Introduction
Prosthetic joint infections (PJI) are a common
complication of joint arthroplasty and may lead to a poor outcome.
The incidence of PJI is 1.41% for knee prostheses and 0.92% for hip
prostheses, according to the Finnish joint registry data involving
112,708 prostheses (1). PJI is one
of the main reasons for the failure of joint arthroplasty (2). The most common pathogens causing PJI,
according to the data from the General Hospital of Chinese People's
Liberation Army (Beijing China), are gram-positive bacteria and, in
particular, coagulase-negative staphylococci (3). PJI caused by fungal pathogens are rare;
between 1966 and July 2012, Kuiper et al (4) reviewed 156 patients and reported eight
cases of fungal PJI.
According to a previous study, the risk factors
associated with the development of fungal PJI include
antibiotic-overuse, diabetes, tuberculosis, the use of
immunosuppressants and diseases associated with immunosuppression,
such as cancer and acquired immunodeficiency syndrome (AIDS)
(5). The potential association
between the surgical history of a patient, infection phase and the
development of fungal PJI has rarely been investigated (6). Therefore, the present study aimed to
investigate the surgical history of patients with fungal PJI at the
General Hospital of Chinese People's Liberation Army in order to
establish whether surgical history may be a potential risk factor
of fungal PJI.
The clinical manifestations of fungal PJI include
local and physical symptoms, such as pain, swelling and dysfunction
of the infected joint (4).
Furthermore, a few of the present cases presented with systematic
symptoms, including a fever, and the levels of c-reactive protein
(CRP) and erythrocyte sedimentation rate (ESR) were elevated in the
majority of patients. The manifestations of an infected joint
following an X-ray may include loosening, osteolysis and/or
swelling of soft tissue, although some patients may have none of
these features (7). The most common
group and species of fungus associated with fungal PJI include
Candida and Candida albicans (7). A PJI may be termed a hybrid infection
when it is caused by multiple pathogens. A number of patients in
the present study exhibited hybrid infections. According to Wimmer
et al, the proportion of hybrid infections in all PJI
patients was 48% (37/77). Hybrid infections have been shown to
reduce the cure rate of PJI, as compared with a PJI caused by a
single pathogen, thus suggesting that more attention should be paid
to these patients (8).
In the case of bacterial PJI, the number of
polymorphonuclear leukocytes in frozen and permanent histological
sections of the infected soft tissue has been shown to increase
(9). Mirra et al (10) reported a strong correlation between
the identification of five polymorphonuclear leukocytes in at least
five separate microscopic fields and infection. However, there have
been no reports regarding the histopathological features of fungal
PJI; thus, it is unclear whether the association between active
infection and an increased number of polymorphonuclear leukocytes
in histological sections also applies to fungal PJI. Therefore, the
present study investigated the histopathological features of fungal
PJI.
At present, there are no guidelines regarding the
protocol for the treatment of fungal PJI. In the case of bacterial
PJI, the standard therapeutic strategy, which has received a high
consensus, is the two stage-exchange protocol (11). The first stage involves removing the
prosthesis, performing débridement and implanting a cement spacer
saturated with antimicrobials, while the second stage involves
removing the spacer and reimplanting the prosthesis (12). The two-stage exchange has shown good
efficacy for the treatment of bacterial PJI. However, there are few
examples in the literature regarding whether this protocol may also
be successfully applied to fungal PJI (4).
The present retrospective study aimed to identify
the risk factors associated with fungal PJI in patients at The
General Hospital of Chinese People's Liberation Army, as well as to
investigate the association between the surgical history of these
patients and the development of fungal PJI. In addition, the
clinical manifestations, in particular the pathological features,
were described, in order to document the characteristics of fungal
PJI. Furthermore, the effectiveness of the two-stage exchange
protocol for the treatment of fungal PJI was evaluated.
Materials and methods
Patients and diagnosis
A total of eight patients (Table I) with fungal PJI, including four
cases of the hip and four of the knee, who were admitted to the
Department of Orthopaedics at The General Hospital of Chinese
People's Liberation Army between May 2000 and March 2012, were
retrospectively analyzed in the present study. Written informed
consent was obtained from all patients for the publication of this
study.
 | Table I.Information regarding the basic
condition, chronic disease history, infection phase and
microbiological features of the eight patients with a fungal
prosthetic joint infection. |
Table I.
Information regarding the basic
condition, chronic disease history, infection phase and
microbiological features of the eight patients with a fungal
prosthetic joint infection.
| Case no. | Age/gender | Chronic disease
history | Site/phase | Fungal species | Other pathogen | Sinus | Positive culture
site |
|---|
| 1 | 42/M | No | Right hip/delayed
phase (9 Mon) | Candida
albicans | Acinetobacter
lwoffii, | Absence CNS | 6x post-op drainage
Intra-op culture |
| 2 | 53/F | Diabetes | Left hip/early
phase (1 W) | Candida
albicans | SA | Presence | Intra-op culture,
Post-op exudate |
| 3 | 43/F | Gallbladder
excision | Left hip/late phase
(7 Y) | Candida
albicans | Enterococcus
faecalis | Presence | Sinus intra-op
culture |
| 4 | 78/M | No | Left hip/delayed
phase (11 Mon) | Candida
glabrata | Gram negative
bacilli, SA | Presence | Sinus intra-op
culture, post-op drainage |
| 5 | 76/F | Hypertension | Right knee/delayed
phase (8 Mon) | Mould | CNS | Absence | Intra-op
culture |
| 6 | 58/F | No | Left knee/early
phase (<1 W) | Candida
freyschussii | No | Presence | Intra-op
culture |
| 7 | 63/F | No | Left knee/delayed
phase (2 Y) | Aspergillus
spp. | No | Absence | Intra-op
culture |
| 8 | 67/M | Hypertension | Left knee/early
phase (3 Mon) | Candida
parapsilosis | No | Presence | Post-op
exudate |
Venous blood samples (2 ml) from all patients were
sent to the Biochemical Laboratory for the detection of plasma
albumin and serum creatinine, urea, alanine transaminase (ALT) and
aspartate aminotransferase (AST) levels using an automated
biochemical analyzer (Olympus AU2700; Olympus Corporation, Tokyo,
Japan). The soft tissue samples, synovial fluid, drainage fluid or
sinus fluid were sent to the Microbiology Department for culture of
the pathogen. Cultured fungi were identified into genus and species
by matrix-assisted laser desorption/ionization-time of flight mass
spectrometry (VITEK® MS; bioMérieux, Marcy l'Etoile,
France). The patients were diagnosed with fungal PJI if the same
fungus was cultured from ≥2 specimens obtained from preoperative
aspirate, a sinus, postoperative drainage fluid, wound exudate
and/or intraoperative tissue or synovial fluid. Culture was
performed preoperatively using fluid from the aspiration in three
cases and fluid from a sinus in two cases. A fungal pathogen was
detected for the two cases in which the fluid from a sinus was
cultured (Table I). The remaining
six cases were diagnosed with PJI via analysis of frozen tissue
sections obtained intraoperatively or by the presence of a sinus.
PJI could not be excluded if the frozen tissue section was negative
due to the high false negative rate of frozen tissue sections with
regard to the diagnosis of PJI (13,14). The
pathogen was confirmed as a fungi post-operatively according to the
intraoperative culture and postoperative drainage culture (Table I).
Treatment protocol
The two-stage exchange protocol involved the removal
of the primary prosthesis and implantation of the cemented spacer
saturated with antimicrobials in the first stage, and the
implantation of the prosthesis in the second stage. The therapeutic
strategy for the eight patients included two-stage exchange and
resection arthroplasty (Fig. 1). A
cemented spacer saturated with antimicrobials was made according to
our previous study (15), and was
used when both the two-stage exchange protocol and resection
arthroplasty were performed. Since five of the eight cases were
diagnosed with hybrid infections, the antimicrobials saturated in
the cemented spacer included vancomycin (Eli Lilly Suzhou
Pharmaceutical Group Co., Ltd., Suzhou, China), meropenem (Sumitomo
Dainippon Pharma Co., Ltd., Osaka, Japan) and/or amphotericin B
(Ben Venue Laboratories, Inc., Bedford, OH, USA). The types and
doses of the antimicrobials are presented in Table II.
 | Table II.Treatment and outcome information for
the eight patients with a fungal prosthetic joint infection. |
Table II.
Treatment and outcome information for
the eight patients with a fungal prosthetic joint infection.
| Case No. |
Surgery/outcome | Antimicrobial
saturated in cement/duration in joint |
Antimicrobials/duration | Pre/post-op Harris
or HSS | Follow-up
duration/results |
|---|
| 1 | Two-stage
revision/success | 4 g Van/40 g/3
Mon | After spacer,
Flu/1.5 Mon after revision, Flu/1 Mon | 48/92 | 3.3 Y/No
recurrence |
| 2 | Two-stage
revision/failure two-stage revision/success | 2nd spacer 4.8 g
Van/40 g/3.6 Mon | After 2nd spacer,
Flu/1.5 Mon after 2nd revision, Flu/1 Mon | 45/87 | 6.6 Y/No
recurrence |
| 3 | Two-stage
revision/success | 4 g Van+2 g Mer/40
g/4.5 Mon | Pre-op Flu, Vor/1
Mon after spacer, Flu/4 Mon after revision, Flu/1Mon | 43/86 | 5.1 Y/No
recurrence |
| 4 | Spacer
implantation/failure resection arthroplasty/success | 4.8 g Van/40 g/6
Mon | After spacer, Flu/3
Mon, Amp/2 Mon after resection arthroplasty Cas/46 days | 37/62 | 2.9 Y/No recurrence
before death |
| 5 | Two-stage
revision/success | 3 g Van+1 g Mer/40
g/3 Mon | After spacer,
Flu/1.5 Mon after revision, Flu/1 Mon | 46/89 | 3.7 Y/No
recurrence |
| 6 | Two-stage
revision/success | 4 g Van/40 g/3
Mon | After spacer,
Flu/1.5 Mon after revision, Cas/1 Mon | 35/91 | 4.7 Y/No
recurrence |
| 7 | Two-stage
revision/success | 3 g Van+1 g Mer/40
g/7 Mon | After spacer, Flu/6
Mon after revision, Flu/1 Mon | 40/91 | 5.2 Y/No
recurrence |
| 8 | Two-stage
revision/success | 2 g Van+200 mg
Amp/40 g/4 Mon | After spacer,
Flu/1.5 Mon after revision, Flu/1 Mon | 55/90 | 4 Y/No
recurrence |
Following implantation of the cemented spacer,
systematic antifungal agents, including fluconazol (400 mg once
daily; intravenous for 7 days and then orally; Shandong Luoxin
Pharmaceutical Group Stock Co., Ltd., Shandong, China),
voriconazole (5 mg/kg intravenously twice daily for 7 days, then
200 mg orally twice daily; Livzon Pharmaceutical Group Inc.,
Zhuhai, China) amphotericin B (initially 5 mg/day, then gradually
increased to 40 mg/day, once daily) and/or caspofungin (70 mg on
the first day, then 50 mg intravenously once daily; Merck Sharp
& Dohme, Clermont-Ferrand, France), were administered for 6
weeks.
Venous blood samples (2 ml) from all patients were
sent to the Clinical Laboratory Department for the detection of CRP
levels using the immune scatter turbidimetry method (16), on a MicroScan Turbidity Meter with
CRP detecting reagents (Siemens AG, Munich, Germany). In addition,
white blood cell (WBC) levels were detected using the ADVIA 2120i
Hematology System with Autoslide (Siemens AG). The levels of CRP
were examined prior to removal of the cemented spacer and
reimplantation of the prosthesis.
Histopathological analysis
Samples of the soft tissue surrounding the
prosthesis, which was highly suspected as the infected tissue, were
obtained intraoperatively from all patients, frozen and sent to the
Pathology Department for histopathological analyses and to
determine whether the pathogen had been completely eradicated.
Briefly, the tissue samples were made into a permanent
paraffin-embedded block (Huayong Paraffin Co., Ltd., Shanghai,
China), sliced into sections (5 µm) using a microtome (RM2235;
Leica Microsystems GmbH, Wetzlar, Germany) and stained with
hematoxylin & eosin (Leica Microsystems GmbH). Subsequently,
the permanent sections were observed under a microscope (CX31;
Olympus Corporation). Five high power fields (HPF; 400x
magnification) of vision for each section were observed to
calculate the average number of polymorphonuclear cells. If the
average number of polymorphonuclear cells was >10, infection
persistence was assumed and another cemented spacer was
implanted.
Outcome evaluation
The treatment was considered a success if the
infection was successfully eradicated without persistence or
recurrence during the ≥2 years follow-up period. Conversely, the
treatment was considered a failure in the case of recurrence or
persistence of fungal PJI, as supported by clinical examination,
signs or symptoms within 2 years post-operatively. The pain and
function of the hip or knee joint were evaluated pre-operatively
and at the final follow-up using the Harris Hip Score (17) or Hospital for Special Surgery (HSS)
score (18). The pain and function
of the joints of patients evaluated by the HHS or HSS scores are
considered good if the scores are between 80–89, and are considered
excellent if the scores are between 90–100 (17,18).
Prior to and following surgery, the patients were sent to the
Medical Image Department for X-ray examination (Discovery XR656; GE
Healthcare Bio-Sciences, Pittsburgh, PA, USA) of the affected
joint.
Statistical analysis
Data are expressed as the mean ± standard deviation,
which was calculated using SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA).
Results
Demography and risk factors
The age, gender, places of residence, history of
chronic diseases and nutritional information were collected for all
patients. The average follow-up duration was 4.4 years (range,
2.9–6.6 years). The potential risk factors for fungal PJI,
including immunosuppression-associated diseases or factors,
duration from primary arthroplasty to the onset of symptoms of
infection, the frequency at which the patient had undergone
surgical procedures prior to the diagnosis of fungal PJI and the
presence of hybrid infection with/without sinus are presented in
Table I. The average age of the
patient was 60 years, of which four patients were older than 60
years. Of the patients, five were female and three were male. Of
the eight cases, seven were residents of urban areas and the
remaining patient was from a rural area. Two of the patients had
hypertension, one had diabetes mellitus and one had undergone a
cholecystectomy. There was no evidence of
immunosuppression-associated diseases in any of the patients. The
average body mass index of the patient's was 22.89±5.33
kg/m2 (normal range, 18.5–23.9 kg/m2). One
patient had plasma albumin levels of 33 g/l (normal range, 35–50
g/l). There were no evidence indicating that any of the patients
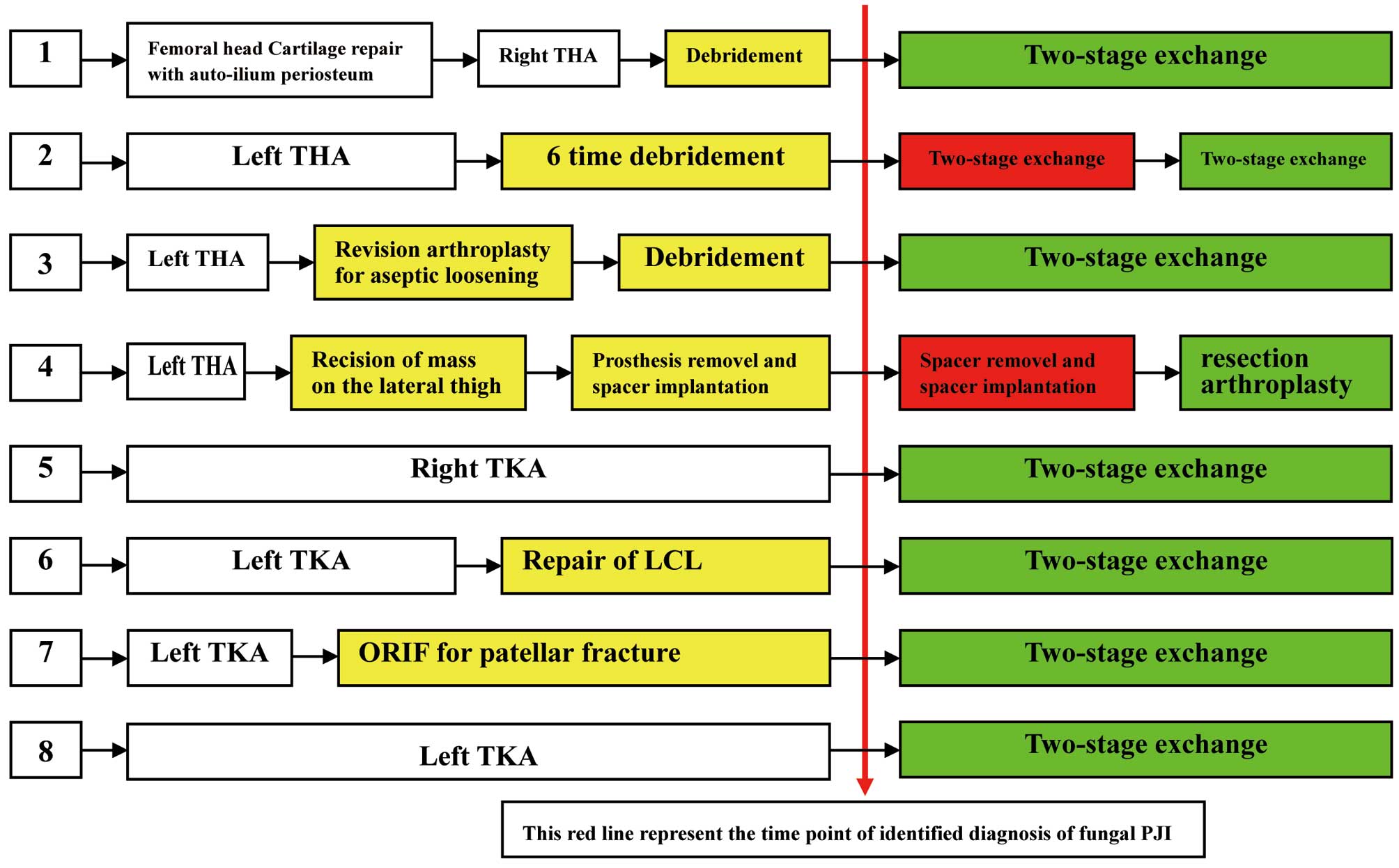
had abnormal nutrition conditions. Of the eight cases, six had
previously undergone additional surgery on the infected joint. This
surgery had been conducted following the primary total joint
arthroplasty and prior to the diagnosis of fungal PJI (Fig. 2).
The duration from primary arthroplasty to the onset
of symptoms of infection in the eight patients are presented in
Table I. Three cases were defined as
early infections (<3 months), four cases were delayed infections
(3 months to 2 years) and one case was of a late infection (>2
years) (7). Typically, early and
delayed phase infections were considered to be associated with
factors of the surgery, whereas the late phase infection was
considered to be associated with a predisposing factor in the
patient. Five cases were hybrid infections, of which three had a
sinus simultaneously.
Clinical manifestation
The symptoms experienced by the eight patients
included swelling, pain, dysfunction and limited mobility of the
infected joint. Five of the eight cases had sinus and effusion of
the infected limb. The temperature of one patient was 37.5°C, which
was around the upper limit of the normal range. All other cases had
a normal body temperature.
Laboratory examination
The CRP levels were elevated above the normal range
(0–0.8 mg/dl) in six patients, but were normal in the remaining two
patients, and the values were 11.8, 6.23, 6.3, 6.02, 0.65, 0.95,
4.99 and 0.48 mg/dl. ESR were higher than normal (normal range,
0–20 mm/h) in seven patients and were on the threshold value in one
patient, and the values were 130, 61, 86, 67, 80, 75, 25 and 20
mm/h. The WBC counts of the eight patients were 12.43, 6.12, 6.95,
9.42, 3.31, 4.87, 5.92 and 4.42×109/l (normal range,
3.5–10×109/l). The WBC count was higher than normal in
one patient, but was normal in seven patients. The plasma albumin
levels were assessed in order to evaluate the nutritional condition
of the patients and were 45.6, 47.8, 43.5, 33.0, 49.8, 44.2, 39.8
and 37.6 g/l (normal range, 35–50 g/l) for the eight patients. The
serum creatinine and urea levels were normal in all patients. The
ALT levels of the eight patients were 10.3, 9.5, 11.3, 50.9, 15.7,
17.4, 12.9 and 15.8 U/l (normal range, 0–40 U/l) and the AST levels
were 24.9, 27.1, 21.6, 58.2, 20.8, 19.1, 18.6 and 21.8 U/l (normal
range, 0–40 U/l). The levels of ALT and AST were slightly elevated
in one patient and were normal in the other seven patients.
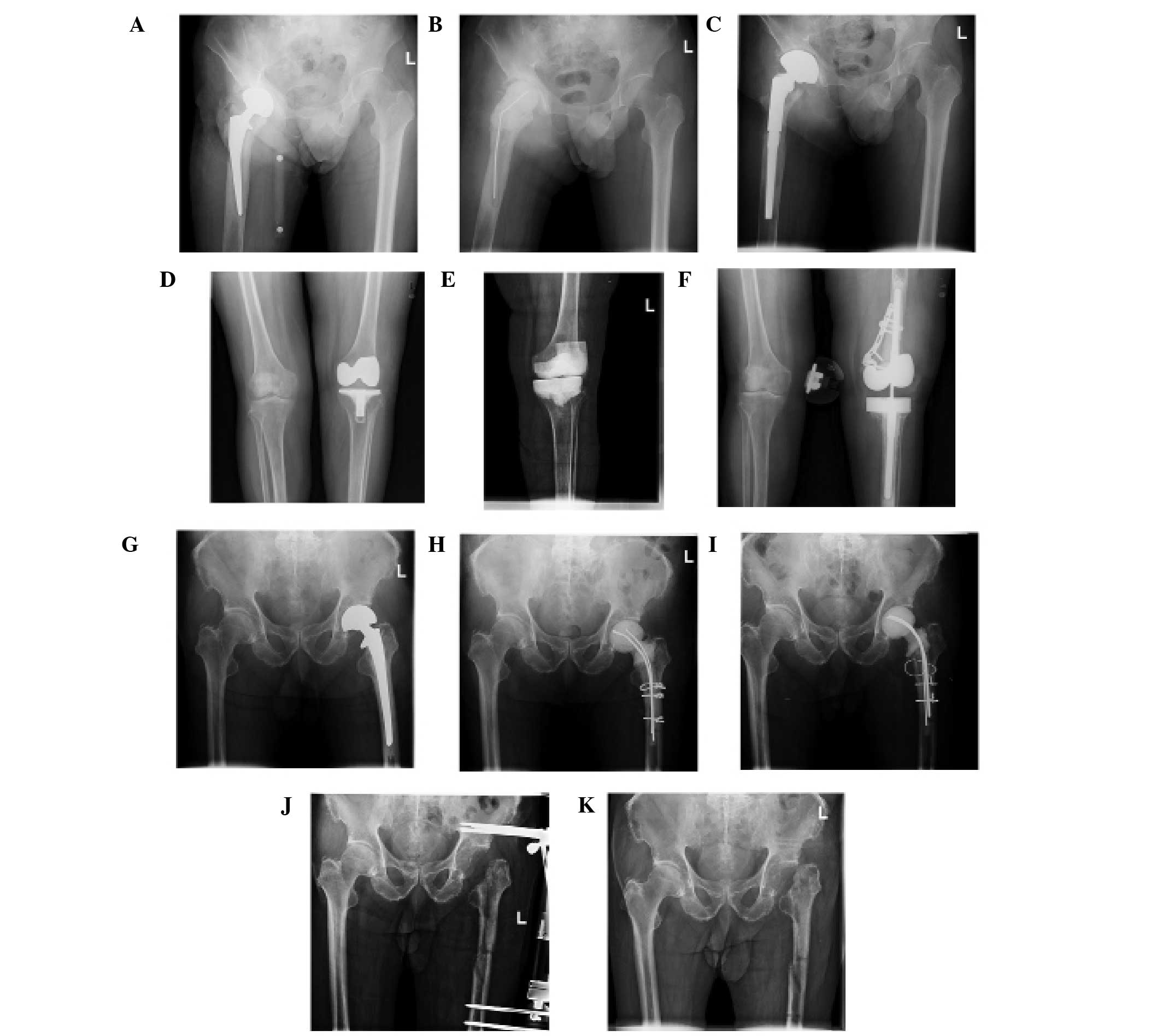
X-ray
The eight cases had no features specific to fungal
PJI. Soft tissue swelling, a lucent line between the prosthesis and
bone, and osteolysis were observed in some of the patients
(Fig. 1).
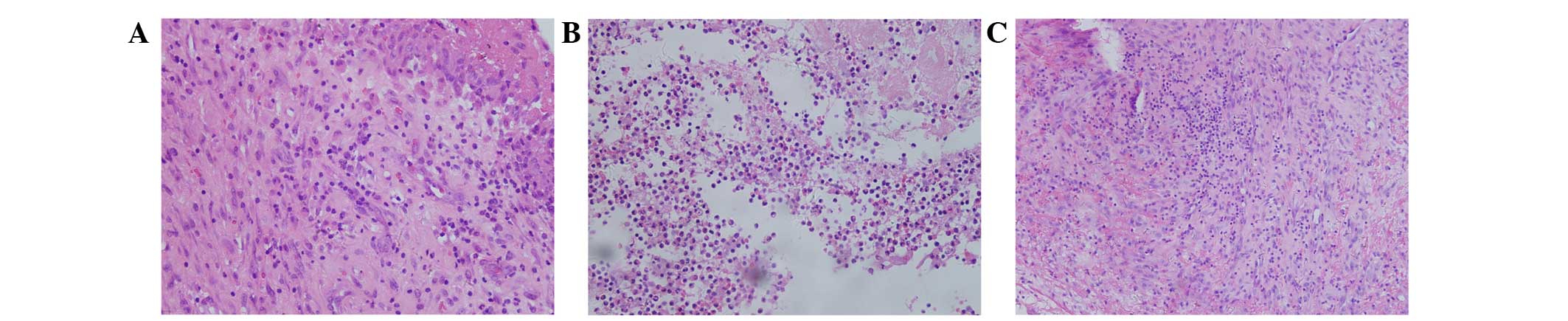
Histopathological features
The permanent tissue sections obtained
intraoperatively from the infected joints were analyzed under a
microscope. Five high-power fields of vision for each section were
used to calculate the average number of polymorphonuclear cells.
The average values of the three patients infected with a single
fungus were 0/HPF, 2/HPF and 3/HPF, corresponding to cases 6, 7 and
8, respectively. The average values of the other five patients
infected with a fungus as well as bacteria were 12/HPF, 4/HPF,
90/HPF, 130/HPF and 12/HPF, corresponding to cases 1, 2, 3, 4 and
5, respectively. Fungal hyphae were observed in the tissue section
of case 5; fungal spores were not observed in any of the cases
(Fig. 3).
Microbiological features
The positively cultured tissue specimens included
eight intraoperative synovia and/or soft tissue, two preoperative
fluid samples from sinuses, two postoperative drainage fluid
samples from the infected joints and two postoperative fluid
samples from wound exudate (Table
I). Of the eight patients, six harbored Candida species
and two harbored moulds. The Candida isolates included three
Candida albicans, one Candida freyschussii, one
Candida glabrata and one Candida parapsilosis. Of the
two molds, one was of the Aspergillus genus. To the best of
our knowledge, no previous cases of PJI have been caused by C.
freyschussii. C. freyschussii and C. glabrata were
susceptible to treatment with fluconazol at the initiation of
antifungal therapy; however, due to the lack of effectiveness of
the fluconazol treatment, the pathogen were once again cultured and
antimicrobial susceptibility testing demonstrated that resistance
to fluconazol was emerging. However, the pathogens remained
susceptible to caspofungin.
Evaluation of treatment
The average preoperative Harris or HSS score was
43.6 and the average postoperative Harris or HSS score was 86. Case
4 was treated by resection arthroplasty without prosthesis
implantation, so the Harris score of this patient was 62 following
the surgical procedure. The remaining seven cases had good or
excellent Harris or HSS scores. The scores for the eight cases are
presented in Table II.
The treatment regimes for the eight cases is
presented in Fig. 2. Case 2 was
initially treated with the two-stage exchange protocol; however,
the infection persisted and so a second two-stage exchange was
performed. Case 4 was initially treated by two time of implantation
of cemented spacer and latest time by resection arthroplasty
without any spacer or prosthesis because the patient refuse to
receive implantation of prosthesis. The remaining six cases were
successfully treated by two-stage exchange. In total, the two-stage
exchange protocol was performed eight times in seven cases, in
which the protocol was successful seven times. The failure rate of
two-stage exchange protocol was 12.5%.
The average duration of antifungal agent usage in
the cases who had undergone two-stage exchange following
implantation of the cement spacer and prior to the implantation of
prosthesis was 2.8 months; in five of the cases, the duration was
1.5 months. In cases 3, 4 and 7, the duration of systematic
antifungal agent administration was prolonged following
implantation of the cement spacer to prevent the persistence of
infection until the CRP levels had decreased to within the normal
range.
Following reimplantation of the prosthesis, the
average duration of antifungal usage was 1 month. The
antimicrobials saturated into the cement implant included
vancomycin, meropenem and amphotericin B. The average duration of
spacer implantation in the infected joint was 4.3 months (Table II). Case 4 was infected with C.
glabrata, which was primarily susceptible to fluconazol and
amphotericin B. However, following administration of fluconazol and
amphotericin B for ~5 months, the infection had not been eradicated
and the treatment was considered a failure. Therefore, this patient
underwent resection arthroplasty without a spacer or prosthesis.
The intraoperative tissue of this patient was cultured again, which
demonstrated that the pathogen was resistant to fluconazol and
amphotericin B, although it was susceptible to caspofungin. Thus,
the patient underwent treatment with caspofungin for 46 days,
permitting eradication of the infection.
Discussion
Fungal PJI are rare; Kuiper et al (4) reviewed 164 cases of fungal PJI between
1966 and July 2012, and Azzam et al (7) conducted a multicentre analysis on
fungal PJI, which included 31 cases between 1999 and 2006. Of the
31 cases reported by Azzam et al (7), 27 had experienced at least one chronic
disease, including renal disease, a malignancy, rheumatoid
arthritis, diabetes mellitus or hepatic and cardiac diseases, and
some cases had a history of prolonged treatment with antibiotics or
chronic steroids. In addition, two patients had undergone multiple
revision surgeries prior to the development of a fungal PJI. Kao
et al (5) reviewed 837 cases
of candidemia and determined that ~24.8% cases had previously been
diagnosed with an immunodeficiency-associated condition, including
lymphoma, leukemia, rheumatic diseases or AIDS, or had undergone an
organ transplantation or extensive treatment with steroids. In the
present study, none of the eight cases were diagnosed with an
immunodeficiency-associated condition. Seven of the eight cases
presented with early or delayed phase infections, which were
considered to be due to factors associated with the surgical
procedure. Conversely, the late phase infection was associated with
a predisposing disease in the patient, such as chronic diseases and
an immunodeficiency-associated condition. Of the eight cases, six
had undergone additional surgery on the infected joint following
the primary total joint arthroplasty and preceding the diagnosis of
fungal PJI. The additional surgery may have increased the
possibility of exposure to the pathogenic fungus.
Case 6 was infected with C. freyschussii,
which, to the best of our knowledge, is the first report of a
fungal PJI caused by this species. The most common pathogenic
fungal group and species in these eight cases were Candida
and C. albicans, which is consistent with previous reports
(7,19). The diagnosis of fungal PJI remains
challenging, due to the difficulty of culturing the pathogen from
the aspiration of the infected joint preoperatively. Three cases in
the present study underwent preoperative aspiration; however, none
of them had a positive culture. In addition, when both fungal and
bacterial pathogens are detected upon culturing, it is difficult to
identify the cause of the infection. Therefore, it may be necessary
to obtain and culture multiple intraoperative specimens, including
both synovial fluid and granulation tissue, from various sites of
the joint. In the present study, the fluid from a sinus was
extracted and cultured in order to identify the causative
pathogen.
To the best of our knowledge, there have been no
previous reports regarding the histopathological features of fungal
PJI, although the histopathological features of general fungal
infections include acute inflammation and chronic granuloma
formation (20). Ritter et al
(21) reported 40 cases of
Exserohilum infections caused by contaminated steroid
injections. The infection site included meninges, epidural,
peripheral joint and injection site and the histopathological
features included suppurative and granulomatous meningitis and
vasculitis. Ayhan et al (22)
reported the incidence of chronic granulomatous Aspergillus
synovitis in a 5-year-old girl with acute lymphoblastic leukemia
undergoing chemotherapy, in which the main histopathological
characteristic was a chronic granuloma. Typically, in the chronic
phase of a fungal infection, the number of polymorphonuclear
leukocytes do not increase and the granuloma is the main
histopathological manifestation (23). In the present study, the
polymorphonuclear leukocyte count was normal in three of the cases
in which the pathogen was a fungus only, which is consistent with
previous reports (21,22). Therefore, it is not possible to
exclude the diagnosis of fungal PJI if the histological frozen
tissue section or permanent tissue section has a normal
polymorphonuclear leukocyte count. Instead, if a fungus PJI is
suspected, Grocott's methenamine silver stain, Periodic acid-Schiff
stain or Fontana-Masson stain may be used, since these are able to
detect most forms of fungi, including hyphae and spores (21).
Kuiper et al (4) reviewed 164 cases of fungal PJI from
1966 to July 2012 and reported a success rate for the two-stage
exchange protocol of 84.8% (67/79). In the 31 cases of fungal PJI
reported by Azzam et al (7),
19 had undergone the two-stage exchange protocol, of which 10 had
recurrence of infection, and so the success rate was 47.4%. Phelan
et al (24) reported four
cases and reviewed six cases of Candidal PJI that were treated by
the two-stage exchange protocol, and reported a success rate of
80%. In addition, Anagnostakos et al (19) reported seven cases of fungal PJI
treated by the two-stage exchange protocol without recurrence in
2012, and Ueng et al (25)
reported 16 patients who had undergone the two-stage exchange
protocol, of which eight relapsed or the infection was not
controlled, and the success rate was 50%. The present study
demonstrated a success rate of 87.5% for the seven cases (a total
of eight times) who had undergone the two-stage exchange protocol.
Sia et al (26) reviewed
1,077 cases of PJI treated by the two-stage exchange protocol in 30
studies, and reported an average success rate of 87% (range,
57–100%). A pattern that emerged from the literature review was
that patients with fungal PJI that were treated by the two-stage
exchange protocol were more likely to relapse, as compared with
patients with bacterial PJI that underwent the same treatment
(7,25,26).
However, at present this remains a hypothesis with no definitive
evidence. There has been only one report of a patient with fungal
PJI who was successfully treated by the one-stage exchange protocol
(27), and the strategy of
débridement with prosthesis retention has had few successful
reports (28–30). Therefore, the two-stage exchange
protocol may be considered the most effective strategy for the
treatment of fungal PJI (4).
At present, the antifungal agents that are used to
saturate the cement spacer are amphotericin B and voriconazole.
Although the elution concentration of amphotericin B surrounding
the cement spacer was undetectable when it was combined with cement
in vivo (31) and in
vitro (32), a poragen, such as
cephazolin, may be added to the cement, together with amphotericin
B, in order to facilitate the elution of amphotericin B. A previous
study reported that 200 mg amphotericin B and 10 g cephazolin may
be added together in 40 g cement (33). Voriconazole was reported to have an
excellent elution concentration and bioactivity both in vivo
(34) and in vitro (35,36).
Deelstra et al (37) reported
a case of a C. albicans prosthetic hip infection
successfully treated by staged exchange with a cement spacer
saturated with voriconazole, amphotericin B and vancomycin.
However, at the time of the present study, there was no evidence to
support the use of amphotericin B and so it was only added to the
cement spacer for one of the eight cases. Nevertheless, the effect
of a cement spacer loaded with voriconazole and amphotericin B on
the eradication of a fungal PJI requires further clinical
investigation.
At present, the duration for which the antifungal
agent should be used following the implantation of the spacer and
prior to reimplantation of the prosthesis is unknown, and there are
no guidelines to address this issue. A previous study recommended
that an antifungal agent should be administered for ≥1 year, in
order to eradicate a fungal infection (30), and the Infectious Diseases Society of
America guidelines recommend between 6 and 12 months (38). However, Anagnostakos et al
(19) suggested that 6 weeks of
administration of an antifungal agent following the implantation of
a spacer was sufficient. Furthermore, Kuiper et al (4) demonstrated that the outcomes of
patients who had received antifungal agent therapy for 0–6 weeks
were no different, as compared with the outcomes of patients who
had received the same therapy for 0–2 months, 0–3 months or 0–6
months. Although five of the eight cases in the present study were
treated for 1.5 months, three underwent an elongated treatment
regime as their CRP levels were higher than normal, which may be
considered a prognostic marker for the persistence of an infection.
Therefore, the duration of which the antifungal agent should be
used following implantation of the spacer and prior to
reimplantation of the prothesis remains unclear and requires
further analysis. It is important to note that antimicrobial
resistance may lead to therapy failure. In the present study, the
pathogens isolated from cases 4 and 6 were resistant to fluconazol,
although they were susceptible to caspofungin. Therefore, the
susceptibility profile of the pathogen causing the fungal PJI
should be analyzed rapidly in order to ensure the effectiveness of
the antifungal agent being used.
In conclusion, the present study demonstrated that
undergoing surgery on a prothesis is a potential risk factor for
the development of fungal PJI. In addition, in the permanent
histological sections from the infected tissue of a fungal PJI, the
polymorphonuclear leukocyte count did not increase, as is typically
observed for bacterial PJI; thus suggesting that the number of
polymorphonuclear leukocytes in the joint may not be used for the
diagnosis of fungal PJI. Finally, a two-stage exchange protocol
with implantation of a cement spacer saturated with antimicrobials
may be considered an efficient strategy for the treatment of fungal
PJI.
References
|
1
|
Huotari K, Peltola M and Jämsen E: The
incidence of late prosthetic joint infections: A registry-based
study of 112,708 primary hip and knee replacements. Acta Orthop.
86:321–325. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Vessely MB, Whaley AL, Harmsen WS, Schleck
CD and Berry DJ: The chitranjan ranawat award: Long-term
survivorship and failure modes of 1000 cemented condylar total knee
arthroplasties. Clin Orthop Relat Res. 452:28–34. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang Y, Yang F, Zhang Z, Li H and Chen J:
Distribution and drug sensitivity of pathogens in patients with
prosthetic joint infection after primary total knee arthroplasty.
Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 28:848–852. 2014.(In
Chinese). PubMed/NCBI
|
|
4
|
Kuiper JW, van den Bekerom MP, van der
Stappen J, Nolte PA and Colen S: 2-stage revision recommended for
treatment of fungal hip and knee prosthetic joint infections. Acta
Orthop. 84:517–523. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kao AS, Brandt ME, Pruitt WR, Conn LA,
Perkins BA, Stephens DS, Baughman WS, Reingold AL, Rothrock GA,
Pfaller MA, et al: The epidemiology of candidemia in two United
States cities: Results of a population-based active surveillance.
Clin Infect Dis. 29:1164–1170. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yilmaz M, Mete B, Ozaras R, Kaynak G,
Tabak F, Tenekecioğlu Y and Oztürk R: Aspergillus fumigatus
infection as a delayed manifestation of prosthetic knee
arthroplasty and a review of the literature. Scand J Infect Dis.
43:573–578. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Azzam K, Parvizi J, Jungkind D, Hanssen A,
Fehring T, Springer B, Bozic K, Della Valle C, Pulido L and Barrack
R: Microbiological, clinical, and surgical features of fungal
prosthetic joint infections: A multi-institutional experience. J
Bone Joint Surg Am. 91(Suppl 6): S142–S149. 2009. View Article : Google Scholar
|
|
8
|
Wimmer MD, Friedrich MJ, Randau TM,
Ploeger MM, Schmolders J, Strauss AA, Hischebeth GT, Pennekamp PH,
Vavken P and Gravius S: Polymicrobial infections reduce the cure
rate in prosthetic joint infections: Outcome analysis with
two-stage exchange and follow-up ≥two years. Int Orthop. Jul
17;(2).
|
|
9
|
Nuñez LV, Buttaro MA, Morandi A, Pusso R
and Piccaluga F: Frozen sections of samples taken intraoperatively
for diagnosis of infection in revision hip surgery. Acta Orthop.
78:226–230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mirra JM, Amstutz HC, Matos M and Gold R:
The pathology of the joint tissues and its clinical relevance in
prosthesis failure. Clin Orthop Relat Res. 221–240. 1976.PubMed/NCBI
|
|
11
|
Leone JM and Hanssen AD: Management of
infection at the site of a total knee arthroplasty. Instr Course
Lect. 55:449–461. 2006.PubMed/NCBI
|
|
12
|
Zimmerli W, Trampuz A and Ochsner PE:
Prosthetic-joint infections. N Engl J Med. 351:1645–1654. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Musso A, Mohanty K and Spencer-Jones R:
Role of frozen section histology in diagnosis of infection during
revision arthroplasty. Postgrad Med J. 79:590–593. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tohtz SW, Müller M, Morawietz L, Winkler T
and Perka C: Validity of frozen sections for analysis of
periprosthetic loosening membranes. Clin Orthop Relat Res.
468:762–768. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Zhou YG, Chen JY, Liu M, Zhang
GQ, Chai W, Fu YM, Wang XL, Dong XY and Wang Y: Treatment of
infected total knee arthroplasty with a self-made,
antibiotic-loaded cement articulating spacer. Zhongguo Gu Shang.
26:119–123. 2013.(In Chinese). PubMed/NCBI
|
|
16
|
Dominici R, Luraschi P and Franzini C:
Measurement of C-reactive protein: Two high sensitivity methods
compared. J Clin Lab Anal. 18:280–284. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Harris WH: Traumatic arthritis of the hip
after dislocation and acetabular fractures: Treatment by mold
arthroplasty. An end-result study using a new method of result
evaluation. J Bone Joint Surg Am. 51:737–755. 1969.PubMed/NCBI
|
|
18
|
Insall JN, Ranawat CS, Aglietti P and
Shine J: A comparison of four models of total knee-replacement
prostheses. J Bone Joint Surg Am. 58:754–765. 1976.PubMed/NCBI
|
|
19
|
Anagnostakos K, Kelm J, Schmitt E and Jung
J: Fungal periprosthetic hip and knee joint infections clinical
experience with a 2-stage treatment protocol. J Arthroplasty.
27:293–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koehler P, Tacke D and Cornely OA: Bone
and joint infections by Mucorales, Scedosporium,
Fusarium and even rarer fungi. Crit Rev Microbiol.
42:158–171. 2016.PubMed/NCBI
|
|
21
|
Ritter JM, Muehlenbachs A, Blau DM,
Paddock CD, Shieh WJ, Drew CP, Batten BC, Bartlett JH, Metcalfe MG,
Pham CD, et al: Exserohilum infections associated with contaminated
steroid injections: A clinicopathologic review of 40 cases. Am J
Pathol. 183:881–892. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ayhan AC, Ozkan K, Timur C, Aktaş B and
Ceyran AB: Chronic granulomatous Aspergillus synovitis: A
case report. Mediterr J Hematol Infect Dis. 5:e20130432013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mukhopadhyay S: Role of histology in the
diagnosis of infectious causes of granulomatous lung disease. Curr
Opin Pulm Med. 17:189–196. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Phelan DM, Osmon DR, Keating MR and
Hanssen AD: Delayed reimplantation arthroplasty for candidal
prosthetic joint infection: A report of 4 cases and review of the
literature. Clin Infect Dis. 34:930–938. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ueng SW, Lee CY, Hu CC, Hsieh PH and Chang
Y: What is the success of treatment of hip and knee candidal
periprosthetic joint infection? Clin Orthop Relat Res.
471:3002–3009. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sia IG, Berbari EF and Karchmer AW:
Prosthetic joint infections. Infect Dis Clin North Am. 19:885–914.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Qin HJ, Feng QM, Fang Y and Shen L: Type-I
interferon secretion in the acute phase promotes Cryptococcus
neoformans infection-induced Th17 cell polarization in vitro. Exp
Ther Med. 7:869–872. 2014.PubMed/NCBI
|
|
28
|
Liu L, Ling J, Ma Z, Yuan Q, Pan J and
Yang H: Changes in von Willebrand factor and ADAMTS-13 in patients
following arthroplasty. Mol Med Rep. 11:3015–3020. 2015.PubMed/NCBI
|
|
29
|
Vasquez JC, Hart M, Denney CF, Pedowitz R
and Ziegler EJ: Fungal arthritis of the knee caused by Candida
parapsilosis in a kidney transplant recipient. J Clin
Rheumatol. 8:147–150. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xia QF, Liu JB, Liu P, Qin X, Qian SY and
Tu ZG: Development of a novel quantitative real-time assay using
duplex mutation primers for rapid detection of Candida
species. Mol Med Rep. 5:207–210. 2012.PubMed/NCBI
|
|
31
|
Marra F, Robbins GM, Masri BA, Duncan C,
Wasan KM, Kwong EH and Jewesson PJ: Amphotericin B-loaded bone
cement to treat osteomyelitis caused by Candida albicans.
Can J Surg. 44:383–386. 2001.PubMed/NCBI
|
|
32
|
Goss B, Lutton C, Weinrauch P, Jabur M,
Gillett G and Crawford R: Elution and mechanical properties of
antifungal bone cement. J Arthroplasty. 22:902–908. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kweon C, McLaren AC, Leon C and McLemore
R: Amphotericin B delivery from bone cement increases with porosity
but strength decreases. Clin Orthop Relat Res. 469:3002–3007. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Denes E, Fiorenza F, Saint-Marcoux F,
Megherbi M, Dupon M and Weinbreck P: Voriconazole stability in
cement spacers. Med Mal Infect. 42:567–568. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Miller RB, McLaren AC, Pauken C, Clarke HD
and McLemore R: Voriconazole is delivered from antifungal-loaded
bone cement. Clin Orthop Relat Res. 471:195–200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Grimsrud C, Raven R, Fothergill AW and Kim
HT: The in vitro elution characteristics of
antifungal-loaded PMMA bone cement and calcium sulfate bone
substitute. Orthopedics. 34:e378–e381. 2011.PubMed/NCBI
|
|
37
|
Deelstra JJ, Neut D and Jutte PC:
Successful treatment of Candida albicans-infected total hip
prosthesis with staged procedure using an antifungal-loaded cement
spacer. J Arthroplasty. 28:374.e5–e8. 2013. View Article : Google Scholar
|
|
38
|
Pappas PG, Kauffman CA, Andes D, Benjamin
DK Jr, Calandra TF, Edwards JE Jr, Filler SG, Fisher JF, Kullberg
BJ, Ostrosky-Zeichner L, et al: Clinical practice guidelines for
the management of candidiasis: 2009 update by the Infectious
diseases society of America. Clin Infect Dis. 48:503–535. 2009.
View Article : Google Scholar : PubMed/NCBI
|