contractility, which maintains RV filling over a long period of
time (6,9). Considering the importance of
interventricular interaction and RA function for RV performance,
dysfunction of both components may be detrimental in PH patients.
Reduced absolute force values in combination with increased calcium
sensitivity in the PH group indicate that RA compensatory
mechanisms are limited in postcapillary PH due to chronic LV
dysfunction, which may lead to an accelerated development of RV
failure. Only limited clinical data are available on the RA
compensatory mechanism for RV-functional adaptation in precapillary
and postcapillary PH (5,22,24).
The results of animal studies investigating the
effect of precapillary hypertension on the RA compensatory
mechanism are inconsistent (9,25). For
instance, Gaynor et al (6)
demonstrated the increase of RA contractility and distensibility as
a result of increased RV strain; however, methodological
limitations exist, since these data were calculated and not
measured. By contrast, studies in rodent models demonstrated that
induced PH resulted in marked reduction of
Ca2+-activated force and in reduced maximal
Ca2+-dependent force values (9,25). To
the best of our knowledge, no animal studies showing the effect of
postcapillary hypertension on the RA compensatory mechanism are
available. Due to the inconsistent results from animal studies, it
can only be speculated whether these data are applicable for the
clinical setting of chronic precapillary or postcapillary PH. The
majority of clinical studies investigated patients subsequent to
heart lung transplantation for severe precapillary PH, and did not
focus on cellular RA compensatory mechanisms (2,5). Rain
et al (5,26) demonstrated increased stiffness of
cardiomyocytes and cardiomyocyte sarcomeres as a result of RV
diastolic dysfunction, and increased calcium sensitivity as a
compensatory mechanism in RV-myocardial specimens in patients with
precapillary PH and impaired RV function. Upon investigation of the
left ventricle, it was demonstrated that reduced force capacity can
be compensated with increased affinity to calcium (27). This compensatory mechanism cannot
utilize full force capacity, since increased calcium sensitivity
leads to incomplete actin-myosin detachment; therefore, this
mechanism compensates only in part for RV myocardial dysfunction
(26).
To the best of our knowledge, this is the first
study in humans to examine RA tissue exposed to chronic volume
overload. However, the present study presented various limitations.
Firstly, the number of patients examined in current study was
limited. To overcome this drawback, a larger sample size is
required to support the current results. In addition, RA tissue was
examined, which may not be representative for RV contractile
dynamics. However, Vannier et al (28) demonstrated that the myofibrils
contractile force of atrial and ventricular fibers show the same
contractile and properties, which may support the assumption that
these results are representative for the right heart. Another
limitation of the present study is that the groups differed
concerning the indication for surgery. In the PH group, mitral
valve regurgitation with chronic volume overload and eccentric
hypertrophy was the main indication for surgery, whereas in the
non-PH group, aortic stenosis with pressure overload and concentric
hypertrophy was the predominant indication for surgery. Myocardial
hypertrophy is a strong factor influencing maximal calcium
activated force and calcium sensitivity in left heart failure in
rodents (29), although it remains
unclear whether this is also the case in humans. Differences in the
manifestation of hypertrophy may have an impact on RA calcium
activation, thus influencing the results of the present study. We
hypothesize that, although RV systolic function was preserved
(TAPSE, >16 mm), the higher prevalence of TR may be a sign of a
latent RV impairment that is not yet clinically apparent. This
hypothesis requires further investigation with magnet resonance
imaging (30).
In conclusion, the present preliminary results in
patients with postcapillary PH demonstrated reduced RA contractile
forces and increased calcium sensitivity. The present clinical
study is the first to show evidence that the RA compensatory
mechanism is already impaired at a point in time when no clinical
and overt echocardiographic signs of RV dysfunction are present in
postcapillary PH. This may have a clinical impact on the timing of
surgical intervention, since occurrence of RV dysfunction in left
heart disease is a powerful predictor of cardiovascular and overall
survival (23,31). The current results may support the
policy of early surgical or interventional treatment in patients
presenting with left heart valvular pathology. Nevertheless, it has
to be considered that these results are only preliminary, and
further clinical studies with larger patient cohorts are mandatory
to determine the clinical importance of these findings.
Acknowledgements
The authors would like to thank Mr. Alexander Graf
at the Department of Cardiothoracic and Vascular Surgery of the
University of Mainz for statistical support.
References
|
1
|
Galiè N, Humbert M, Vachiery JL, Gibbs S,
Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A,
Beghetti M, et al: 2015 ESC/ERS Guidelines for the diagnosis and
treatment of pulmonary hypertension: The Joint Task Force for the
Diagnosis and Treatment of Pulmonary Hypertension of the European
Society of Cardiology (ESC) and the European Respiratory Society
(ERS): Endorsed by: Association for European Paediatric and
Congenital Cardiology (AEPC), International Society for Heart and
Lung Transplantation (ISHLT). Eur Respir J. 46:903–975. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Trammell AW, Pugh ME, Newman JH, Hemnes AR
and Robbins IM: Use of pulmonary arterial hypertension-approved
therapy in the treatment of non-group 1 pulmonary hypertension at
US referral centers. Pulm Circ. 5:356–363. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Moraes DL, Colucci WS and Givertz MM:
Secondary pulmonary hypertension in chronic heart failure: The role
of the endothelium in pathophysiology and management. Circulation.
102:1718–1723. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murch SD, La Gerche A, Roberts TJ, Prior
DL, MacIsaac AI and Burns AT: Abnormal right ventricular relaxation
in pulmonary hypertension. Pulm Circ. 5:370–375. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rain S, Handoko ML, Trip P, Gan CT,
Westerhof N, Stienen GJ, Paulus WJ, Ottenheijm CA, Marcus JT,
Dorfmüller P, et al: Right ventricular diastolic impairment in
patients with pulmonary arterial hypertension. Circulation.
128:2016–2025. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gaynor SL, Maniar HS, Prasad SM, Steendijk
P and Moon MR: Reservoir and conduit function of right atrium:
Impact on right ventricular filling and cardiac output. Am J
Physiol Heart Circ Physiol. 288:H2140–H2145. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gaynor SL, Maniar HS, Bloch JB, Steendijk
P and Moon MR: Right atrial and ventricular adaptation to chronic
right ventricular pressure overload. Circulation. 112(Suppl 9):
S1212–S1218. 2005.
|
|
8
|
Leeuwenburgh BP, Helbing WA, Steendijk P,
Schoof PH and Baan J: Biventricular systolic function in young
lambs subject to chronic systemic right ventricular pressure
overload. Am J Physiol Heart Circ Physiol. 281:H2697–H2704.
2001.PubMed/NCBI
|
|
9
|
Fan D, Wannenburg T and de Tombe PP:
Decreased myocyte tension development and calcium responsiveness in
rat right ventricular pressure overload. Circulation. 95:2312–2317.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shiina Y, Funabashi N, Lee K, Daimon M,
Sekine T, Kawakubo M, Takahashi M, Yajima R, Tanabe N, Kuriyama T
and Komuro I: Right atrium contractility and right ventricular
diastolic function assessed by pulsed tissue Doppler imaging can
predict brain natriuretic peptide in adults with acquired pulmonary
hypertension. Int J Cardiol. 135:53–59. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
World Medical Association: World Medical
Association Declaration of Helsinki: Ethical Principles for Medial
Research Involving Human Subjects. JAMA. 284:3043–3045. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kossman CE: The Criteria of the New York
Heart Association: Diseases of the Heart and Blood Vessels:
Nomenclature and Criteria for Diagnosis (6th). Little, Brown and
Co. Boston: 110–114. 1964.
|
|
13
|
Simonneau G, Gatzoulis MA, Adatia I,
Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar
R, Landzberg M, Machado RF, et al: Updated clinical classification
of pulmonary hypertension. J Am Coll Cardiol. 62(Suppl 25):
D34–D41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rudski LG, Lai WW, Afilalo J, Hua L,
Handschumacher MD, Chandrasekaran K, Solomon SD, Louie EK and
Schiller NB: Guidelines for the echocardiographic assessment of the
right heart in adults: A report from the American Society of
Echocardiography endorsed by the European Association of
Echocardiography, a registered branch of the European Society of
Cardiology and the Canadian Society of Echocardiography. J Am Soc
Echocardiogr. 23:685–713; quiz 786–788. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lang RM, Bierig M, Devereux RB,
Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward
J, Shanewise JS, et al: Recommendations for chamber quantification:
A report from the American Society of Echocardiography's Guidelines
and Standards Committee and the Chamber Quantification Writing
Group, developed in conjunction with the European Association of
Echocardiography, a branch of the European Society of Cardiology. J
Am Soc Echocardiogr. 18:1440–1463. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Morano I, Arndt H, Gärtner C and Rüegg JC:
Skinned fibers of human atrium and ventricle: Myosin isoenzymes and
contractility. Circ Res. 62:632–639. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fabiato A and Fabiato F:
Excitation-contraction coupling of isolated cardiac fibers with
disrupted or closed sarcolemmas. Calcium-dependent cyclic and tonic
contractions. Circ Res. 31:293–307. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zakaria D, Sachdeva R, Gossett JM, Tang X
and O'Connor MJ: Tricuspid annular plane systolic excursion is
reduced in infants with pulmonary hypertension. Echocardiography.
32:834–838. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bano M, Kanaan UB, Ehrlich AC, McCracken
C, Morrow G, Oster ME and Sachdeva R: Improvement in tricuspid
annular plane systolic excursion with pulmonary hypertension
therapy in pediatric patients. Echocardiography. 32:1228–1232.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Damy T, Kallvikbacka-Bennett A, Goode K,
Khaleva O, Lewinter C, Hobkirk J, Nikitin NP, Dubois-Randé JL,
Hittinger L, Clark AL and Cleland JG: Prevalence of, associations
with, and prognostic value of tricuspid annular plane systolic
excursion (TAPSE) among out-patients referred for the evaluation of
heart failure. J Card Fail. 18:216–225. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Guazzi M, Bandera F, Pelissero G,
Castelvecchio S, Menicanti L, Ghio S, Temporelli PL and Arena R:
Tricuspid annular plane systolic excursion and pulmonary arterial
systolic pressure relationship in heart failure: An index of right
ventricular contractile function and prognosis. Am J Physiol Heart
Circ Physiol. 305:H1373–H1381. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Marcus JT, Vonk Noordegraaf A, Roeleveld
RJ, Postmus PE, Heethaar RM, Van Rossum AC and Boonstra A: Impaired
left ventricular filling due to right ventricular pressure overload
in primary pulmonary hypertension: Noninvasive monitoring using
MRI. Chest. 119:1761–1765. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Le Tourneau T, Deswarte G, Lamblin N,
Foucher-Hossin C, Fayad G, Richardson M, Polge AS, Vannesson C,
Topilsky Y, Juthier F, et al: Right ventricular systolic function
in organic mitral regurgitation: Impact of biventricular
impairment. Circulation. 127:1597–1608. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ferrari R, Böhm M, Cleland JG, Paulus WJ,
Pieske B, Rapezzi C and Tavazzi L: Heart failure with preserved
ejection fraction: Uncertainties and dilemmas. Eur J Heart Fail.
17:665–671. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Pérez NG, Hashimoto K, McCune S, Altschuld
RA and Marbán E: Origin of contractile dysfunction in heart
failure: Calcium cycling versus myofilaments. Circulation.
99:1077–1083. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rain S, Bos Dda S, Handoko ML, Westerhof
N, Stienen G, Ottenheijm C, Goebel M, Dorfmüller P, Guignabert C,
Humbert M, et al: Protein changes contributing to right ventricular
cardiomyocyte diastolic dysfunction in pulmonary arterial
hypertension. J Am Heart Assoc. 3:e0007162014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wankerl M, Böhm M, Morani I, Rüegg JC,
Eichhorn M and Erdmann E: Calcium sensitivity and myosin light
chain pattern of atrial and ventricular skinned cardiac fibers from
patients with various kinds of cardiac disease. J Moll Cell
Cardiol. 22:1425–1438. 1990. View Article : Google Scholar
|
|
28
|
Vannier C, Veksler V, Mekhfi H, Mateo P
and Ventura-Clapier R: Functional tissue and development
specifities of myofibrils and mitochondria in cardiac muscle. Can J
Physiol Pharmacol. 74:23–31. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perreault CL, Bin OH, Brooks WW, Ransil BJ
and Morgan JP: Differential effects of cardiac hypertrophy and
failure on right versus left ventricular calcium activation. Circ
Res. 67:707–712. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Naito H, Arisawa J, Harada K, Yamagami H,
Kozuka T and Tamura S: Assessment of right ventricular regional
contraction and comparison with the left ventricle in normal
humans: A cine magnetic resonance study with presaturation
myocardial tagging. Br Heart J. 74:186–191. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Haddad F, Doyle R, Murphy DJ and Hunt SA:
Right ventricular function in cardiovascular disease, part II:
Pathophysiology, clinical importance, and management of right
ventricular failure. Circulation. 117:1717–1731. 2008. View Article : Google Scholar : PubMed/NCBI
|