Introduction
In recent years, the coal mining industry has been
developing at a high speed, which has significantly increased the
incidence of occupational disease (1). Coal miners are at a risk for certain
lung diseases, including silicosis, coal worker's pneumoconiosis
(CWP) and mixed dust pneumoconiosis, along with chronic airway
diseases and dust-associated diffuse fibrosis (2). CWP, a progressive and irreversible lung
disease occurring worldwide, mainly results from the inhalation and
accumulation of respirable coal and silica dusts (3). According to statistics, there were
~24,206 new cases of CWP diagnosed in 2012, which accounted for
~88.28% of all reported occupational diseases (4). Similar to other lung diseases, a
person's risk of developing CWP results from various factors, such
as free silica content, concentration of respirable coal dust,
particle size with its composition of coal dust, age, duration of
exposure and work environment of workers (5). CWP is reported to greatly influence
patients' health, since it can exacerbate other diseases or even
result in disability and mortality, but also increases the burdens
of the national health care system (6). Currently, not all the active coal
miners with CWP have worked under dust standards based on the
Federal Coal Mine Health and Safety Act of 1969, implying that
workers in the coal mining industry still lack adequate protection
from coal mine dust-associated disease (7).
Pulmonary function tests (PFTs) are usually applied
to identify and quantify abnormalities and defects in the function
of the respiratory system (8). PFTs
evaluate the entire respiratory system through physical
examinations, investigation of patient history, tests of pulmonary
function, arterial blood gas analysis, as well as chest X-ray
examinations (9). The forced vital
capacity (FVC), forced expiratory volume in 1 second (FEV1) and
FEV1/FVC are three common measures that clinically relevant to
pulmonary function (10). FEV1 and
FVC are affected by the size of the lung and are also reduced by
restrictive lung diseases, which lead to a greater reduction in
FEV1 compared with FVC (11). In
addition, reduced FEV1/FVC, independent of lung size and indicative
of airflow obstruction, is the main criterion for defining
obstructive hypoventilation (12).
PFTs have an important guiding significance for the early detection
of lung and airway diseases, the assessment of severity and
prognosis of disease, and the identification of the reasons for
expiratory dyspnea (13).
It is well-known that smoking can lead to the
occurrence and development of various diseases, such as
cerebrovascular disease, cancer, respiratory system disease and
cardiovascular disease (14). A
previous study proposed that the combined effect of smoking and
dust is an important factor causing a decline in pulmonary function
of dust-exposed workers (15).
Respirable coal dust concentration in the working area, and
cumulative total dust exposure (CTE) is a significant risk factor
for abnormal pulmonary function, and a previous study observed that
CTE may lead to COPD, including chronic bronchitis and emphysema
(2). Therefore, in order to
increasing the life span of coal-mine workers, the present study
aimed to investigate the correlation of smoking with CTE and
cumulative abnormal rate of pulmonary function in 376 coal-mine
workers.
Materials and methods
Ethics statement
The study was performed with the approval of the
Institutional Review Board of the Experimental Teaching
Demonstration Center, Central Laboratory, College of Public Health
of North China University of Science and Technology (Tangshan,
China). Informed written consent was collected from each eligible
worker and the entire study was performed based on the Declaration
of Helsinki (16).
Subjects
A total of 376 male workers specialized in mining
operations, who did not change occupation since they began coal
mining operations, were selected from a coal mine in Tangshan as
the observation group. These male subjects were aged between 21 and
59 years, with a mean age of 40.67±11.17 years, body weight of
47–86 kg, mean body weight of 66.47±11.37 kg, mean height of
170.8±6.24 cm and mean working duration of 22.53±9.56 years.
Workers were considered to be dust-exposed and were included into
the study if they met the following criteria: i) Exposure to dust
for >1 year; ii) exposure time in the specific coal mine
accounted for >50% of their total exposure time; iii) health
examination results within the past 2 years were available; and iv)
the history of changes in work were clear and complete, or could be
completed through archives.
In addition, 179 healthy male workers in the
instrument and meter plant and electrical power plant, which were
in the same area as the coal mine, were selected as the control
group of non-dust exposure workers. Control subjects were aged
between 20 and 58 years, with a mean age of 38.99±11.62 years, body
weight of 46–87 kg, mean body weight of 66.65±11.81 kg, and mean
height of 169.3±5.32 cm. There was no statistically significant
difference in the age, gender, body weight and height between the
two groups (P>0.05). The selected subjects were free of active
tuberculosis, short-term (within 2 weeks) bronchial-pulmonary
infection history, bronchial asthma, and heart, liver, kidney,
immune system or endocrine system diseases. Furthermore, the
subjects had not received treatments affecting the immune and
endocrine functions. A professional physician performed unified
health inspection using a unified health examination form, which
was completed individually for each participant. The main
information collected included the following: Name, gender, age,
height, body weight, temperature, pressure, smoking status, type of
dust exposed to, dust work history, work environment, protection
measures and past medical history.
PFT
The determination and analysis of PFT results were
performed by respiration physicians using Beijing AS.507 type
spirometer (Beijing Kangxin Tongchuang Science and Technology Co.
Ltd., Beijing, China). Prior to the examination, the subjects were
instructed to rest for 5–10 min and to breath calmly 4–5 times
before each test. The subjects inhaled deeply, aligning the inlet
of the equipment with the oral cavity, and subsequently exhaled
deeply as fast as possible. The pulmonary function measurement
parameters included FVC, FEV1 and FEV1/FVC. The optimum results
obtained from three or more measurements in each subject were
selected as the final results of the pulmonary function.
Evaluation criteria
Smoking was evaluated based on the smoking index,
which was calculated by the number of cigarettes smoked per day per
smoking year. Subjects with a smoking index ≥20 were classified as
smokers, while subjects with an index <20 were classified as
non-smokers.
Abnormal pulmonary functions were evaluated
according to the relative values of FVC and FEV1, with the
exception of FEV1/FVC. The relative value was calculated as
follows: (Actual measured value / theoretical value) × 100%. The
theoretical value was obtained using a PFT instrument and a
computational formula (17) based on
the age, gender, height, weight, temperature, pressure and other
factors, while the relative value eliminated the influence of the
aforementioned factors. Generally, an index with a theoretical
value of ≥80% was considered as normal, according to the techniques
and methods of compiled normal values of pulmonary function
reported nationwide (18).
Dust-exposure time was also evaluated. The
dust-exposed working duration was considered to be 1 year in
subjects exposed to coal dust for 1 full year, without changes in
work history. Subjects were divided into the following four groups,
according to the dust-exposure working duration: <10 years,
10–20 years, 20–30 years and >30 years.
CTE
According to the detection data of dust-exposure
levels reported between 1970 and 2013 by the Safety and
Environmental Protection Department of the coal mine (total dust
concentration is mainly measured by the filter membrane method)
(19), the work history of subjects
was carefully investigated, including details on the work hours or
the length of time working in the mine in their career, and any
changes in the type of work. The CTE of each coal-mine worker was
calculated as follows: CTE = ∑(Ci × Ti), where Ci is the dust
concentration the worker was exposed to at a certain period of
time, and Ti is the working year during which the worker was
exposed to this concentration. CTE is expressed in terms of
mg/m3.years.
Cumulative abnormal rate of pulmonary
function
The cumulative abnormal rate of pulmonary function
was calculated as follows: Cumulative abnormal rate = 1 −
cumulative normal rate; cumulative normal rate = (1 − abnormal
rate) × (1 − higher abnormal rate); abnormal rate = morbidity
[number of subjects at the beginning of the period – (number of
subjects at the end of the period / 2)]. Number of subjects at the
beginning of the period refers to all the individuals with a
certain initial CTE, while the number at the end of the period
refers to all the individuals with a certain CTE at the end.
Statistical analysis
The calculation formula according to quantitative
results of sample size was as follows: n =
[2σ2/(µ2−µ1)2] ×
f+(α, β), and this formula was used to calculate whether
the research object met the required sample size. µ1 and
µ2 refer to the mean values in non-dust exposed and
dust-exposed workers, respectively. σ refers to standard deviation,
f(α,β) refers to the function. α refers to the probability of the
first type of error and β is the probability of second type of
error. Considering the relative value of FEV1 as an example, if
α=0.05, β=0.10 and f+(α, β)=10.5, and the results of the
present study were µ1=87.46, µ2=79.07 and
σ=14.63, then the obtained sample size would be 63.85. Therefore, a
sample size of 64 cases is required in this test, while the actual
sample size of the control group was 179 cases, and the sample size
of the dust-exposed worker group was 376 cases; thus, the sample
sizes included in the present study satisfy the required
number.
In the current study, the associated information was
collected and a database was established. SPSS version 16.0
software (SPSS, Inc., Chicago, IL, USA) was used for data analysis
and processing. Measurement data are presented as the mean ±
standard deviation, and were analyzed using t test and F test.
Enumeration data are presented as %, and were analyzed using
χ2 test. Correlations were analyzed by Pearson's
correlation. A P-value of <0.05 was considered to indicate a
statistically significant difference.
Results
Distribution of smoking status in the
observational and control groups
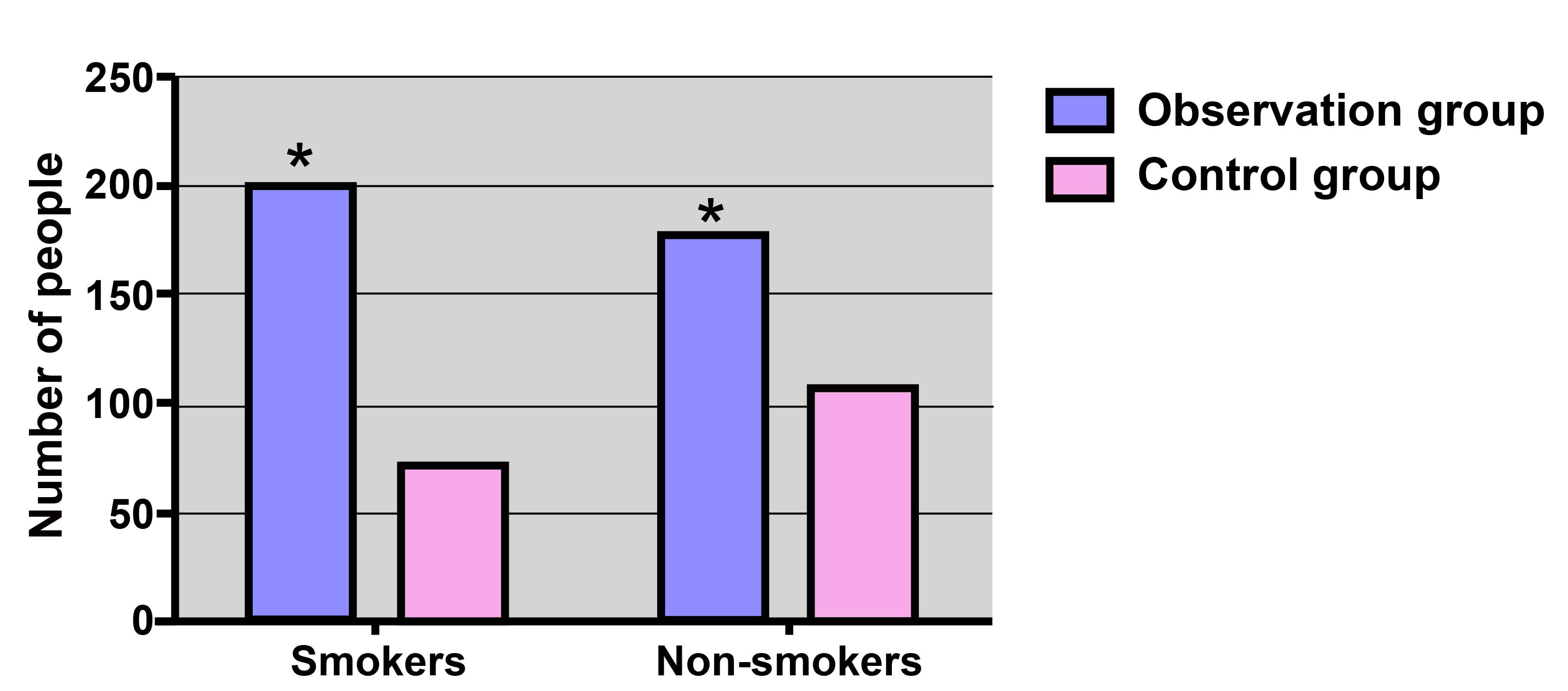
The distribution of smoking status in the
observational and control groups is displayed in Fig. 1. In the observational group, 200/376
subjects (53.19%) were smokers, which was higher compared with the
number of smokers in the control group (72/179 subjects; 40.22%),
with a significant difference observed (χ2=8.16;
P=0.004).
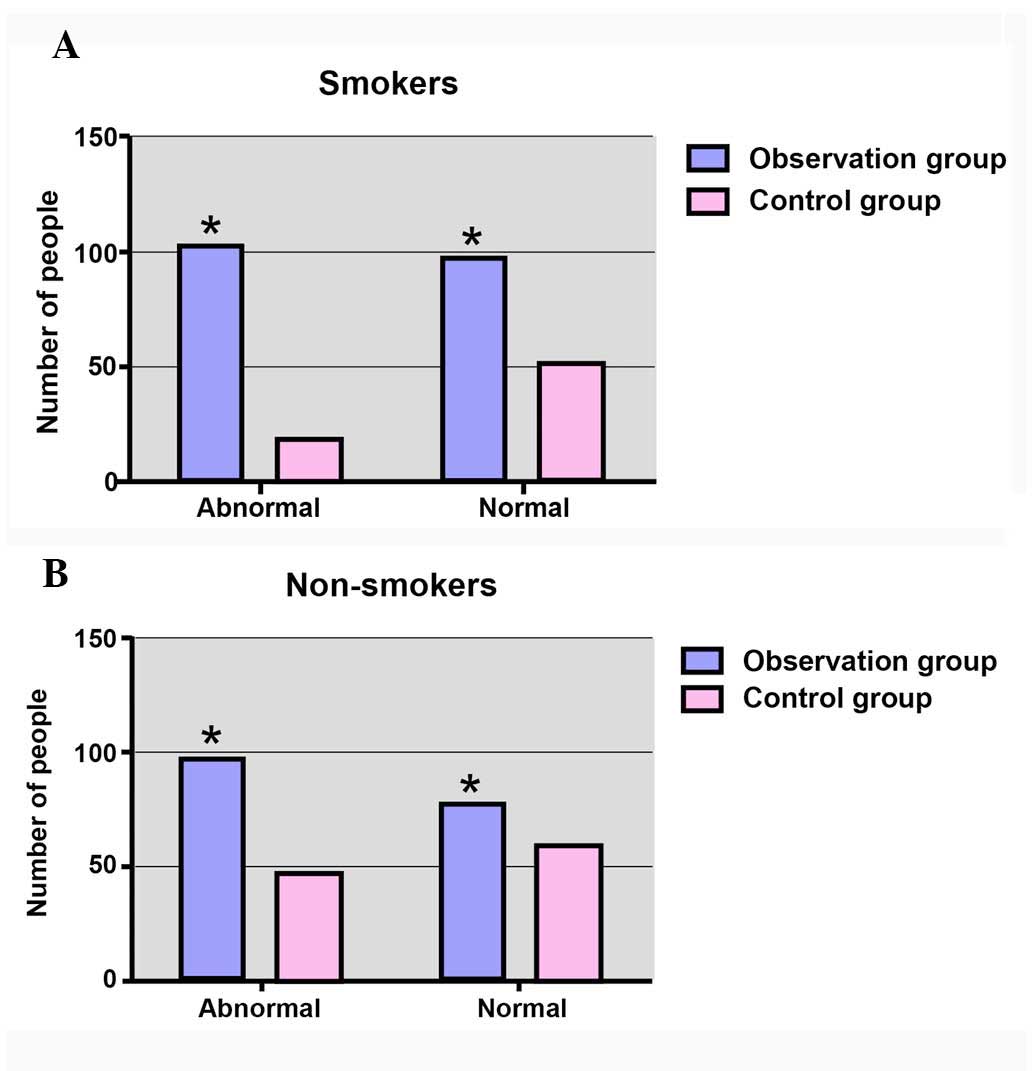
Comparison of cumulative abnormal rate
of pulmonary function in the observational and control groups
As shown in Fig. 2,
the cumulative abnormal rate of pulmonary function within smokers
in the observational group (102/200; 51.00%) was evidently higher
compared with that of smokers in the control group, with a
statistically significant difference identified
(χ2=12.98; P=0.003). However, in the non-smoking
subgroup, the cumulative abnormal rate of pulmonary function in the
control group was 44.86% (48/107), while the rate was 55.68%
(98/176) in the observational group, with no significant difference
observed (χ2=3.121; P=0.077).
Comparison of FVC, FEV1 and FEV1/FVC
values
In the smoking and non-smoking subgroups, FVC and
FEV1 in the observational group were markedly lower compared with
those in the control group (both P<0.05). In addition, FEV1/FVC
in the observational group was weakly lower compared with that in
the control group; however, no significant difference was detected
(P>0.05; Table I).
 | Table I.Comparison of FVC, FEV1 and FEV1/FVC
between the observational and control groups. |
Table I.
Comparison of FVC, FEV1 and FEV1/FVC
between the observational and control groups.
|
| Non-smoking
subgroup | Smoking subgroup |
|---|
|
|
|
|
|---|
| Group | n | FVC | FEV1 | FEV1/FVC | n | FVC | FEV1 | FEV1/FVC |
|---|
| Observational | 176 | 82.29±15.06 | 79.07±20.03 | 95.43±16.58 | 200 | 76.81±17.99 | 72.57±19.40 | 94.67±15.62 |
| Control | 107 | 87.68±14.69 | 84.25±18.47 | 97.09±14.73 | 72 | 91.19±15.01 | 87.30±13.65 | 96.18±20.37 |
| t-test |
| 2.947 | 2.172 | 1.144 |
| 6.063 | 5.932 | 0.338 |
| P-value |
| 0.004 | 0.031 | 0.254 |
| <0.001 | <0.001 | 0.734 |
As shown in Table
II, in the smoking subgroup, FVC and FEV1 in the group with a
working duration of >30 years were evidently lower compared with
the values in the <10-year, 10–20-year and 20–30-year working
duration group (all P<0.05). Comparison of FEV1/FVC in different
working duration groups showed no significant difference (P=0.169).
However, in the non-smoking subgroup, the comparison of FVC, FEV1
and FEV1/FVC in different working duration groups also showed no
significant difference (all P>0.05).
 | Table II.Comparison of FVC, FEV1 and FEV1/FVC
in various working durations between the smoking and non-smoking
subgroups. |
Table II.
Comparison of FVC, FEV1 and FEV1/FVC
in various working durations between the smoking and non-smoking
subgroups.
|
| Smoking subgroup | Non-smoking
subgroup |
|---|
|
|
|
|
|---|
| Working age
(years) | n | FVC | FEV1 | FEV1/FVC | n | FVC | FEV1 | FEV1/FVC |
|---|
| <10 | 26 | 90.29±14.66 | 88.42±16.64 | 99.76±22.52 | 10 | 89.40±14.41 | 85.81±14.54 | 97.67±21.44 |
| 10–20 | 63 |
80.96±17.98a |
76.78±16.95a | 95.82±13.23 | 35 | 88.66±14.44 | 84.22±11.37 | 97.05±19.43 |
| 20–30 | 70 |
74.16±15.60ab |
69.68±18.90ab | 93.55±16.59 | 86 | 87.97±15.01 | 83.34±14.70 | 96.80±21.72 |
| >30 | 41 |
66.42±16.96abc |
60.97±17.15abc | 91.61±10.96 | 45 | 85.97±14.64 | 81.77±16.34 | 97.12±22.72 |
| F-test |
| 13.05 | 14.69 | 1.696 | | 0.125 | 0.311 | 0.011 |
| P-value |
| <0.001 | <0.001 | 0.169 |
| 0.945 | 0.817 | 0.999 |
Correlation of smoking with pulmonary
function in coal-mine workers with different working durations
Table III
demonstrates the correlation of smoking with pulmonary function in
the observational group. The results indicated that FVC, FEV1 and
FEV1/FVC in the smoking subgroup were negatively correlated with
the time length of dust exposure (all P<0.05). By contrast, FVC,
FEV1 and FEV1/FVC in the non-smoking subgroup of coal-mine workers
were not found to be correlated with dust-exposure working duration
(all P>0.05).
 | Table III.Correlation of smoking with pulmonary
function in coal-mine workers with different working durations. |
Table III.
Correlation of smoking with pulmonary
function in coal-mine workers with different working durations.
|
| Smoking
subgroup | Non-smoking
subgroup |
|---|
|
|
|
|
|---|
| Index | r-value | P-value | r-value | P-value |
|---|
| FVC | −0.407 | <0.001 | −0.069 | 0.365 |
| FEV1 | −0.426 | <0.001 | −0.073 | 0.338 |
| FEV1/FVC | −0.156 |
0.027 | −0.003 | 0.996 |
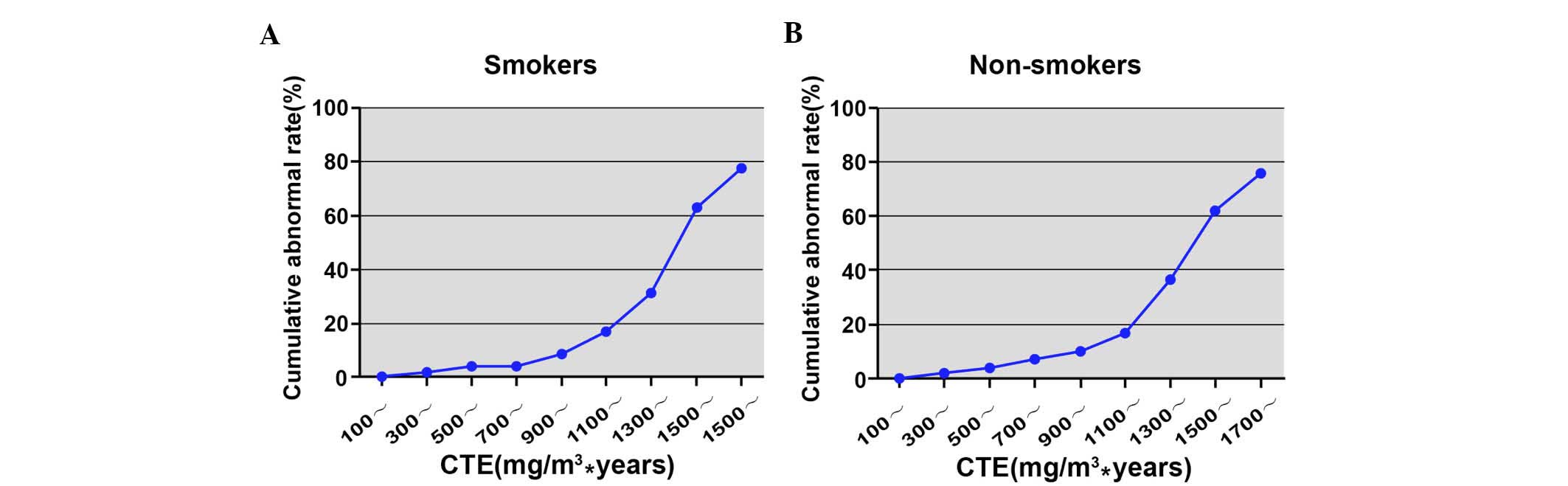
Correlation of CTE and cumulative
abnormal rate of pulmonary function
The cumulative abnormal rate of pulmonary function
in the smoking subgroup increased from 0.54% (100
mg/m3.years group) to 77.78% (1,700
mg/m3.years group) as shown in Table IV and Fig. 3. Similarly, the cumulative abnormal
rate in the non-smoking subgroup increased from 0.58% (100
mg/m3·years group) to 75.76% (1,700
mg/m3·years group; Table
V and Fig. 3).
 | Table IV.Correlation of cumulative total dust
exposure and cumulative abnormal rate of pulmonary function in
smoking subgroup. |
Table IV.
Correlation of cumulative total dust
exposure and cumulative abnormal rate of pulmonary function in
smoking subgroup.
| Cumulative total
dust exposure (mg/m3 years) | Subject no. at the
beginning of the period | Subject no. at the
end of the period | Morbidity | Abnormal rate | Accumulative
abnormal rate |
|---|
| 0 | 200 | 0 | 0 | 0.0000 | 0.0000 |
| 100 | 200 | 7 | 1 | 0.0051 | 0.0054 |
| 200 | 193 | 6 | 1 | 0.0053 | 0.0103 |
| 300 | 187 | 6 | 2 | 0.0109 | 0.0161 |
| 400 | 181 | 8 | 3 | 0.0169 | 0.0276 |
| 500 | 173 | 10 | 4 | 0.0238 | 0.0404 |
| 600 | 163 | 8 | 3 | 0.0189 | 0.0422 |
| 700 | 155 | 9 | 4 | 0.0266 | 0.0449 |
| 800 | 146 | 9 | 5 | 0.0353 | 0.0610 |
| 900 | 137 | 14 | 7 | 0.0538 | 0.0873 |
| 1,000 | 123 | 13 | 8 | 0.0687 | 0.1188 |
| 1,100 | 110 | 21 | 11 | 0.1106 | 0.1716 |
| 1,200 | 89 | 19 | 11 | 0.1384 | 0.2336 |
| 1,300 | 70 | 23 | 12 | 0.2051 | 0.3151 |
| 1,400 | 47 | 17 | 13 | 0.3377 | 0.4735 |
| 1,500 | 30 | 15 | 10 | 0.4444 | 0.6320 |
| 1,600 | 15 | 6 | 4 | 0.3333 | 0.6296 |
| 1,700 | 9 | 9 | 3 | 0.6667 | 0.7778 |
 | Table V.Correlation of cumulative total dust
exposure and cumulative abnormal rate of pulmonary function in the
non-smoking subgroup. |
Table V.
Correlation of cumulative total dust
exposure and cumulative abnormal rate of pulmonary function in the
non-smoking subgroup.
| Cumulative total
dust exposure (mg/m3 years) | Subject no. at the
beginning of the period | Subject no. at the
end of the period | Morbidity | Abnormal rate | Accumulative
abnormal rate |
|---|
| 0 | 176 | 0 | 0 | 0.0000 | 0.0000 |
| 100 | 176 | 6 | 1 | 0.0058 | 0.0058 |
| 200 | 170 | 5 | 1 | 0.0060 | 0.0117 |
| 300 | 165 | 6 | 2 | 0.0123 | 0.0182 |
| 400 | 159 | 7 | 2 | 0.0129 | 0.0250 |
| 500 | 152 | 9 | 4 | 0.0271 | 0.0396 |
| 600 | 143 | 7 | 5 | 0.0358 | 0.0620 |
| 700 | 136 | 8 | 5 | 0.0379 | 0.0724 |
| 800 | 128 | 7 | 5 | 0.0402 | 0.0765 |
| 900 | 121 | 13 | 7 | 0.0611 | 0.0988 |
| 1,000 | 108 | 11 | 8 | 0.0780 | 0.1344 |
| 1,100 | 97 | 19 | 9 | 0.1029 | 0.1729 |
| 1,200 | 78 | 16 | 12 | 0.1714 | 0.2567 |
| 1,300 | 62 | 21 | 12 | 0.2330 | 0.3645 |
| 1,400 | 41 | 15 | 10 | 0.2985 | 0.4620 |
| 1,500 | 26 | 13 | 9 | 0.4615 | 0.6223 |
| 1,600 | 13 | 4 | 3 | 0.2727 | 0.6084 |
| 1,700 | 9 | 9 | 3 | 0.6667 | 0.7576 |
Correlation of smoking with pulmonary
function in coal-mine workers with different CTE
CTE was positively correlated with cumulative
abnormal rate of pulmonary function in both the smoking and
non-smoking subgroups (smoking subgroup: r=0.884, P<0.001;
non-smoking subgroup: r=0.901, P<0.001). As shown in Table VI, FEV1 was negatively correlated
with CTE in the smoking subgroup (r=−0.184, P=0.009); however, FVC
and FEV1/FVC showed no correlation with CTE (both P>0.05). In
the non-smoking subgroup, FVC, FEV1 and FEV1/FVC had no significant
association with CTE (all P>0.05; Table VI).
 | Table VI.Correlation of smoking with pulmonary
function in coal-mine drillers with different cumulative total dust
exposure. |
Table VI.
Correlation of smoking with pulmonary
function in coal-mine drillers with different cumulative total dust
exposure.
|
| Smoking
subgroup | Non-smoking
subgroup |
|---|
|
|
|
|
|---|
| Index | r | P-value | r | P-value |
|---|
| FVC | −0.013 | 0.064 | −0.049 | 0.519 |
| FEV1 | −0.184 | 0.009 | −0.031 | 0.681 |
| FEV1/FVC | −0.083 | 0.241 | −0.035 | 0.645 |
Discussion
The present study demonstrated that the cumulative
abnormal rate of pulmonary function in smoking coal-mine workers
was significantly higher when compared with that in the control
group, indicating that smoking is an important risk factor for the
damage of pulmonary function in coal-mine workers. This may be
explained by the fact that cigarette smoke contains various harmful
substances, including nicotine, tobacco tar, nitrosamine and carbon
monoxide, which causes great harm to the respiratory system
(20). Inhalation of these harmful
substances can activate alveolar macrophages, T lymphocytes and
neutrophils, and these activated inflammatory cells subsequently
release a variety of compounds, including leukotriene B4,
interleukin-8 and tumor necrosis factor-α (21). These can damage the lung structure
and promote the inflammatory reaction of neutral granulocyte, and
lead to chronic inflammation of airway, lung parenchyma and
pulmonary blood vessels (21).
Furthermore, shorter and irregular bronchial epithelial cilia
caused by smoking can hinder the ciliary movement, reduce local
resistance, weaken phagocytosis and sterilization effects of
alveolar phagocytic cells, and are able to cause bronchospasm and
increase in airway resistance (22).
A previous study has shown that smoking causes the increase of
secretion in the airway through the sensory nerve endings, and the
excessive secretion of mucus has been found to be an important risk
factor of airflow obstruction (23).
With the exception of airway obstruction, long-term smoking also
can damage the vascular system. Carbon monoxide produced by
cigarette smoking can damage the endothelial cells of the arterial
wall and accelerate the development of atherosclerosis, which
further decrease the gas exchange function of lungs (24). The effect of smoking on pulmonary
function has been demonstrated by a previous study, and it is
reported that smoking may cause a decline in pulmonary function,
especially in small airway dysfunction. The combined effects of
smoking and CTE on FEV1 were significantly greater than that of the
smoking only and CTE only (25).
The current study indicated that FEV1 was negatively
correlated with CTE in the smoking subgroup, suggesting that
smoking was positively correlated with the damage of pulmonary
function in coal-mine workers and CTE. Additionally, in both the
smoking and non-smoking subgroups, FVC, FEV1 and FEV1/FVC were
significantly lower compared with the control group, indicating
that long-term exposure to coal dust significantly affected the
pulmonary function of workers, with a marked decrease observed in
FVC, FEV1 and FEV1/FVC. These decreased value may be due to the
fact that dust inhaled through the nose or mouth can reach any part
of the respiratory tract, from the nose to alveoli, and affect the
entire respiratory system; similarly, respirable dust can enter the
alveolar region of the lung and result in fibrosis or
pneumoconiosis that may be represented by changes in FVC (26). In addition, a low FEV1/FVC value
suggests the existence of airflow obstruction, while FEV1 serves an
important role in classifying the severity and in following the
progression of obstructive lung disease for a long time (27). Notably, a low FVC is an indicator of
a restrictive disorder, and patients with a low FVC will also tends
to have a low FEV1, which indicates an obstructive impairment.
Generally, a healthy individual exhales approximately 70–80% of the
FVC in the first second of exhalation, while people with airway
obstruction exhale only 60% or less of the FVC in the first second
(28).
The present study also demonstrated that FVC and
FEV1 in smoking workers with a >30-year working duration were
evidently lower when compared with those in the <10-year,
10–20-year and 20–30-year working duration groups. This suggested
that with the increase of workers' exposure time to coal dust, the
FVC, FEV1 and FEV1/FVC decreased more significantly, thus
indicating a close association between the dust-exposure time and
pulmonary function indexes in coal-mine workers. In the current
study, the cumulative abnormal rate of pulmonary function increased
with the increase in the amount of accumulated dust, implying that
abnormal pulmonary function is directly associated with the CTE
value of the worker. Therefore, a greater CTE results in a higher
risk of abnormal pulmonary function. Correlation analysis
demonstrated that there was a positive correlation between CTE and
the cumulative abnormal rate of pulmonary function; thus,
controlling the CTE of workers to a certain level range may help to
control the abnormal rate of pulmonary function. Coal workers with
respirable dust exposure for a long period of time are more likely
to be affected by mortality from respirable diseases, indicating
that the risk of mortality from respirable dust increases with the
increase of respirable dust exposure (29). In agreement with the present study
results, a previous study reported that CWP is primarily controlled
by reducing dust exposure in coal mines with technological
improvements and the establishment of dust standards (1).
In conclusion, the results of the present study
indicated that smoking is an important risk factor for the damage
of pulmonary function in coal-mine workers, and it is positively
correlated with dust-exposure time and CTE in these
individuals.
References
|
1
|
Lira M, Rabbani E Kohlman, Junior B
Barkokebas and Lago E: Risk evaluation and exposure control of
mineral dust containing free crystalline silica: A study case at a
quarry in the Recife Metropolitan Area. Work. 41(Suppl 1):
S3109–S3116. 2012.
|
|
2
|
Petsonk EL, Rose C and Cohen R: Coal mine
dust lung disease. New lessons from old exposure. Am J Respir Crit
Care Med. 187:1178–1185. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ji X, Wu B, Jin K, Luo C, Han R, Chen M,
Hou Z, Fan J and Ni C: MUC5B promoter polymorphisms and risk of
coal workers' pneumoconiosis in a Chinese population. Mol Biol Rep.
41:4171–4176. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xia Y, Liu J, Shi T, Xiang H and Bi Y:
Prevalence of pneumoconiosis in Hubei, China from 2008 to 2013. Int
J Environ Res Public Health. 11:8612–8621. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hung YP, Teng CJ, Liu CJ, Hu YW, Hung MH,
Tzeng CH, Liu CY, Yeh CM, Chen TJ and Chiou TJ: Cancer risk among
patients with coal workers' pneumoconiosis in Taiwan: A nationwide
population-based study. Int J Cancer. 134:2910–2916. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu B, Ji X, Han R, Han L, Wang T, Yang J,
Zhu B and Ni C: GITR promoter polymorphism contributes to risk of
coal workers' pneumoconiosis: A case-control study from China.
Immunol Lett. 162:210–216. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Blackley DJ, Halldin CN, Wang ML and Laney
AS: Small mine size is associated with lung function abnormality
and pneumoconiosis among underground coal miners in Kentucky,
Virginia and West Virginia. Occup Environ Med. 71:690–694. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Crim C, Celli B, Edwards LD, Wouters E,
Coxson HO, Tal-Singer R and Calverley PM: ECLIPSE investigators:
Respiratory system impedance with impulse oscillometry in healthy
and COPD subjects: ECLIPSE baseline results. Respir Med.
105:1069–1078. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zuccarini G, Bocchino M, Assante LR, Rea G
and Sanduzzi A: Metformin/glibenclamide-related interstitial lung
disease: A case report. Sarcoidosis Vasc Diffuse Lung Dis.
31:170–173. 2014.PubMed/NCBI
|
|
10
|
van Dalen C, Harding E, Parkin J, Cheng S,
Pearce N and Douwes J: Suitability of forced expiratory volume in 1
second/forced vital capacity vs percentage of predicted forced
expiratory volume in 1 second for the classification of asthma
severity in adolescents. Arch Pediatr Adolesc Med. 162:1169–1174.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan S, He H, Guan B, Liu T, Yuan X, Ma W
and Xie Y: The effect of endoscopic sinus surgery on pulmonary
function of chronic rhinosinusitis patients with asthma. Lin Chung
Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 28:1118–1121. 2014.PubMed/NCBI
|
|
12
|
Dashti HS, Shea MK, Smith CE, Tanaka T,
Hruby A, Richardson K, Wang TJ, Nalls MA, Guo X, Liu Y, et al:
Meta-analysis of genome-wide association studies for circulating
phylloquinone concentrations. Am J Clin Nutr. 100:1462–1469. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pellegrino R, Viegi G, Brusasco V, Crapo
RO, Burgos F, Casaburi R, Coates A, van der Grinten CP, Gustafsson
P, Hankinson J, et al: Interpretative strategies for lung function
tests. Eur Respir J. 26:948–968. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kallberg H, Ding B, Padyukov L, Bengtsson
C, Rönnelid J, Klareskog L and Alfredsson L: EIRA Study Group:
Smoking is a major preventable risk factor for rheumatoid
arthritis: Estimations of risks after various exposures to
cigarette smoke. Ann Rheum Dis. 70:508–511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Churg A, Hall R and Bilawich A:
Respiratory bronchiolitis with fibrosis-interstitial lung disease:
A new form of smoking-induced interstitial lung disease. Arch
Pathol Lab Med. 139:437–440. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
MPN, . World medical association publishes
the revised declaration of helsinki. Natl Med J India.
27:562014.
|
|
17
|
Jin Y: Data acquisition and processing
system of pulmonary function instrument. Electronic Technology and
Software Engineering. 4:220–221. 2014.(In Chinese).
|
|
18
|
Mu KJ: Compiled normal values of pulmonary
function nationwide. United Press of Beijing Medical University and
Peking Union Medical College; 1990, (In Chinese).
|
|
19
|
Gooch JW: Membrane Filter Method.
Springer; New York: 2011, (In Chinese).
|
|
20
|
Talhout R, Schulz T, Florek E, van Benthem
J, Wester P and Opperhuizen A: Hazardous compounds in tobacco
smoke. Int J Environ Res Public Health. 8:613–628. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cazzola M, Page CP, Calzetta L and Matera
MG: Emerging anti-inflammatory strategies for COPD. Eur Respir J.
40:724–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Brekman A, Walters MS, Tilley AE and
Crystal RG: FOXJ1 prevents cilia growth inhibition by cigarette
smoke in human airway epithelium in vitro. Am J Respir Cell Mol
Biol. 51:688–700. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Caramori G, Kirkham P, Barczyk A, Di
Stefano A and Adcock I: Molecular pathogenesis of cigarette
smoking-induced stable COPD. Ann N Y Acad Sci. 1340:55–64. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Leone A and Landini L: Vascular pathology
from smoking: Look at the microcirculation! Curr Vasc Pharmacol.
11:524–530. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu X, Salter A, Thomas P, Leigh J and
Wang H: Exhaled nitric oxide levels and lung function changes of
underground coal miners in Newcastle, Australia. J Toxicol Environ
Health A. 73:437–444. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shieh TS, Chung JJ, Wang CJ, Tsai PJ, Kuo
YC and Guo HR: Pulmonary function, respiratory symptoms, and dust
exposures among workers engaged in early manufacturing processes of
tea: A cohort study. BMC Public Health. 12:1212012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hancock DB, Eijgelsheim M, Wilk JB, Gharib
SA, Loehr LR, Marciante KD, Franceschini N, van Durme YM, Chen TH,
Barr RG, et al: Meta-analyses of genome-wide association studies
identify multiple loci associated with pulmonary function. Nat
Genet. 42:45–52. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rao DR, Gaffin JM, Baxi SN, Sheehan WJ,
Hoffman EB and Phipatanakul W: The utility of forced expiratory
flow between 25% and 75% of vital capacity in predicting childhood
asthma morbidity and severity. J Asthma. 49:586–592. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Miller BG and MacCalman L: Cause-specific
mortality in British coal workers and exposure to respirable dust
and quartz. Occup Environ Med. 67:270–276. 2010. View Article : Google Scholar : PubMed/NCBI
|