Introduction
Diabetic keratopathy is an ocular complication that
occurs with diabetes. Previous findings showed that 47–64% of
diabetics may be affected by primary keratopathy (1). Long-term hyperglycemia affects every
structure of the patients' cornea, including recurrent corneal
ulcer, persistent corneal epithelial defect, reduced sensitivity,
corneal edema, corneal opacity and endothelial fluorescence leakage
(2). However, there is a lack of
evidence on diabetic keratopathy primarily because of a lack of
effective diagnostic methods, particularly in the early period when
the patients exhibit no classical symptoms and conventional
slit-lamp did not identify abnormalities (2). It was also challenging to
quantitatively detect and repetitively measure the abnormal changes
of the cornea.
Corneal optical density is used to describe the
biological and histological characteristic of the cornea. Corneal
optical density, as unique biological and histological material, is
closely associated with corneal transparency and may be used to
describe the degree of corneal transparency (3). Previous findings showed that the
corneal optical density in the area of inflammation was higher than
that of the normal corneal optical density, even when the damages
were repaired (one month later). Thus, corneal optical density is
used to examine the inflammatory reaction and guide objective
examination after corneal surgery (3). Pentacam is a camera that was designed
on the basis of the Scheimpflug theory. Pentacam is capable of
obtaining a three-dimensional image to evaluate various parameters,
including the cornea, crystalline lens, and atria (4–6). It has
been confirmed that Pentacam objectively assesses the nubecula
through a quantitative measurement of cornea density (7).
In the present study, the Pentacam was used to
detect the corneal optical density of the diabetic patients and
alterations of transparency on diabetic mellitus patients during
the disease were examined. A correlation analysis was subsequently
conducted between the central corneal thickness and corneal
endothelial cell density. The results offer a new course for early
diagnosis and the pathogenesis of diabetic keratopathy.
Materials and methods
Materials
In total, 180 diabetic (360 eyes) patients, treated
at the Department of Ophthalmology at the Xiangyang Hospital
Affiliated to Hubei University of Medicine (Hubei, China) from
March, 2012 to March, 2013 were enrolled in the present study.
There were 94 male and 86 female patients, aged 41–77 years, with
an average age of 59.27 years. The course of diabetes was between 1
and 20 years, with an average of 9.02 years. Simultaneously,
another 60 healthy cases (120 eyes) were enrolled in the study as
the normal control group. There were 26 male and 34 female
subjects, aged 41–75 years, with an average age of 59 and 17 years.
The differences in age and gender between the two groups had no
statistical significance (P>0.05). Patients with a history of
eye surgery, laser treatment, contact lens wearing, eye traumas,
keratonosus, uvea disease and intraocular hypertension disease were
excluded from the study. All patients accepted to undergo a
split-lamp examination and were confirmed as normal without any
lesions in the eye. The diabetes group was divided into the <5,
5–10 and >10 groups, each with 60 patients (120 eyes).
The study was approved by the ethics committee of
Xiangyang Hospital Affiliated to Hubei University of Medicine.
Written informed consent was obtained from the patients and/or
guardians.
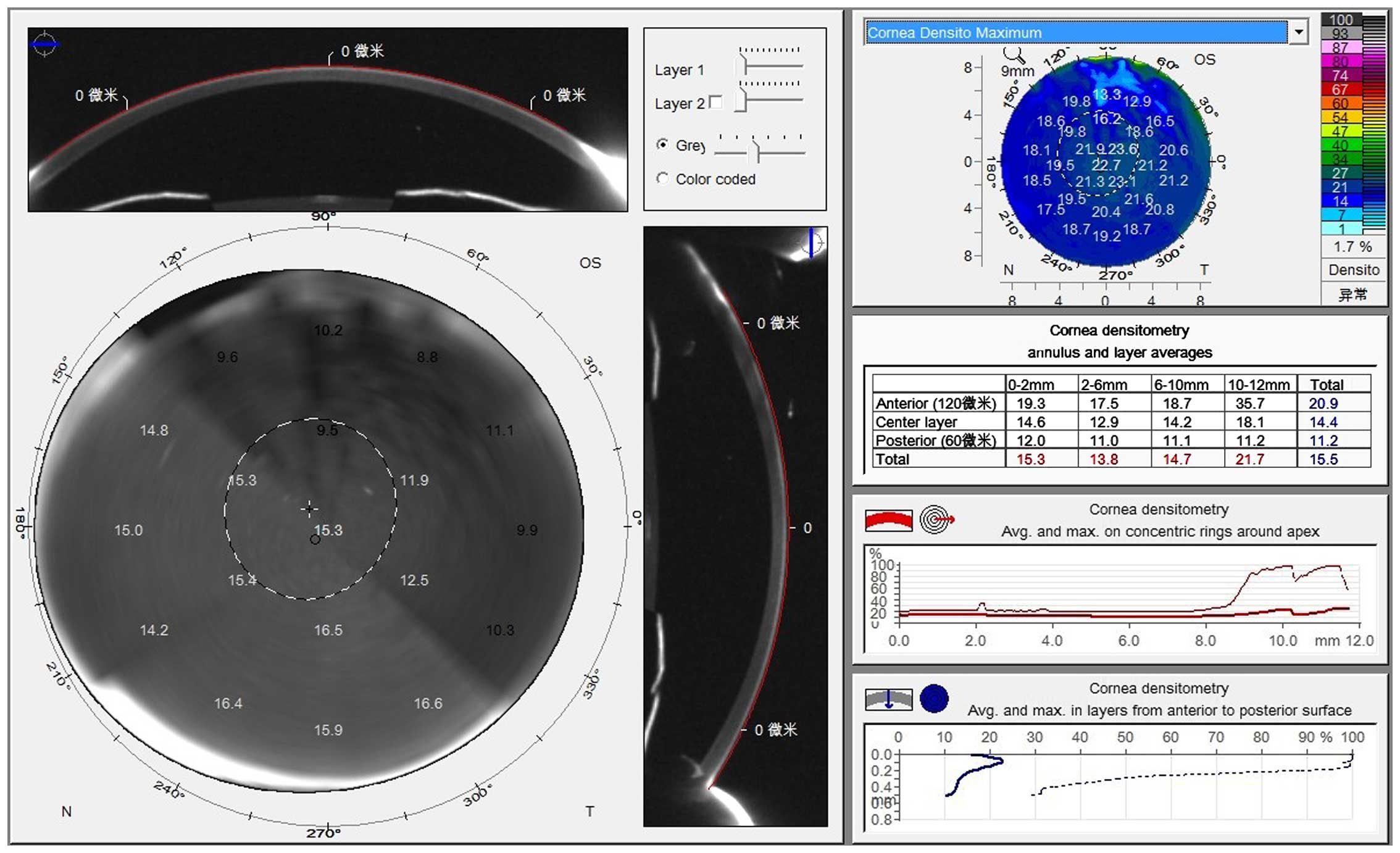
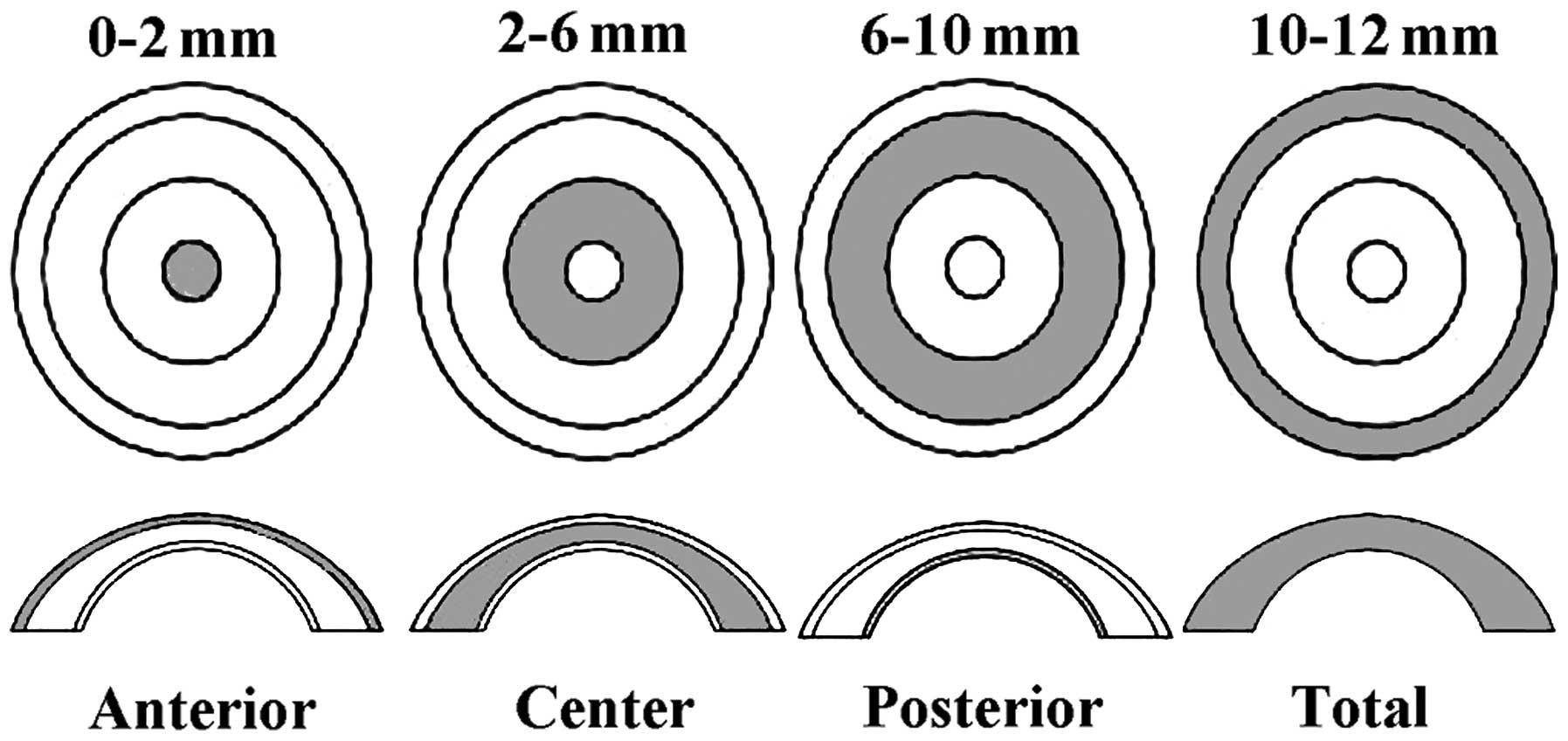
Corneal optical density and corneal
thickness
The Pentacam (OCULUS Optikgeräte GmbH, Wetzlar,
Germany) was used for the examination. Patients remained in a dark
room for 5–10 min during the examination and accepted to be tested
under the natural state of the pupil. The patients were kept in a
dark room, asked to be seated and fix their lower jaw on a mandible
support and stare at the fixation target in the center of the blue
streak of the Pentacam. Patients were not allowed to blink or move
their eyes. The examiner chose the measurement pattern (25
sections/sec) to scan automatically and obtained the data in 2 sec
in a non-contact manner. After the examination, the examiner
manually measured the corneal density, and recorded the maximum
density value on the vertical axis at the corneal vertex, using the
gray value to represent the corneal density and propose 0 as
complete transparency, and 100 as total nubecula. Simultaneously,
the Pentacam was used to measure the minimum thickness of the
central cornea (Figs. 1 and 2).
Corneal endothelial cell count
The EM-3000 fully-automatic corneal endothelial cell
analyzer produced by TOMEY Corp. (Nagoya, Japan) was used to detect
the amount of corneal endothelial cells. The patients were required
to stare at the indicator light. The examiner adjusted the corneal
endothelial meter to capture images of the corneal endothelium
automatically. Its built-in computer analysis system was used to
analyze the endothelial images, calculate and print the results.
The procedures were completed by the same professional examiner to
reduce intraobserver bias. After controlling the age factors by
partial correlation coefficient in the diabetes group, the
relationship between the corneal optical density and course of
diabetes, corneal optical density and number of endothelium,
corneal optical density and corneal thickness were measured.
Statistical analysis
SPSS 18.0 software (Chicago, IL, USA) was used to
analyze the data of the different groups. After the age factors
were excluded, a covariance analysis was applied to test the series
of data. P<0.05 was considered statistically significant.
Results
Corneal optical density value, central
corneal thickness and corneal endothelial cell density of the
diabetics and normal controls
In the two groups, the corneal optical density
increased as the distance from the center increased. The corneal
optical density of the same cornea gradually decreased from the
epithelium of the outer cornea to the corneal endothelium. Compared
to the normal control group, the corneal optical density and
central corneal thickness of the diabetics increased, while the
corneal endothelial cell density decreased (P<0.05) (Tables I and II).
 | Table I.Corneal optical density of the normal
control group. |
Table I.
Corneal optical density of the normal
control group.
| Control | 0–2 mm | 2–6 mm | 6–10 mm | 10–12 mm | Total |
|---|
| Anterior |
22.87±2.91 |
21.32±2.80 |
26.37±8.67 |
35.69±11.22 |
25.81±5.14 |
| Center |
14.63±1.62 |
13.69±1.69 |
19.68±6.78 |
24.90±6.50 |
17.47±3.60 |
| Posterior |
12.75±1.46 |
12.03±1.55 |
16.92±5.10 |
21.75±5.74 |
15.39±2.99 |
| Total |
14.77±1.46 |
15.81±1.97 |
20.81±6.74 |
27.36±7.47 |
19.74±3.89 |
 | Table II.Corneal optical density of the
diabetics. |
Table II.
Corneal optical density of the
diabetics.
| DM | 0–2 mm | 2–6 mm | 6–10 mm | 10–12 mm | Total |
|---|
| Anterior |
25.32±5.71 |
21.48±3.73 |
27.18±11.39 |
35.79±13.71 |
28.04±10.83 |
| Center |
19.80±7.18 |
16.30±6.19 |
28.73±10.43 |
35.03±13.42 |
23.62±10.11 |
| Posterior |
30.75±9.476 |
27.79±12.35 |
31.72±12.04 |
37.37±11.06 |
29.75±11.38 |
| Total |
15.11±3.05 |
18.01±6.53 |
23.55±09.58 |
28.72±10.91 |
21.52±5.58 |
Association between the course of
diabetes and central corneal thickness, corneal endothelial cell
count and corneal optical density
As the duration of diabetes was extended, the
corneal optical density and central corneal thickness increased
while the corneal endothelial cell density decreased. The result of
the correlation analysis between the course of diabetes and the
central corneal thickness, corneal endothelial cell number and
corneal optical density showed that the central corneal thickness
and the medial corneal optical density were positively correlated
with the duration of diabetes (P<0.05). However, the corneal
endothelial cell density, superficial and total corneal optical
density were not significantly associated with the duration of
diabetes (P>0.05) (Tables
III–VI).
 | Table III.Comparison of the central corneal
thickness, corneal endothelial cell density, and corneal optical
density (0–2 mm) on diabetics at different stages of the
disease. |
Table III.
Comparison of the central corneal
thickness, corneal endothelial cell density, and corneal optical
density (0–2 mm) on diabetics at different stages of the
disease.
| Item | <5 years | 5–10 years | >10 years |
|---|
| CCT |
557.21±3.51 |
569.41±4.01 |
581.72±3.17 |
| Cell density |
3015±34.92 |
2401±26.74 |
2092.58±52.36 |
| Anterior |
25.28±4.94 |
24.97±5.61 |
25.41±4.98 |
| Center |
16.20±2.56 |
18.97±4.83 |
22.36±6.05 |
| Posterior |
20.37±7.61 |
34.29±9.84 |
41.02±11.36 |
| Total |
14.75±3.49 |
15.08±6.62 |
16.03±5.37 |
 | Table VI.Correlation between central corneal
thickness and intimal corneal optical density (0–2 mm). |
Table VI.
Correlation between central corneal
thickness and intimal corneal optical density (0–2 mm).
| Item (years) | Posterior | CCT | r | P-value |
|---|
| <5 |
20.37±7.61 |
557.21±3.51 | 0.221 | 0.036 |
| 5–10 |
34.29±9.84 |
569.41±4.01 | 0.042 | 0.017 |
| >10 |
41.02±11.36 |
581.72±3.17 | 0.049 | 0.001 |
Discussion
Improvement in living standards and changes to
lifestyle have led to an increase in the incidence of diabetes
(6). Long-term hyperglycemia has
toxic effects on all cells in the body, and its most profound
effects on eye tissues are on the cornea and retina. Seventy
percent of diabetes is complicated by keratopathy, including
recurrent corneal epithelial erosions, delayed wound healing,
ulcers and edema (1). Corneal
nervous lesion diabetes, which would result in the loss of corneal
sensitivity and innervation, may be associated with corneal
epithelial erosion. Thus, corneal neuropathy is considered an
important reason for corneal epithelial defects. Current studies on
diabetic ocular complications are focused on diabetic retinopathy
and diabetic cataract. To the best of our knowledge, few studies
are available on diabetic keratopathy (7–11). Those
few studies are carried out using confocal microscopy in the study
of diabetic corneal neuropathy (7–11). Major
clinical symptoms of diabetic retinopathy (DK) include corneal
epithelium damages, decreased corneal perception, thickened corneal
edema and nubecula, descemet's membrane folds, and corneal
autofluorescence leakage and dry eye (3,7,12–14).
Thus, hyperglycemia causes damage of different degrees to each
layer of the corneal structure. Corneal optical density has a
sensitive reflection on various traumas, inflammation and nubecula
incurred by denaturation, and is widely used to describe corneal
transparency. In the present study, we used the Pentacam to measure
and identify changes in corneal optical density on each layer of
the diabetics' cornea to reveal the pathogenesis of diabetic
keratopathy.
Corneal optical density value in the
diabetic and normal control groups
In the present study, we set the center point of the
cornea as the object of reference and measured the optical density
of each layer in each corneal section whose diameter was <2,
2–6, 6–10 and 10–12 mm, including the superficial, middle, interior
layers, and in total. We found that in the same section, the
optical density increased from the centre to the periphery while in
the same layer, the optical density decreased from the superficial
layer to the interior layer. Such alterations were associated with
the distribution of the corneal collagen fiber. Compared with the
normal control group, the diabetics' corneal optical density in the
same layer of the same section increased, and such an increase was
most obvious in the middle and interior layer.
The finding indicated that the decline of
transparency of the cornea was potentially due to the degeneration
of the corneal collagen fiber leading to an increase in the corneal
optical density. It has been previously shown that as patient age,
the corneal collagen fiber was glycosylated and became thickened
and hardened (15). The change in
corneal collagen fiber and the extracellular matrix increased the
corneal optical density. In a high-glucose environment, advanced
glycation end products (AGEs) accumulated in the corneal epithelial
basement membrane, or matrix. The descemet's membrane, which leads
to abnormal protein cross-linking and damage of the cell structure,
resulting in the clinical symptoms, includes local incrassation,
multiple stratification, and discontinuity (16,17).
Thus, long-term hyperglycemia may lead to the degeneration of
corneal collagen, leading to an increase in the corneal optical
density and a decrease in transparency. In addition, damage of the
pumping function of the corneal endothelium and the liquid
epithelial barrier resulted in edema and nubecula, decreased
transparency and increased optical density on the corneal stroma
and epithelium. Corneal endothelial morphology becomes abnormal in
patients with a long course of diabetes and their activity of
Na+/K+-ATPase enzyme declines, resulting in
pumping dysfunctions of corneal endothelium, edema and nubecula of
corneal stroma and the increase of corneal optical density.
The normal cornea is a transparent tissue without
blood vessels but full of nerve endings (18,19). The
central region of the cornea is associated with eyesight and has a
relatively stable structure. Previous studies have shown that the
thickness of the central regions is not associated with age
(20). Thus, this region became the
focus of the present study and the correlation of its optical
density and the duration of diabetes was investigated. The results
showed that as the duration of diabetes extended, the optical
density in the middle and interior layer gradually increased.
Therefore, the intimal and medial corneal optical density were
positively correlated with the course of diabetes, while the
optical density in the surface and total layer had no significant
correlation with the course of diabetes. Thus, the transparency of
the middle and interior layers of the cornea were the first to have
abnormal changes, which indicated that in the early period of
diabetes, the corneal endothelium and matrix may be the first to
have lesions. It may be because of the dysfunction of corneal
endothelial and the weakening of the water aspirator function
(7,21–23).
Thus, a correlation analysis was carried out between the course of
diabetes and central corneal density and corneal endothelial cell
count.
Correlation between central corneal
thickness, corneal endothelial cell number, and corneal optical
density
Through correlation analysis of the corneal
thickness, corneal endothelial cell density and the course of
diabetes, we found that as the duration of diabetes extended, the
central corneal thickness increased while the corneal endothelial
cell density decreased. However, the corneal endothelial cell
density had no correlation with the stage of the disease. It was
positively correlated with the central corneal thickness.
Therefore, we conducted a correlation analysis between the central
and intimal corneal optical density and central corneal thickness.
The result showed that in the early period of diabetes (<5
years), the medial and intimal corneal optical density had no
correlation with central corneal thickness. In patients with
long-term diabetes (>5 years), the medial corneal optical
density was positively correlated with central corneal thickness,
while the intimal corneal optical density was positively associated
with central corneal thickness in patients with short-term
diabetes. Thus, in the early period of diabetic keratopathy,
corneal optical density and central corneal thickness
increased.
Generally speaking, the change of corneal thickness
is an important indicator for a low corneal endothelial function.
Apparent corneal incrassation indicated the malfunction of the
cornea (20). The maintenance of
corneal thickness depended on the degree of dehydration of strong
water-absorbing base. It was mainly dependent on the epithelial
liquid barrier and endothelial pump mechanism to maintain the
cornea, and on the pumping function of the
Na+/K+-ATPase enzyme of the endothelial cells
to keep the balance between substrate ion and hydrodynamic force,
in order that the cornea always be kept dehydrated and transparent
(7,11). When the activity of the
Na+/K+-ATPase enzyme was reduced, the ionic
pump function of the corneal endothelial cells was impaired and
this was an important mechanism for diabetic corneal edema
(24–27). Subsequently, corneal thickness was
positively correlated with corneal intimal optical density. As
corneal edema became thickened, corneal transparency was reduced
and corneal optical density was increased accordingly (28). In terms of the course of the disease,
intimal and medial corneal optical density was the most altered.
Thus, intimal optical density was the first to become abnormal on
diabetic keratopathy patients and this was associated with corneal
endothelial dysfunction. The result of the present study showed
that corneal endothelial cell density had no significant
correlation with the stage of the disease. In the early period of
diabetic keratopathy, the endothelial lesion was manifested in the
abnormity of the morphology and function, and therefore a primary
research orientation in the future.
In conclusion, in the early stage of diabetic
keratopathy, corneal optical density in the interior and middle
layers changed abnormally, and the change was closely associated
with the duration of diabetes. The corneal thickness increased and
central corneal thickness was positively correlated with the length
of diabetes. The intimal corneal optical density was positively
correlated with corneal thickness. Thus, the corneal optical
density (especially the intimal optical density) is a sensitive
indicator for diabetic keratopathy. Corneal optical density and
central corneal density may be an important basis for the diagnosis
of diabetic keratopathy.
References
|
1
|
Wang Y, Zhou Q and Xie L: Diabetic
keratopathy: new progresses and challenges. Zhonghua Yan Ke Za Zhi.
50:69–72. 2014.(In Chinese). PubMed/NCBI
|
|
2
|
Kaji Y: Prevention of diabetic
keratopathy. Br J Ophthalmol. 89:254–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cennamo G, Forte R, Aufiero B and La Rana
A: Computerized Scheimpflug densitometry as a measure of corneal
optical density after excimer laser refractive surgery in myopic
eyes. J Cataract Refract Surg. 37:1502–1506. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Rosales P and Marcos S: Pentacam
Scheimpflug quantitative imaging of the crystalline lens and
intraocular lens. J Refract Surg. 25:421–428. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lanza M, Cennamo M, Iaccarino S, Romano V,
Bifani M, Irregolare C and Lanza A: Evaluation of corneal
deformation analyzed with a Scheimpflug based device. Cont Lens
Anterior Eye. 38:89–93. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kolb H and Mandrup-Poulsen T: The global
diabetesepidemic as a consequence of lifestyle-induced low-grade
inflammation. Diabetologia. 53:10–20. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Takacs AI, Mihaltz K and Nagy ZZ: Corneal
density with the Pentacam after photorefractive keratectomy. J
Refract Surg. 27:269–277. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hillenaar T, van Cleynenbreugel H, Verjans
GM, Wubbels RJ and Remeijer L: Monitoring the inflammatory process
in herpetic stromal keratitis: the role of in vivo confocal
microscopy. Ophthalmology. 119:1102–1110. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hamrah P, Cruzat A, Dastjerdi MH, Zheng L,
Shahatit BM, Bayhan HA, Dana R and Pavan-Langston D: Corneal
sensation and subbasal nerve alterations in patients with herpes
simplex keratitis: an in vivo confocal microscopy study.
Ophthalmology. 117:1930–1936. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Vivino MA, Chintalagiri S, Trus B and
Datiles M: Development of a Scheimpflug slit lamp camera system for
quantitative densitometric analysis. Eye (Lond). 7:791–798. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Greenstein SA, Fry KL, Bhatt J and Hersh
PS: Natural history of corneal haze after collagen crosslinking for
keratoconus and corneal ectasia: Scheimpflug and biomicroscopic
analysis. J Cataract Refract Surg. 36:2105–2114. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Fares U, Otri AM, Al-Aqaba MA, Faraj L and
Dua HS: Wavefront-optimized excimer laser in situ keratomileusis
for myopia and myopic astigmatism: refractive outcomes and corneal
densitometry. J Cataract Refract Surg. 38:2131–2138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Otri AM, Fares U, Al-Aqaba MA and Dua HS:
Corneal densitometry as an indicator of corneal health.
Ophthalmology. 119:501–508. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Constantinou M, Jhanji V, Tao LW and
Vajpayee RB: Clinical review of corneal ulcers resulting in
evisceration and enucleation in elderly population. Graefes Arch
Clin Exp Ophthalmol. 247:1389–1393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kaji Y, Usui T, Oshika T, Matsubara M,
Yamashita H, Araie M, Murata T, Ishibashi T, Nagai R, Horiuchi S
and Amano S: Advanced glycation end products in diabetic corneas.
Invest Ophthalmol Vis Sci. 41:362–368. 2000.PubMed/NCBI
|
|
16
|
Konstantopoulos A, Kuo J, Anderson D and
Hossain P: Assessment of the use of anterior segment optical
coherence tomography in microbial keratitis. Am J Ophthalmol.
146:534–542. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Konstantopoulos A, Hossain P and Anderson
DF: Recent advances in ophthalmic anterior segment imaging: a new
era for ophthalmic diagnosis? Br J Ophthalmol. 91:551–557. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Daxer A, Misof K, Grabner B, Ettl A and
Fratzl P: Collagen fibrils in the human corneal stroma: structure
and aging. Invest Ophthalmol Vis Sci. 39:644–648. 1998.PubMed/NCBI
|
|
19
|
Wegener A and Laser-Junga H: Photography
of the anterior eye segment according to Scheimpflug's principle:
options and limitations - a review. Clin Experiment Ophthalmol.
37:144–154. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Alsbirk PH: Corneal thickness. I. Age
variation, sex difference and oculometric correlations. Acta
Ophthalmol (Copenh). 56:95–104. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Keoleian GM, Pach JM, Hodge DO, Trocme SD
and Bourne WM: Structural and functional studies of the corneal
endothelium in diabetes mellitus. Am J Ophthalmol. 113:64–70. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cho YK, Chang HS, La TY, Ji D, Kim H, Choi
JA and Kim MS: Anterior segment parameters using Pentacam and
prediction of corneal endothelial cell loss after cataract surgery.
Korean J Ophthalmol. 24:284–290. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Buehl W, Stojanac D, Sacu S, Drexler W and
Findl O: Comparison of three methods of measuring corneal thickness
and anterior chamber depth. Am J Ophthalmol. 141:7–12. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Matsuda J, Hieda O and Kinoshita S:
Quantification of corneal opacity after refractive corneal surgery
using the anterior segment analyzer. Nippon Ganka Gakkai Zasshi.
111:447–453. 2007.(In Japanese). PubMed/NCBI
|
|
25
|
Shankar H, Taranath D, Santhirathelagan CT
and Pesudovs K: Anterior segment biometry with the Pentacam:
comprehensive assessment of repeatability of automated
measurements. J Cataract Refract Surg. 34:103–113. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Vemuganti GK, Reddy K, Iftekhar G, Garg P
and Sharma S: Keratocyte loss in corneal infection through
apoptosis: a histologic study of 59 cases. BMC Ophthalmol.
4:162004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vesaluoma M, Pérez-Santonja J, Petroll WM,
Linna T, Alió J and Tervo T: Corneal stromal changes induced by
myopic LASIK. Invest Ophthalmol Vis Sci. 41:369–376.
2000.PubMed/NCBI
|
|
28
|
Erie JC, Patel SV, McLaren JW, Hodge DO
and Bourne WM: Keratocyte density in the human cornea after
photorefractive keratectomy. Arch Ophthalmol. 121:770–776. 2003.
View Article : Google Scholar : PubMed/NCBI
|