Introduction
Allergic asthma is a chronic respiratory disease
that is characterized by obstruction of airflow as a response to
certain triggers, airway hyperresponsiveness and increased airway
inflammation. The chronic inflammation is associated with airway
hyperresponsiveness resulting in recurrent episodes of coughing,
chest tightness, breathlessness and wheezing, particularly in the
early morning or at night (1).
Allergic asthma has become one of the most common public health
problems worldwide due to its rapidly increasing prevalence
(2).
Allergen-specific CD4+ T cells are
considered to be significantly involved in the development of
asthma (3). In addition, the balance
between the T-helper 1 (Th1) and Th2 cells responses are central to
the pathogenesis of allergic asthma (4). Synthesis of increased levels of
interleukin (IL)-4, IL-5 and IL-13 by Th2 cells results in the
production of allergen-specific immunoglobulin (Ig)E and the
release of mediators from mast cells. By contrast, Th1 cells
secrete interferon (IFN)-γ, thus suppressing Th2 immune responses.
IFN-γ is also involved in IgG2a class switching. However, in recent
years, it was recognized that Th1/Th2 imbalance does not entirely
explain the etiology of asthma, since the reversal of Th1/Th2
imbalance does not fully control asthmatic symptoms in humans
(5).
Th17 cells and regulatory T (Treg) cells have been
previously described as two T cell population subsets that are
distinct from Th1 and Th2. Th17 cells serve a crucial role in the
induction of autoimmune tissue injuries and chronic inflammation
through the production of various cytokines, including IL-6, IL-17
and tumor necrosis factor (TNF)-α (6). By contrast, Treg cells are involved in
the anti-inflammatory process and maintain tolerance to
self-components by producing anti-inflammatory cytokines, such as
transforming growth factor (TGF)-β and IL-10 (7). Imbalances of Th17/Treg cells have been
identified in patients with allergic asthma, and were shown to be
associated with moderate to severe asthma (8,9). This
suggested that the balance between Th17 and Treg cells may be
important in the development of autoimmune diseases, such as asthma
(5).
Gu-Ben-Fang-Xiao-Tang (GBFXT) is a mixture based on
an empirical traditional Chinese medicine (TCM) formula that
comprises of 11 medicinal plants (Table
I). GBFXT has been used to treat bronchial asthma for 30 years
at the Jiangsu Provincial Hospital of Traditional Chinese Medicine,
Nanjing, China (10,11). A randomized, multicenter, parallel
controlled study revealed that GBFXT combined with acupoint
application had evident benefits for treating chronic asthmatic
children (10). Furthermore, a
previous animal study showed that GBFXT treatment was able to
decrease the airway hyperresponsiveness, reduce the number of
inflammation cells in the bronchoalveolar lavage fluid (BALF), and
reduce the ratio of eosinophil and neutrophil granulocytes in
asthma mice at the remission stage (11), implying a therapeutic effect of GBFXT
on asthma. However, the precise mechanisms of GBFXT in the
treatment of asthma are not fully understood. Accordingly, the
present study was designed in order to determine whether GBFXT
exerts an anti-inflammatory effect through the regulation of
Th17/Treg balance in an ovalbumin (OVA)-induced murine asthma
model.
 | Table I.Ratio of the components in
Gu-Ben-Fang-Xiao-Tang. |
Table I.
Ratio of the components in
Gu-Ben-Fang-Xiao-Tang.
| Component | Quantity (g) |
|---|
| Radix Astragali
preparata | 15 |
| Ginseng radix | 10 |
| Largehead
Atractylodes rhizome | 10 |
| Glabrous greenbrier
rhizome | 10 |
| Calcined oyster
shells | 15 |
| Periostracum
cicadae | 6 |
| Pericarpium citri
reticulatae | 6 |
| Sileris radix | 3 |
| Flos magnoliae | 6 |
| Schisandra
chinensis (Turcz.) bail | 6 |
| Radix
Glycyrrhizae | 3 |
| Total quantity | 90 |
Materials and methods
Reagents and animals
A total of 50 female BALB/c mice aged 6–8 weeks and
weighing 20–22 g were purchased from Shanghai Laboratory Animal
Center (Shanghai, China). The mice were maintained in a specific
pathogen-free room at the Animal Facilities of Taizhou University
(Taizhou, China) at 24°C and 55–65% humidity, under a 12-h
light/dark cycle with ad libitum access to standard chow and
water. The experimental procedures were approved by the Laboratory
Animal Care Committee at the Medical College of Taizhou University.
All animals received care according to the Guide for the Care and
Use of Laboratory Animals (National Institutes of Health, Bethesda,
MD, USA). OVA and methacholine (MCH) were obtained from
Sigma-Aldrich (St. Louis, MO, USA), aluminum hydroxide was
purchased from Thermo Fisher Scientific, Inc. (Pierce
Biotechnology; Rockford, IL, USA), and a Wright-Giemsa staining kit
was purchased from Nanjing Jiancheng Bioengineering Institute
(Nanjing, China).
GBFXT preparation
GBFXT was supplied by the Pharmaceutical Preparation
Section of Taizhou Municipal Hospital, and was prepared according
to a traditional herbal formula, as listed in Table I. In brief, the chopped herbs were
combined in the listed ratios and immerged in cold water for 30
min, then boiled twice for 30 min. The poaching liquid was filtered
and concentrated as a decoction of 2 g/ml, and stored at 4°C prior
to administration to mice.
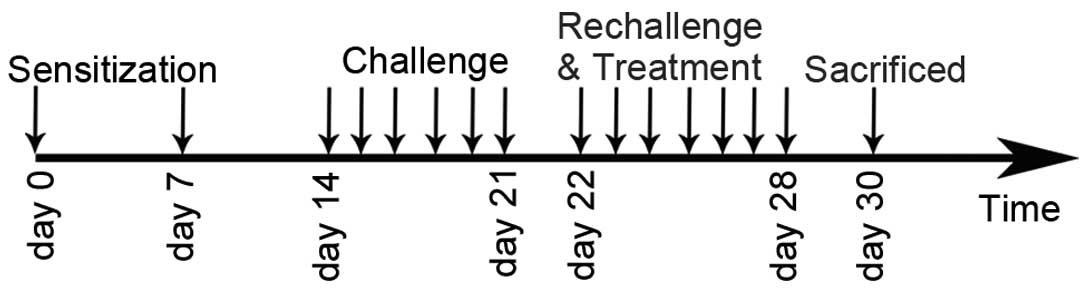
Experimental protocol and
administration of GBFXT
A mouse asthmatic model was established as described
previously (12). The sensitization,
challenge and treatment schedules are shown in Fig. 1. In brief, mice (with the exception
of those in the normal control group) were sensitized by
intraperitoneal injection of 10 µg OVA and 1 mg aluminum hydroxide.
The mice were sensitized twice, on days 0 and 7. One week after the
second sensitization, mice (except those in the normal control
group) were anesthetized with 2% isoflurane (Sinopharm Chemical
Reagent Co., Ltd., Shanghai, China) and intranasally challenged
with 100 µg OVA in 0.05 ml PBS once per day between days 14 and 21.
Subsequently, mice were rechallenged with 2.5% OVA-PBS between days
22 and 28. In the GBFXT treatment groups (n=10/group), 12 or 36
g/kg GBFXT was administered orally once daily on days 22–28. Mice
in the normal control group (n=10) were sensitized with PBS and
were treated with PBS on days 22–28, whereas mice in the model
control and in the positive control (termed montelukast group)
groups (n=10/group) were treated orally with PBS and montelukast
(2.6 mg/kg; Sigma-Aldrich), respectively, once daily on days 22–28.
Animals were sacrificed by overdose with pentobarbital sodium (50
mg/kg) 48 h after the last challenge (on day 30) in order to
characterize the effects of GBFXT.
Measurement of airway
responsiveness
An AniRes 2005 Lung Function System (Bestlab,
Beijing, China) was used to determine the airway responsiveness at
48 h after the last OVA challenge using the forced oscillation
technique, as previously described (13). Prior to the measurement, the mice
were anesthetized with intraperitoneal injection of 40 mg/kg
pentobarbital sodium (Sinopharm Chemical Reagent Co., Ltd.).
Subsequently, the trachea was surgically exposed and connected to a
computer-controlled ventilator via an intratracheal tube. The time
ratio of expiration/inspiration was pre-set at 1.5:1, while the
respiratory rate was pre-set at 90/min. Mice were stabilized for 5
min, and then increasing concentrations of MCH aerosol
(Sigma-Aldrich) between 6.25 and 50 mg/ml were administered for 20
sec. Lung airway resistance (RL) values were recorded by the system
following the MCH administration. Subsequent to each MCH dose, data
were continuously collected for 3 min and maximum values of RL were
recorded to determine changes in murine airway function.
Collection of BALF
Following sacrifice, tracheotomy was performed and
0.4 ml cold PBS was instilled into the lung. Next, the BALF was
collected by three successive aspirations (total volume, 1.2 ml)
via tracheal cannulation. This procedure recovered ~90% of the
infused fluid. BALF was centrifuged at 250 × g at 4°C for 5 min,
and the total number of cells in BALF was counted using a
hemocytometer. Subsequently, 100 µl BALF was placed on a slide and
centrifuged at 200 × g at 4°C for 10 min using a cell cytospin
machine, after which the slides were dried, cells were fixed with
10% formaldehyde and stained using the Wright-Giemsa staining kit,
according to the manufacturer's instructions. A total of 200
cells/slide were randomly selected to calculate the percentage of
eosinophils, neutrophils, lymphocytes and macrophages in the sample
under the microscope. The supernatants of BALF were collected by
centrifugation at 250 × g for 5 min at 4°C for ELISAs.
Histological assessment of lung
tissue
Lung tissues were detached from the, fixed in 10%
formalin, embedded in paraffin and cut into 4-µm sections with a
microtome (RM2255; Leica Biosystems, Nussloch, Germany). Next, the
tissue samples were placed on glass sides and deparaffinized. The
sections were then stained with hematoxylin-eosin and examined
under a light microscope. The degree of cell infiltration in the
airway was scored in a double-blind screen by two independent
investigators. As previously described (14), the degree of perivascular and
peribronchial inflammation was evaluated using scores of 0–3, as
follows: 0, little or no detectable inflammation; 1, occasional
cuffing with inflammatory cells; 2, the majority of vessels or
bronchi were surrounded by a thin layer of inflammatory cells (1–5
cells); and 3, the majority of vessels or bronchi were surrounded
by a thick layer of inflammatory cells (>5 cells).
Flow cytometric analysis
The spleen from each sacrificed mouse was removed,
and splenocytes were prepared by gently crushing the tissues with a
glass slide to release the cells. Cell preparations were filtered
to remove debris and washed twice in phosphate-buffered saline
(PBS), prior to resuspending in complete RPMI-1640 medium (Hyclone;
GE Healthcare Life Sciences, Logan, UT, USA). For Th17-cell
staining, the splenocytes were stimulated for 5 h with PMA (50
ng/ml; Sigma-Aldrich) and ionomycin (500 ng/ml; Sigma-Aldrich) in
the presence of brefeldin A (10 µg/ml; Bioscience, Inc., San Diego,
CA, USA). Next, the cells were harvested, washed and stained with
fluorescein isothiocyanate (FITC)-conjugated rat anti-mouse CD4
(1:500; cat. no. 553046) and allophycocyanin-conjugated hamster
anti-mouse CD3 (1:600; cat. no. 553066) monoclonal antibodies (BD
Biosciences, San Jose, CA, USA), in the presence of FcR-Block
(eBioscience, Inc., San Diego, CA, USA) at 4°C for 30 min. After
washing with PBS, cells were fixed using CytoFix/CytoPerm buffer
(BD Biosciences), according to the manufacturer's instructions, and
stained with phycoerythrin (PE)-conjugated rat anti-mouse IL-17A
monoclonal antibody (1:300; cat. no. 559502; BD Biosciences) at 4°C
for 30 min. For Treg-cell staining, the splenocytes were initially
stained with FITC-conjugated rat anti-mouse CD4 (1:400; cat. no.
11-0042-81; eBioscience, Inc.) and PE-conjugated rat anti-mouse
CD25 (1:800; cat. no. 12-0251-81) monoclonal antibodies
(eBioscience, Inc.) at 4°C for 30 min. Intracellular staining for
FoxP3 was performed by using a FoxP3 staining kit (eBioscience,
Inc.). Isotype controls were treated to enable correct compensation
and to confirm antibody specificity. Data were acquired on a FACS
Calibur flow cytometer (BD Bioscience) and analyzed using FlowJo
software, version 7.6 (FlowJo LLC, Ashland, OR, USA).
ELISA
BALF cell-free supernatants (50 µl) were collected
by centrifugation at 250 × g for 5 min at 4°C. The concentrations
of IL-17A and IL-10 in the BALF were measured using ELISA kits
(cat. nos. 88-7371-22 and 88-7105-22, respectively; eBioscience,
Inc.), according to the manufacturer's instructions. All assays
were performed in triplicate. The concentration of each protein was
calculated from the standard curve.
Statistical analysis
Data were analyzed using a two-tailed Student's
t-test and the Graphpad Prism version 5 software (GraphPad
Software, Inc., La Jolla, CA, USA). The results are expressed as
the mean ± standard deviation, and differences with a P<0.05
were regarded as statistically significant.
Results
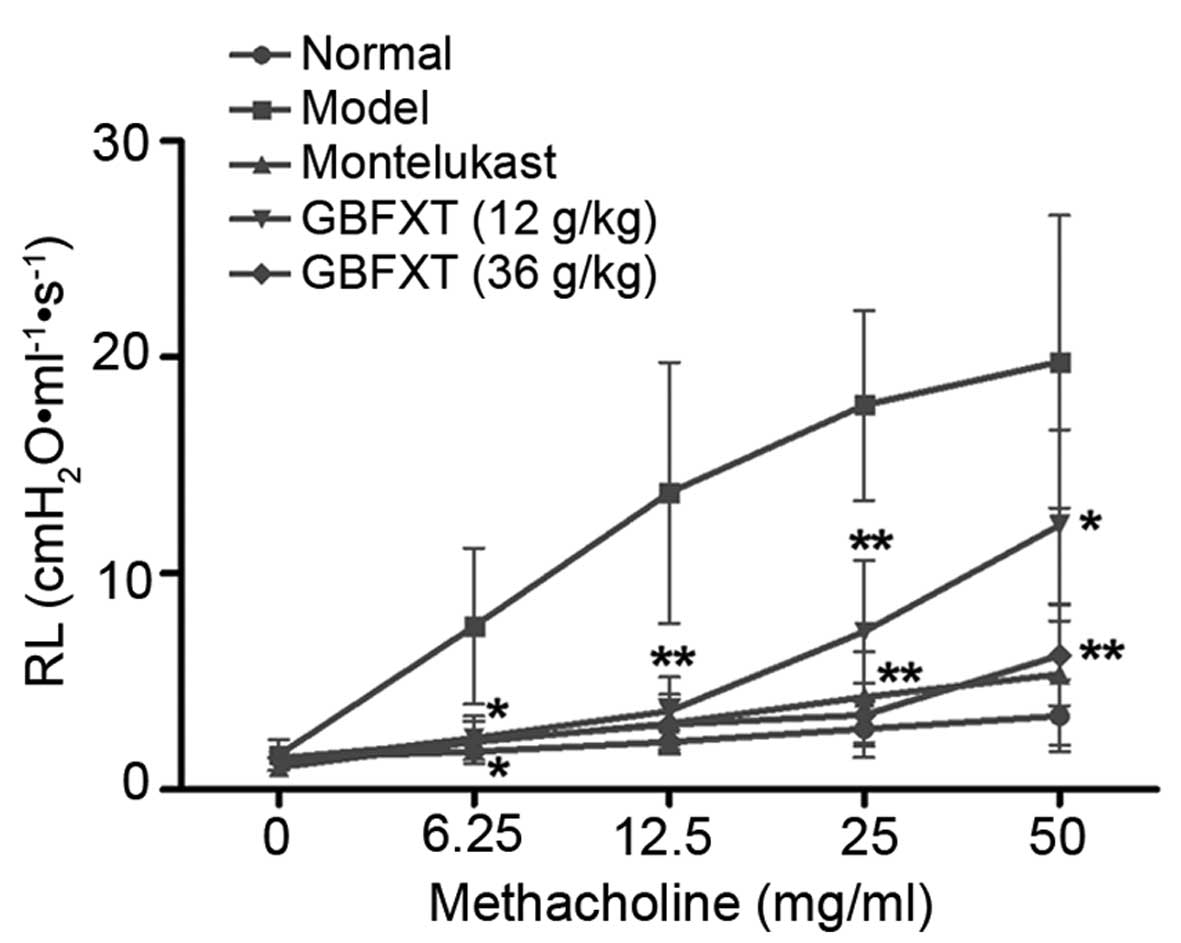
Effect of GBFXT on airway
hyperresponsiveness
Fig. 2 shows the
airway hyperresponsiveness, as recorded during MCH-induced
constriction. The results indicated that the RL value of the
OVA-challenged group (model group) was significantly higher
compared with that of the normal group (P<0.05) when using
concentrations of 6.25–50 mg/ml MCH. However, mice sensitized and
challenged with OVA, followed by treatment with different doses of
GBFXT, exhibited a reduced mean RL values upon exposure to 6.25–50
mg/ml MCH when compared with the RJ values in the model group. In
addition, positive control mice administered montelukast (2.6
mg/kg) showed markedly decreased RL values compared with the model
group, which were similar to the values achieved using GBFXT
(P<0.05). These results suggest that GBFXT suppresses airway
hyperresponsiveness during MCH-induced constriction.
Effect of GBFXT on OVA-induced
eosinophilia in BALF
Alterations in the total cell levels in the BALF
were then investigated in order to determine the effect of GBFXT
treatment on asthma. Suppression of eosinophilia following GBFXT
treatment in OVA-challenged mice was estimated by counting the
percentage of eosinophils and other cells present in the BALF at 48
h after the last challenge. As shown in Table II, OVA induced a marked influx of
leukocyte and eosinophil levels in the BALF in OVA-challenged mice.
By contrast, OVA-sensitized and -challenged mice treated with GBFXT
displayed a significantly reduced proportion of leukocytes
(P<0.05) and eosinophils (P<0.05), while a significant
decrease in absolute neutrophils was observed compared with the
model group upon asthma induction (P<0.01). These results
suggest that GBFXT reduces the percentage of leukocytes and
eosinophils, and decreases absolute neutrophil infiltration, in
BALF.
 | Table II.Total cell number and cellular
composition in BALF of mice. |
Table II.
Total cell number and cellular
composition in BALF of mice.
| Group | Total cells
(×104/ml) | Eosinophils (%) | Neutrophils (%) | Lymphocytes (%) | Macrophages (%) |
|---|
| Normal |
71.3±12.3 |
0.81±0.7 |
21.1±7.4 |
50.4±14.7 |
26.3±5.7 |
| Model |
135.0±30.7 |
4.5±1.7 |
31.8±6.7 |
30.4±4.1 |
34.2±5.1 |
| Montelukast |
86.2±24.8a |
1.37±0.9b |
27.8±5.4 |
33.4±8.3 |
36.4±6.1 |
| GBFXT (12 g/kg) |
95.1±23.1a |
3.4±1.4 |
24.2±5.1b |
32.5±6.8 |
36.9±6.8 |
| GBFXT (36
g/kg) |
84.4±21.3a |
1.9±0.8a |
22.7±4.3b |
31.9±7.2 |
38.1±7.3 |
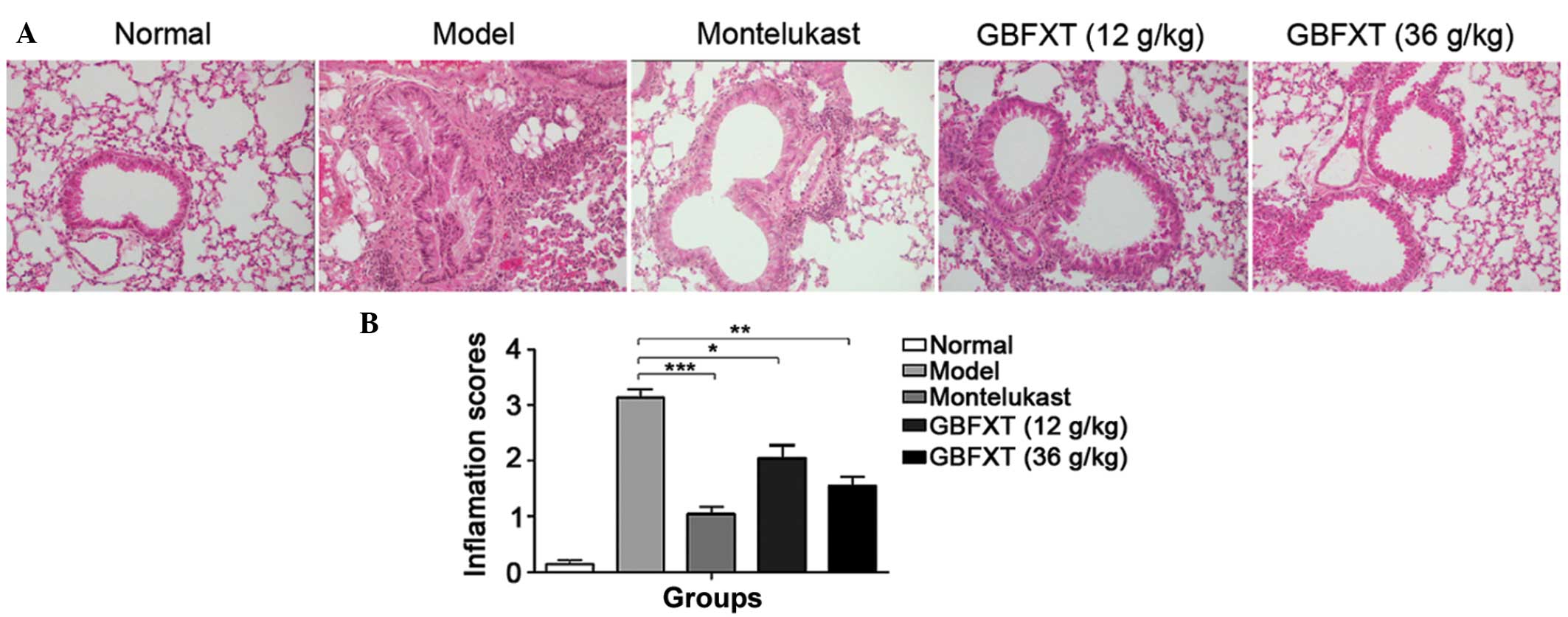
Effect of GBFXT on OVA-induced
eosinophilia in lung tissue
As shown in Fig. 3, a
significant infiltration of inflammatory cells into the
peribronchial and perivascular connective tissues was observed in
OVA-induced asthmatic lung tissues when compared with the normal
lung tissue (P<0.01). Furthermore, the majority of leukocytes
present were eosinophils. The normal group presented no alteration
in the extent of inflammatory cell infiltration, and similar
findings were observed in the montelukast-treated positive control
mice. However, eosinophilia in OVA-sensitized and -challenged mice
treated with GBFXT was significantly attenuated compared with the
level of eosinophils observed in OVA-challenged mice, as shown in
Fig. 3 (P<0.05 and P<0.01,
respectively). These results suggest that GBFXT inhibits
inflammatory cell recruitment to lung tissues.
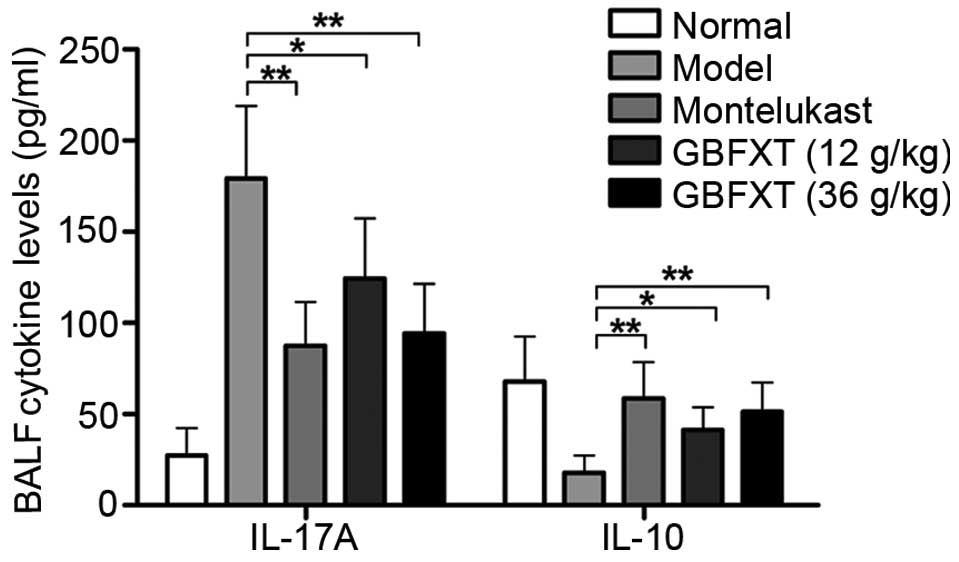
Effect of GBFXT on cytokine levels in
BALF
As shown in Fig. 4,
OVA challenging triggered a significant increase in IL-17A
expression levels in the BALF, compared with the levels in normal
fluid (P<0.01). In OVA-sensitized and -challenged mice treated
with GBFXT, the increase in IL-17A cytokine levels was
significantly suppressed (P<0.05 and P<0.01, respectively),
when compared with the levels observed in the asthmatic model
group. Regarding IL-10 expression, OVA challenging resulted in a
significant reduction in the cytokine levels in the BALF
(P<0.01), when compared with the levels in normal fluid. IL-10
cytokine levels were significantly enhanced in mice with
OVA-induced asthma that were treated with GBFXT (P<0.05), when
compared with the concentration detected in the model group. These
results suggest that GBFXT treatment significantly decreases the
IL-17A cytokine levels and increases the IL-10 cytokine levels in
the BALF.
Effect of GBFXT on Th17 and Treg
cells
In the present study, flow cytometric analysis was
used to determine the effect of GBFXT on the proportion of Th17 and
Treg cells. As shown in Fig. 5, the
percentage of Th17 cells in the model group (5.54±2.04%) was
significantly higher compared with that in the normal group
(2.01±0.64%; P<0.01). In addition, the proportion of Th17 cells
was significantly lower in the montelukast (2.14±0.57%; P<0.01)
and 36 g/kg GBFXT (3.61±1.34%; P<0.05) treatment groups compared
with those in the model group. Furthermore, the proportion of Treg
cells in the model group (1.67±0.87%) was significantly lower
compared with that in the normal group (5.54±2.34%; P<0.01),
while it was significantly higher in the montelukast (5.14±1.31%;
P<0.05) and 36 g/kg GBFXT (3.29±1.07%; P<0.05) treatment
groups, when compared with those in the model group. These results
suggest that GBFXT significantly suppresses Th17 and increases Treg
cell proportions in asthmatic mice.
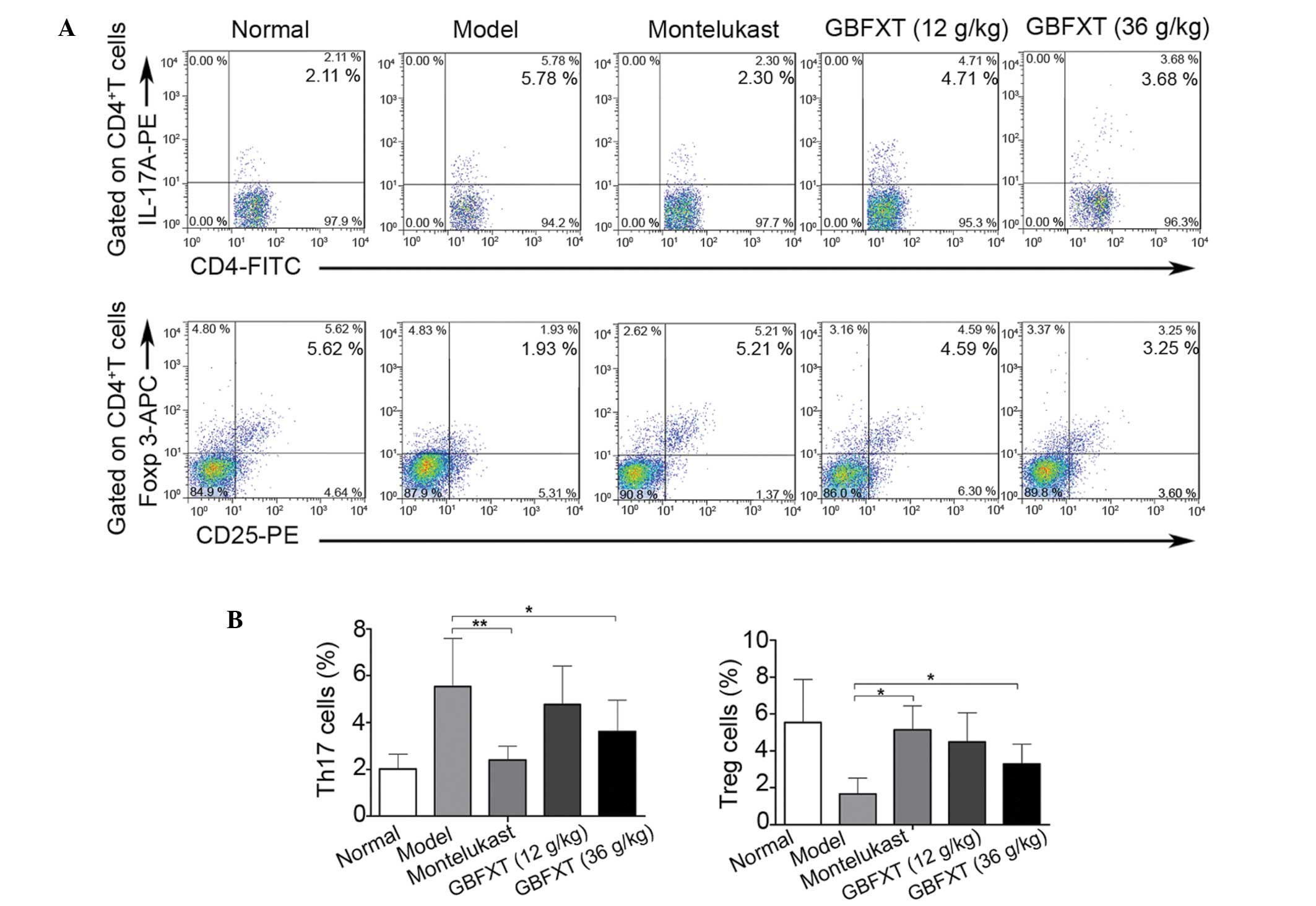 | Figure 5.Effect of GBFXT on Th17 and Treg
cells. (A) Representative FACS flow cytometry profiles; and (B)
percentages of Th17 cells and Treg cells in various groups. One
representative of 10 mice in each group is shown in the FACS
profiles. Values represent the mean ± standard deviation of 10
mice. *P<0.05 and **P<0.01, vs. model group. GBFXT,
Gu-Ben-Fang-Xiao-Tang; FACS, fluorescence-activated cell sorting;
IL, interleukin; PE, phycoerythrin; FITC, fluorescein
isothiocyanate; Foxp 3, forkhead box P3; APC, allophycocyanin;
Th17, T helper 17; Treg, regulatory T. |
Discussion
In the present study, the immunoregulatory effects
and possible mechanism of GBFXT were investigated in a OVA-induced
chronic allergic asthma murine model. The results showed that GBFXT
treatment suppressed the airway hyperresponsiveness and eosinophil
granulocyte infiltration, while it significantly decreased IL-17A
and increased IL-10 cytokine levels. Furthermore, GBFXT treatment
significantly suppressed the proportion of Th17 cells and increased
the proportion of Tregs in asthmatic mice. Therefore, to the best
of our knowledge, the present study demonstrated for the first time
that GBFXT treatment may attenuate the airway inflammation in an
asthma animal model by regulating the Th17/Treg balance.
Allergic disorders, such as asthma, represent a
severe public health problem in children. The chronic nature of
these diseases and the fear of known side effects of synthetic
drugs result in numerous families seeking complementary and
alternative medicine (CAM) (15).
The use of TCM is a major component of the CAM modality, and TCM
formulas have been used for centuries for the treatment of asthma,
with a number of successful cases previously reported (12,16,17).
GBFXT, a formula designed following the TCM theories and clinical
experience, has been used to treat asthmatic patients in China for
three decades (10). Unlike the side
effect occurring when using corticosteroids, GBFXT relieves
asthmatic syndrome without total immune suppression, and also has
an increased heightening effect to the immune function of mice
(18). In support of the present
study findings, a preclinical study performed by Yuan et al
(11) reported that GBFXT treatment
was able to decrease airway hyperresponsiveness, reduce BALF
inflammation cell count, and the ratio of eosinophil and neutrophil
granulocytes. In addition, GBFXT resulted in an increase was
observed in the IFN-γ level in the BALF and relieved the
infiltration of inflammatory cells in the airway in asthmatic mice
at the remission stage (11),
implying a therapeutic effect of GBFXT on asthma.
Previous clinical studies have demonstrated that the
proportion of Th17 cells in the BALF, sputum and lung tissue, as
well as the serum levels of IL-17, in asthma patients were
significantly increased compared with those in normal control
patients, and were positively associated with the severity of
asthma symptoms (8,19–21). In
addition, certain studies using animal asthma models have also
shown that Th17 cells and their cytokines, including IL-17A and
IL-17F, are involved in antigen-induced neutrophil recruitment, as
well as in the enhancement of Th2 cell-mediated eosinophil
recruitment into the airways (22,23). In
human allergy patients, Treg cell numbers decrease in the
peripheral blood and BALF, and cannot suppress the cell
proliferation and cytokine production of allergen-stimulated
CD4+ T cells (24–26).
Furthermore, several murine studies have demonstrated that the
induction of Treg cell function reverses the airway
hyperresponsiveness and protects against experimentally-induced
asthma (27,28). In the current study, the results of
flow cytometric analysis revealed that OVA challenge was able to
significant increase the proportion of Th17 cells and decrease the
proportion of Treg cells, resulting in imbalance of the Th17/Treg
cell ratio; this suggests that the Th17/Treg imbalance serves an
important role in the pathogenesis of asthma. Finally,
administration of GBFXT significantly suppressed Th17 cells and
increased Treg cells in mice with OVA-induced asthma, and attenuate
the airway inflammation.
In conclusion, the present study demonstrated a
novel mechanism of GBFXT in the treatment of asthma. The current
findings suggest that GBFXT may effectively inhibit the progression
of airway inflammation in allergic asthma. The anti-inflammatory
effects of GBFXT may be mediated partially by modulation of the
Th17/Treg balance.
Acknowledgements
The current study was supported by grants from the
Administration of Traditional Chinese Medicine of Zhejiang Province
(nos. 2012ZB174 and 2013ZA134) and the Science and Technology
Planning Project of Taizhou City (no. 1201KY21).
References
|
1
|
Bice JB, Leechawengwongs E and Montanaro
A: Biologic targeted therapy in allergic asthma. Ann Allergy Asthma
Immunol. 112:108–115. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pawankar R: Allergic diseases and asthma:
A global public health concern and a call to action. World Allergy
Organ J. 7:122014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pascual RM and Peters SP: Airway
remodeling contributes to the progressive loss of lung function in
asthma: An overview. J Allergy Clin Immunol. 116:477–486; quiz 487.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Umetsu DT and DeKruyff RH: Th1 and Th2
CD4+ cells in the pathogenesis of allergic diseases.
Proc Soc Exp Biol Med. 215:11–20. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schmidt-Weber CB: Th17 and treg cells
innovate the TH1/TH2 concept and allergy research. Chem Immunol
Allergy. 94:1–7. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oboki K, Ohno T, Saito H and Nakae S: Th17
and allergy. Allergol Int. 57:121–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Choi IS: Immune tolerance by induced
regulatory T cells in asthma. Allergy Asthma Immunol Res.
4:113–115. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shi YH, Shi GC, Wan HY, Jiang LH, Ai XY,
Zhu HX, Tang W, Ma JY, Jin XY and Zhang BY: Coexistence of Th1/Th2
and Th17/Treg imbalances in patients with allergic asthma. Chin Med
J (Engl). 124:1951–1956. 2011.PubMed/NCBI
|
|
9
|
Toldi G, Molvarec A, Stenczer B, Müller V,
Eszes N, Bohács A, Bikov A, Rigó J Jr, Vásárhelyi B, Losonczy G and
Tamási L: Peripheral T(h)1/T(h)2/T(h)17/regulatory T-cell balance
in asthmatic pregnancy. Int Immunol. 23:669–677. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yuan XJ, Sun YQ, Wang SM, Li YN, Wang MQ
and Liu XR: Clinical research of Gubenfangxiao decoction combined
with point application on chronic asthmatic children in 100 cases.
Zhong Hua Zhong Yi Yao Za Zhi. 21:2306–2309. 2010.(In Chinese).
|
|
11
|
Yuan X, Xia C and Wang S: Therapeutic
effects of Guben Fangxiao Decoction on asthma mice at remission
stage. Zhong Yao Xin Yao Yu Lin Chuang Yao Li Bian Ji Bu.
3:257–260. 2010.(In Chinese).
|
|
12
|
Lee MY, Shin IS, Lim HS and Shin HK: A
water extract of Samchulkunbi-tang attenuates airway inflammation
by inhibiting inos and MMP-9 activities in an ovalbumin-induced
murine asthma model. BMC Complement Altern Med. 12:2572012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du Q, Chen Z, Zhou LF, Zhang Q, Huang M
and Yin KS: Inhibitory effects of astragaloside IV on
ovalbumin-induced chronic experimental asthma. Can J Physiol
Pharmacol. 86:449–457. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tournoy KG, Kips JC, Schou C and Pauwels
RA: Airway eosinophilia is not a requirement for allergen-induced
airway hyperresponsiveness. Clin Exp Allergy. 30:79–85. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li XM: Complementary and alternative
medicine in pediatric allergic disorders. Curr Opin Allergy Clin
Immunol. 9:161–167. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wen MC, Wei CH, Hu ZQ, Srivastava K, Ko J,
Xi ST, Mu DZ, Du JB, Li GH and Wallenstein S: Efficacy and
tolerability of anti-asthma herbal medicine intervention in adult
patients with moderate-severe allergic asthma. J Allergy Clin
Immunol. 116:517–524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lin CH, Yeh CH, Lin LJ, Wang JS, Wang SD
and Kao ST: The Chinese herbal medicine formula
Sheng-Fei-Yu-Chuan-Tang suppresses Th2 responses and increases IFN
γ in Dermatophagoides pteronyssinus induced chronic
asthmatic mice. Evid Based Complement Alternat Med.
2013:9841212013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao S, Yuan X and Sun Y: Effects of Guben
Fangxiao Yin on immune function in mice. J Liaoning Univ Trad
Chinese Med. 5:162013.
|
|
19
|
Albano GD, Di Sano C, Bonanno A, Riccobono
L, Gagliardo R, Chanez P, Gjomarkaj M, Montalbano AM, Anzalone G,
La Grutta S and Profita M: Th17 immunity in children with allergic
asthma and rhinitis: UA pharmacological approach. PLoS One.
8:e588922013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chien JW, Lin CY, Yang KD, Lin CH, Kao JK
and Tsai YG: Increased IL-17A secreting CD4+ T cells,
serum IL-17 levels and exhaled nitric oxide are correlated with
childhood asthma severity. Clin Exp Allergy. 43:1018–1026. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Silverpil E and Lindén A: IL-17 in human
asthma. Expert Rev Respir Med. 6:173–186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wakashin H, Hirose K, Maezawa Y, Kagami S,
Suto A, Watanabe N, Saito Y, Hatano M, Tokuhisa T, Iwakura Y, et
al: IL-23 and Th17 cells enhance Th2-cell-mediated eosinophilic
airway inflammation in mice. Am J Respir Crit Care Med.
178:1023–1032. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao Y, Feng X, Wang Y, Zhao L and Han T:
An increased of Th17 cells and IL-23 and IL-17 in the lymph and
blood of rats with bronchial asthma. Xi Bao Yu Fen Zi Mian Yi Xue
Za Zhi. 29:1281–1284. 2013.(In Chinese). PubMed/NCBI
|
|
24
|
Hartl D, Koller B, Mehlhorn AT, Reinhardt
D, Nicolai T, Schendel DJ, Griese M and Krauss-Etschmann S:
Quantitative and functional impairment of pulmonary
CD4+CD25hi regulatory T cells in pediatric asthma. J
Allergy Clin Immunol. 119:1258–1266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ling EM, Smith T, Nguyen XD, Pridgeon C,
Dallman M, Arbery J, Carr VA and Robinson DS: Relation of
CD4+CD25+ regulatory T-cell suppression of
allergen-driven T-cell activation to atopic status and expression
of allergic disease. Lancet. 363:608–615. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strickland DH, Stumbles PA, Zosky GR,
Subrata LS, Thomas JA, Turner DJ, Sly PD and Holt PG: Reversal of
airway hyperresponsiveness by induction of airway mucosal
CD4+CD25+ regulatory T cells. J Exp Med.
203:2649–2660. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lewkowich IP, Herman NS, Schleifer KW,
Dance MP, Chen BL, Dienger KM, Sproles AA, Shah JS, Köhl J, Belkaid
Y and Wills-Karp M: CD4+CD25+ T cells protect
against experimentally induced asthma and alter pulmonary dendritic
cell phenotype and function. J Exp Med. 202:1549–1561. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park BS, Hong GU and Ro JY:
Foxp3(+)-Treg cells enhanced by repeated low-dose
gamma-irradiation attenuate ovalbumin-induced allergic asthma in
mice. Radiat Res. 179:570–583. 2013. View
Article : Google Scholar : PubMed/NCBI
|