Introduction
Fungal keratitis has an prominently increasing
morbidity, accounting for 46.7% of purulent corneal infection in
China (1). It has been reported that
filamentary fungi are the most prevalent pathogens in China,
wherein the genus Fusarium is the most common cause (77.6%)
followed by Aspergillus (10.8%) (2). A well-known contributing factor for the
development of fungal keratitis is ocular trauma, specifically
contamination of corneal lesions by soil and other vegetative
material (3). Notably, the majority
of the patients suffering from fungal keratitis in China are
farmers in whom early diagnosis is easily missed, and whose
residences are commonly distant from well-equipped eye care
facilities. Delays in diagnosis and the initiation of prompt
antifungal medical therapy are the major reasons that many patients
from rural areas present with advanced corneal infection (4). Since approximately one-third of cases
of fungal keratitis result in either medical treatment failures or
corneal perforations (5), it has
become a serious disease in China. When fungal corneal infections
become unresponsive to medical therapy, surgical intervention
offers a second chance for eradicating the infection and
maintaining the globe integrity.
Although therapeutic penetrating keratoplasty (TPK)
and lamellar keratoplasty (LK) have been shown to be effective in
the management of recalcitrant fungal keratitis (6–9), the
lack of donor corneas in China has forced ophthalmologists to
investigate other treatment strategies. Given the massive rejection
reaction and high graft failure rates associated with keratoplasty
in the treatment of recalcitrant fungal keratitis, any modality
that avoids the requirement for a donor cornea would be of
significant value in countries such as China (1,10,11).
Historically, debulking the organism and necrotic
material by daily debridement at the slit lamp was the mainstay of
therapy for fungal keratitis. The removal of active infection and
devitalized tissue was conducted with the aim of enhancing the
penetration of topical antifungal medications. However, removal of
the necrotic corneal tissue combined with conjunctival flap
excisional keratectomy combined with conjunctival flap inlay
(EKCFI) is now becoming widely used in many tertiary eye care
facilities in China (12).
Additionally, cryotherapy combined with antifungal agents and/or
corneoscleral grafting has been used successfully in cases of
fungal scleritis and keratoscleritis (13,14).
Several groups have reported promising results using human amniotic
membrane as an adjunct for the treatment of active microbial
keratitis (15–17). In a study conducted by Chen et
al in 2006, human amniotic membrane was successfully used in
active cases of fungal keratitis, even in some cases in which
perforation had previously occurred (18). These earlier reports demonstrate that
non-keratoplasty modalities may be effective alternatives for the
treatment of recalcitrant fungal keratitis.
In 2006, the present authors began employing human
amniotic membrane and cryotherapy as adjuncts to surgical
interventions in the management of fungal keratitis using
excisional keratectomy combined with focal cryotherapy and amniotic
membrane inlay (EKCAI). The present study is a retrospective
analysis of all confirmed cases of filamentary fungal keratitis at
a single institution, in which biostatistical analysis was
attempted to evaluate the efficacy of EKCAI compared with that of
conventional surgical therapies for recalcitrant fungal
keratitis.
Materials and methods
Patient enrolment and ethics
The charts of all patients with a diagnosis of
fungal keratitis who were enrolled in the General Hospital of
Shenyang Military Command (Shenyang, China) in-patient
ophthalmology service from January 2006 to January 2011, were
reviewed and the cases that received surgical intervention were
retrieved from the records. Inclusion criteria in this analysis
were as follows: i) Filamentary keratitis confirmed by corneal
scrape culture or potassium hydroxide staining; ii) any cases of
urgent surgical intervention due to corneal perforation either at
presentation or within the first week of admission; iii) cases
deemed as medical treatment failure, defined as documentation of
progression of corneal ulcers after at least 1 week of appropriate
medical treatment; iv) patients who underwent surgical
interventions and had ≥1 year of follow-up after surgery.
Medical treatment was standardized to topical 5%
natamycin eye drops (5%; Alcon; Novartis International AG, Basel,
Switzerland) every 2 h and 150 mg intravenous infusion of
fluconazole (Changchun Dirui Pharmacy, Inc., Changchun, China) once
a day from the time of admission. A total of 128 eyes (128
patients) met the inclusion criteria and were selected in this
analysis. The study was approved by the ethics committee of the
General Hospital of Shenyang Military Command and conducted in
accordance with the Declaration of Helsinki. Written informed
consent was obtained from each subject.
Surgical triage of fungal keratitis
cases
As a general rule, cases with corneal perforation
either at presentation or during medical treatment were managed
with TPK if donor corneas were available. Otherwise, such patients
were treated with EKCFI or EKCAI, based on the availability of
amniotic membranes at the time of surgeries or the surgeon's
personal preference. If patients were deemed to be a medical
treatment failure, cases with corneal ulcers with the depth >70%
of the corneal stroma were also preferentially treated with TPK if
donor corneas were available. Therefore, relatively severe cases in
the clinic tended to be treated with TPK. For cases involving
perforation as a result of medical treatment failure that did not
receive TPK, the assignment of either EKCFI or EKCAI was not
randomized either, with more EKCAI procedures performed in later
phase of the reviewed time frame.
Surgical procedures and postoperative
treatment
All surgical interventions were performed under
general or local retrobulbar anesthesia with 2.5 ml 2% lidocaine.
Intraoperatively, 2 mg/ml fluconazole solution was used to irrigate
the corneal ulcers for subconjunctival injection. The operated eyes
were bandaged for 12 h postoperatively prior to the initiation of
treatment. The postoperative medical antifungal regimen was the
same in all three treatment groups and consisted of 5% natamycin
drops topically 6 times daily for the first week, 4 times daily for
the second week, and twice daily for the following 2 additional
weeks during waking hours. In addition, 1% atropine sulfate
ointment (1%; Alcon; Novartis International AG) was applied once
daily.
EKCFI procedure
Details of the EKCFI procedure have been described
previously by Sun et al (12). Briefly, the necrotic corneal tissue
was removed with sharp dissection and two 5-mm bulbar conjunctival
incisions were made perpendicular to the limbus at the 3:00 and
9:00 o'clock positions, respectively. The incisions were extended
circumferentially along the limbus to create superior and inferior
conjunctival flaps, and then undermined to the fornices. Two
conjunctival flaps were then pulled centripetally onto the corneal
surface and sutured in an interrupted manner to cover the debrided
stromal ulcer bed.
EKCAI procedure
In the EKCFI procedure, after the ulcer bed was
debrided and dried with a surgical sponge, the entire area of the
ulcer was treated with a 3-mm cryoprobe, with involvement of the
junctional region 1 mm beyond the edge of the ulcer. The duration
of each treatment was 10 sec once the probe reached-70°C. Dry
amniotic membrane (Ruiji Biotechnology Co. Ltd., Jiangxi, China)
was first hydrated for 10 min in balanced salt solution (BSS)
solution. Multilayers of the amniotic membrane were placed as
inlays to cover the ulcer bed and sutured onto the stroma with
interrupted 10–0 nylon sutures.
TPK procedure
The specific TPK process was reported by Lim et
al in 2011 (9). In summary, a
trephine that was ≥0.5 mm larger in diameter than the affected area
was applied to each case to perform the penetrating keratoplasty.
Prior to graft placement, the hypopyon in the anterior chamber was
thoroughly irrigated with 2 mg/ml fluconazole solution and any
fibrovascular membrane present on the anterior iris surface was
carefully removed. The donor corneal grafts were generally
oversized by 0.25-0.5 mm. Large corneal or corneoscleral grafts
were required for ulcers with limbal or scleral involvement.
Off-center grafts were placed when required to adequately cover the
defects.
Outcome evaluation
Therapeutic success, recurrence of the cornea or
sclera infection, postoperative best-corrected visual acuity
(BCVA), postoperative complications, corneal neovascularization in
the EKCFI and EKCAI groups, and graft survival (clarity) in the TPK
group, were all reviewed during a 1-year follow-up. Therapeutic
success was defined as no evidence of recurrent infection following
the cessation of postoperative antifungal medical therapy and the
maintenance of globe integrity without subsequent secondary
surgical intervention during 1-year follow-up. Therapeutic failure
was defined as: i) Necessity to continue medical treatment for ≥3
months following the primary surgery; ii) recurrence of the
original infection during the 1-year follow-up period following the
cessation of antifungal medical therapy, requiring repeated medical
therapy or secondary surgical intervention. Corneal
neovascularization was graded on a scale of 0 to IV, based on its
involvement of the cornea by quadrants at 1 year postoperatively in
cases with therapeutic success. Postoperative complications
included corneal perforation, development of endophthalmitis and
secondary glaucoma within 1 year of the primary surgeries. The
conversion of the original decimal notation visual acuity to LogMAR
vision was performed to facilitate statistical analysis. For vision
CF or worse, the following conversions were made: CF=1.6; hand
movements = 2.0; light perception = 2.5; and no light perception =
3.0 LogMAR units, according to Arroyo et al (19).
Statistical analysis
The dataset, including patient's demographic
characteristics, preoperative findings and postoperative results
among the three surgical interventions, was carefully evaluated by
expert statisticians. Analysis of variance was used for normally
distributed continuous variables to test for mean differences
within the surgical groups. Pearson Chi-square test was used to
test the frequency differences between groups for categorical
variables. However, when categorical variables had a small number
of expected counts within at least one of the three surgical
categories, the Fisher's exact test was used instead. In addition,
pairwise comparisons of preoperative data and postoperative results
among the three surgical groups were conducted, with Bonferroni
adjustment.
Odds ratios and 95% confidence intervals were
determined by Firth logistic regression, a penalized likelihood
method to solve quasi-complete separation problems in regular
logistic regression models. Three logistic regression models were
used to explore the association between surgical success and
surgical interventions. Unadjusted results were derived from the
initial univariate model. Model 1 was adjusted for gender. Model 2
was adjusted for both gender and a history of steroid use (positive
vs. negative). P<0.05 was considered significant to indicate a
statistically significant difference. All analyses were conducted
using SAS software, version 9.4 (SAS Institute, Inc., Cary, NC,
USA).
Results
Preoperative characteristics
The preoperative characteristics of all selected
patients, included pertinent history, infection characteristics and
vision are shown in Table I, There
was a male predominance, and histories of steroid use were not
evenly distributed among the three intervention groups. There were
predominantly more males in the EKCFI group and more cases of
steroid use in the EKCAI group, in comparison with the other two
groups respectively (Table II). No
statistical differences were found among the groups in terms of
disease characteristics and severity. All patients who met the
selection criteria had vision defined as count fingers (CF) or
worse at presentation.
 | Table I.Patient characteristics in the three
groups. |
Table I.
Patient characteristics in the three
groups.
| Index | EKCAI (n=43) | EKCFI (n=24) | TPK (n=61) | P-valuea |
|---|
| Male gender | 25 (58.14) | 21 (87.50) | 38 (62.30) | 0.0396 |
| Age (years) | 46.02±5.51 | 43.42±6.03 | 44.69±7.18 | 0.2713 |
| History |
|
|
|
|
|
Traumab | 19 (44.19) | 15 (62.50) | 27 (44.26) | 0.2713 |
|
Surgeryb | 1 (2.33) | 1 (4.17) | 2 (3.28) | 1.0000 |
| Steroid
useb | 14 (32.56) | 6 (25.00) | 2 (3.28) | <0.0001 |
| Contact
lens useb | 1 (2.33) | 1 (4.17) | 1 (1.64) | 0.7695 |
| No
positive historyb | 4 (9.30) | 4 (16.67) | 15 (24.59) | 0.1507 |
| Presentation |
|
|
|
|
| LogMAR
vision | 1.81±0.20 | 1.77±0.20 | 1.80±0.20 | 0.6458 |
| Ulcer
diameter, mm | 6.51±1.71 | 6.50±1.72 | 6.97±1.47 | 0.2720 |
| Ulcer
depth (%) | 69.53±24.39 | 71.25±26.59 | 73.61±23.88 | 0.7028 |
| Infection WLI | 9 (20.93) | 4 (16.67) | 15 (24.59) | 0.7166 |
| Hypopyon
presentb | 38 (88.37) | 20 (83.33) | 55 (90.16) | 0.6225 |
|
Perforationb | 10 (23.26) | 5 (20.83) | 19 (31.15) | 0.5215 |
 | Table II.Postoperative outcome distributions
among surgical intervention groups. |
Table II.
Postoperative outcome distributions
among surgical intervention groups.
| Surgical
intervention | EKCAI (n=43) | EKCFI (n=24) | TPK (n=61) | P-value |
|---|
| Therapeutic
successa | 38 (88.37) | 14 (58.33) | 57 (93.44) |
0.0002 |
| Secondary
glaucomab | 5 (11.63) | 6 (25.00) | 3 (4.92) |
0.0235 |
|
Neovascularizationb |
|
|
| <0.0001 |
| Grade
0 | 14 (32.56) | 7 (29.16) | 18 (29.50) |
|
| Grade
I | 10 (23.26) | 0 (0.00) | 35 (57.38) |
|
| Grade
II | 13 (30.23) | 1 (4.17) | 7 (11.48) |
|
| Grade
III | 6 (13.95) | 15 (62.50) | 1 (1.64) |
|
| Grade
IV | 0 (0.00) | 1 (4.17) | 0 (0.00) |
|
|
LogMARvisionc | 1.33±1.03 | 1.85±1.01 | 0.84±0.73 | <0.0001 |
Treatment outcomes
The treatment outcomes of the three interventions
are summarized in Table II. TPK
achieved the highest rate of infection control (therapeutic
success, 93.44%) compared with EKCAI (88.37%) and EKCFI (58.33%).
TPK also had the lowest incidence of secondary glaucoma, the least
neovascularization of the cornea, and better postoperative vision
at 1 year postoperatively, compared with EKCAI and EKCFI. A
pairwise comparison was then performed between surgical
intervention groups (Table III).
Significant differences were observed between the EKCAI and EKCFI
groups in infection control (P=0.0141) and postoperative
neovascularization (P<0.0001). However, there was no significant
difference between these two interventions in rates of secondary
glaucoma and postoperative vision at 1 year. Compared with EKCAI,
TPK showed superior postoperative vision at 1 year (P=0.0171) and
significantly less corneal neovascularization (P<0.0001).
However, no difference was identified in infection control and
secondary glaucoma between TPK and EKCAI. TPK also performed
significantly better than EKCFI in all outcome measures analyzed
(P<0.0001), with the exception of the rate of secondary glaucoma
(P=0.0402). Typical cases of filamentary fungal keratitis cases
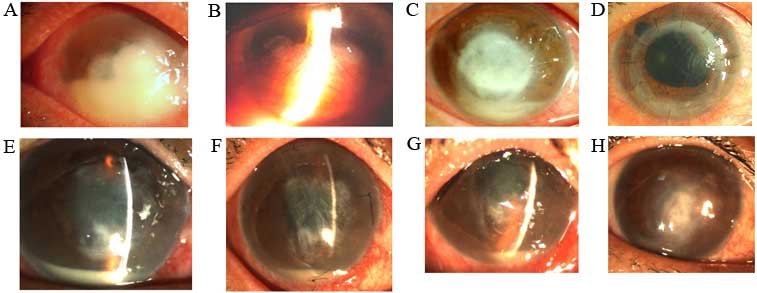
treated with different surgical modalities were shown in Fig. 1.
 | Table III.Pairwise comparison of postoperative
results. |
Table III.
Pairwise comparison of postoperative
results.
|
| P-value |
|---|
|
|
|
|---|
| Treatment
outcome | EKCAI vs.
EKCFI | EKCAI vs. TPK | EKCFI vs. TPK |
|---|
| Therapeutic
successa |
0.0141 | 1 | <0.0001 |
| Secondary
glaucomab |
0.5472 |
0.8109 |
0.0402 |
|
Neuvascularizationb | <0.0001 | <0.0001 | <0.0001 |
| LogMAR
visionc |
0.1440 |
0.0171 | <0.0001 |
Since the study was not randomized, odds ratios of
therapeutic success among surgical interventions were calculated,
adjusting for gender and steroid use (Table IV). Univariate modeling shows that
EKCAI had superior infection control compared with EKCFI
(P=0.0073), but had an equivalent outcome compared with TPK
(P=0.3712). After adjusting for gender (model 1), gender and
steroid use (model 2), the statistical significance remained
unchanged.
 | Table IV.Odds ratios of therapeutic success
among surgical interventions. |
Table IV.
Odds ratios of therapeutic success
among surgical interventions.
|
| Univariate
model | Model
1a | Model
2b |
|---|
|
|
|
|
|
|---|
| Surgical
intervention | OR | 95% CI | P-value | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| EKCAI | 1 (Ref) |
|
| 1 (Ref) |
|
| 1 (Ref) |
|
|
| EKCFI | 0.184 | 0.054–0.634 | 0.0073 | 0.087 | 0.019–0.404 | 0.0018 | 0.077 | 0.016–0.378 | 0.0016 |
| TPK | 1.874 | 0.473–7.431 | 0.3712 | 1.806 | 0.440–7.420 | 0.4121 | 1.451 | 0.339–6.219 | 0.6161 |
Discussion
Filamentous fungal keratitis has a high incidence in
China where farmers constitute the majority of the affected
patients, and have poor awareness of the signs and symptoms of the
disease. In addition, limited availability of services in primary
hospitals accounts for the majority of advanced and refractory
cases in this region (20).
Broad-spectrum antifungal medication is the acknowledged first-line
treatment during the earliest stage of the infection. However, a
delay in presentation and treatment results in a worse prognosis
(21). Analysis of treatment
outcomes in cases where treatment had been delayed for 10 days
showed significantly higher rates of surgical interventions. In the
patients included in the present study, the duration of infection
prior to presentation in the majority of cases was >10 days, and
thus the infection was refractory to antifungal therapy, and
required surgical intervention.
Standard surgical modalities, including TPK and LK,
have been shown to be effective in the management of recalcitrant
fungal keratitis (6–9). However, the low availability of donor
corneas has remained a formidable challenge to ophthalmologists in
China. Alternative methods are constantly sought for managing these
difficult cases. In recent years, EKCFI has been widely adopted in
many tertiary eye care facilities in China (12).
In the treatment of serious fungal keratitis, EKCFI
is a useful surgical technique, particularly in eye care facilities
without keratoplasty capabilities, or when corneal donor tissue is
unavailable. It has been theorized that the conjunctival flap
provides additional blood supply to the affected area, allowing
anti-inflammatory and immunomodulatory substances to be delivered
locally, promoting better wound healing. Sun et al (12) reported a total of 10 cases treated
with EKCFI, which had a success rate of 100% with no recurrence.
Outcomes of the present study showed that EKCFI had a lower success
rate of 58.33% (14/24) compared with that in this earlier study.
The severity of disease observed in the present study, combined
with the late presentations, and ineffectiveness of antifungal
medications may account for the difference in success rates.
EKCAI is a relatively new surgical approach that may
be an excellent therapeutic option in the management of refractory
fungal keratitis. With outcomes on par with standard TKP in
controlling infection and maintaining globe integrity, this
technique offers the combined benefits of cryotherapy and amniotic
membrane tissue, and does not require donor corneas. Cryotherapy
has been used for many years in the treatment of numerous eye
diseases with increasing popularity, not only in the United States
but also in some developing countries such as China, in the
management of infectious keratitis. The therapeutic effects
observed with cryotherapy are considered to have several
mechanisms, and may be particularly useful in difficult cases of
fungal keratitis. Through the destruction of small-caliber blood
vessels, tissue ischemia begins to develop. As the freezing
continues, osmotic forces produce cellular dehydration which
facilitates intracellular ice crystal formation. The ice crystals
and intracellular dehydration lead to pH changes and the formation
of a toxic concentration of salts in the cell. Slow thawing
produces a longer exposure to this toxic substrate concentration
and leads to re-crystallization which increases cell destruction.
The ultimate mechanism underlying the effects of cryotherapy is the
denaturation of lipid-protein complexes, cytomembrane rupture and
eventually cell death (22–24). Cryotherapy has been used in the past
to treat acanthamoeba keratitis with variable success, however, its
impact in fungal ulcers is not clearly understood (25–28).
Early studies have shown promising results in cases of fungal
scleritis and keratoscleritis (12,13), and
the present study lends further support to its role in these
difficult cases. The present authors have previously studied rabbit
models with fungal keratitis, which showed the topical cryotherapy
is able to destroy fungal elements, and the effect of topical
cryotherapy combined with antifungal drugs was observed to be
better than that of antifungal drugs alone (29).
The biological properties attributed to amniotic
membrane tissues include lack of immunogenicity, promotion of
epithelialization, and inhibition of fibrosis, angiogenesis and
inflammation (30–35). The amniotic membrane is the innermost
layer of the fetal membrane, and is composed of an avascular
epithelial layer over a thick basement membrane. Use of amniotic
membrane has been shown to promote epithelialization and reduce
inflammation and scarring, possibly through the regulation of
growth factors or by acting as a barrier to infiltrating
lymphocytes (36). Bauer et
al (36) reported that the
mechanism underlying the effects of amniotic membrane is associated
with the modulation of macrophages. Apoptotic cells induced in the
environment of an amniotic membrane support the presence and
survival of such macrophages (36).
The use of amniotic membrane in ophthalmology has
been extensively reported (18,37,38). The
inhibitory effect on inflammation, proteolysis, angiogenesis and
fibrosis, and the promoting effect on epithelialization following
amniotic membrane transplantation have been well recognized, as
well as the potential advantages over traditional penetrating
keratoplasty in terms of graft rejection. The application of
amniotic membranes for infectious keratitis was reported by Kim
et al in early 2001 (39).
Unlike TPK or LK, there is no risk of rejection following amniotic
membrane transplantation. If the perforation is tiny and, notably,
if there is some residual stroma around the perforation site,
amniotic membrane transplantation, particularly multilayered,
performed at the residual stroma is possible to prevent further
leakage of aqueous humor.
With regard to EKCAI, amniotic membrane is extremely
valuable for the management of fungal keratitis associated with
poor wound healing and impending perforation. Double amniotic
membrane and an additional layer of fibrin sealant patch
(TachoSil®; Baxter, Deerfield, IL, USA) has been
reported to be useful for the therapy of large perforations that
require urgent treatment (40).
Several case reports have also employed the amniotic membrane to
cover the ocular surface in the acute stages (36,39–41).
This method is aimed at preventing the cicatricial conjunctival and
corneal complications that frequently occur in these patients
(41).
EKCAI, taking the advantage of the combined benefits
of cryotherapy and amniotic membrane, is an effective therapeutic
option for curing recalcitrant fungal keratitis. When compared with
the traditional method TPK in the present study, there was similar
rate of infection control, 93.44% with TPK compared to 88.37% in
EKCAI. This appeared to be better than EKCFI which had a
therapeutic success rate in this study of 58.33%. Additionally,
although TPK resulted in a superior postoperative vision at 1 year,
and less corneal neovascularization compared with EKCAI, there was
no significant difference in the rates of secondary glaucoma. In
summary, EKCAI with equivalent therapeutic success, should be
considered a valuable alternative in the management of recalcitrant
fungal keratitis, particularly in areas where donor corneal tissue
is limited.
The major limitation of the present study is the
non-randomization of the treatment groups. Due to the limited
availability of donor corneas and even amniotic membranes at the
time of surgeries, conducting a prospective, randomized study would
be problematic. Additionally, corneal ulcers with depth >70%
were preferentially treated with TPK, if donor corneas were
available. Therefore, these patients already had a relatively
severe condition. To overcome this possible bias, professional
statisticians were invited to this study for the analysis of the
data. First, a fairly even distribution of cases was noted in many
of the pre-operative characteristics. Furthermore, analysis was
also conducted with unevenly distributed parameters being adjusted,
such as gender and steroid use, as a cause of infection (Table IV). It appears that the final
outcomes were unaffected, with or without the adjustment.
In conclusion, this retrospective study showed that
TPK is the first choice for fungal keratitis with late presentation
refractory to antifungal drugs, while EKCAI is a better alternative
in progressive cases when donor corneas are not available. Such
cases have poor prognosis, highlighting the fact that early
diagnosis and administration of antifungal treatment is
imperative.
References
|
1
|
Xie L, Dong X and Shi W: Treatment of
fungal keratitis by penetrating keratoplasty. Br J Ophthalmol.
85:1070–1074. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie L, Zhai H, Zhao J, Sun S, Shi W and
Dong X: Antifungal susceptibility for common pathogens of fungal
keratitis in Shandong Province, China. Am J Ophthalmol.
146:260–265. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wong TY, Ng TP, Fong KS and Tan DT: Risk
factors and clinical outcomes between fimgal and bacterial
keratitis: A comparative study. CLAO J. 23:275–281. 1997.PubMed/NCBI
|
|
4
|
Shi WY and Wang T: Several problems of
diagnosis and treatment in fungal keratitis in China. Zhonghua Yan
Ke Za Zhi. 49:2–5. 2013.(In Chinese). PubMed/NCBI
|
|
5
|
Xie L, Zhai H and Shi W: Penetrating
keratoplasty for corneal perforations in fungal keratitis. Cornea.
26:158–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Anshu A, Parthasarathy A, Mehta JS, Htoon
HM and Tan DT: Outcomes of therapeutic deep lamellar keratoplasty
and penetrating keratoplasty for advanced infectious keratitis: A
comparative study. Ophthalmology. 116:615–623. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mandell KJ and Colby KA: Penetrating
keratoplasty for invasive fungal keratitis resulting from a thorn
injury involving Phomopsis species. Cornea. 28:1167–1169. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xie L, Qi F, Gao H, Wang T, Shi W and Zhao
J: Major shifts in corneal transplantation procedures in north
China: 5316 eyes over 12 years. Br J Ophthalmol. 93:1291–1295.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lim LS, Arundhati A and Tan DT: Sequential
therapeutic penetrating keratoplasty with cryopreserved and fresh
corneal tissue for severe infectious keratitis: A case-control
study. Cornea. 30:739–743. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xie L, Zhai H and Shi W: Penetrating
keratoplasty for corneal perforations in fungal keratitis. Cornea.
26:158–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie L, Hu J and Shi W: Treatment failure
after lamellar keratoplasty for fungal keratitis. Ophthalmology.
115:33–36. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sun GH, Li SX, Gao H, Zhang WB, Zhang MA
and Shi WY: Clinical observation of removal of the necrotic corneal
tissue combined with conjunctival flap covering surgery under the
guidance of the AS-OCT in treatment of fungal keratitis. Int J
Ophthalmol. 5:88–91. 2012.PubMed/NCBI
|
|
13
|
Reynolds MG and Alfonso E: Treatment of
infectious scleritis and keratoscleritis. Am J Ophthalmol.
112:543–547. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodriguez-Ares MT, De Rojas Silva MV,
Pereiro M, Sampayo B Fente, Chamas G Gallegos and S-Salorio M:
Aspergillus fumigatus scleritis. Acta Ophthalmol Scand. 73:467–469.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Heiligenhaus A, Li H, Galindo EE
Hernandez, Koch JM, Steuhl KP and Meller D: Management of acute
ulcerative and necrotising herpes simplex and zoster keratitis with
amniotic membrane transplantation. Br J Ophthalmol. 87:1215–1219.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Al-Kharashi S, Al-Khawaja A, Gonnah El-S,
Al-Assiri A, Al-Motowa S, Al-Towerki AE and Wagoner MD: Microbial
keratitis after amniotic membrane transplantation. Int Ophthalmol.
26:73–76. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen JH, Ma DH and Tsai RJ: Amniotic
membrane transplantation for pseudomonal keratitis with impending
perforation. Chang Gung Med J. 25:144–152. 2002.PubMed/NCBI
|
|
18
|
Chen HC, Tan HY, Hsiao CH, Huang SC, Lin
KK and Ma DH: Amniotic membrane transplantation for persistent
corneal ulcers and perforations in acute fungal keratitis. Cornea.
25:564–572. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arroyo JG, Postel EA, Stone T, McCuen BW
and Egan KM: A matched study of primary scleral buckle placement
during repair of posterior segment open globe injuries. Br J
Ophthalmol. 87:75–78. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xie L, Zhong W, Shi W and Sun S: Spectrum
of fungal keratitis in north China. Ophthalmology. 113:1943–1948.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rautaraya B, Sharma S, Kar S, Das S and
Sahu SK: Diagnosis and treatment outcome of mycotic keratitis at
tertiary eye care center in eastern India. BMC Ophthalmol.
11:392011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Fraunfelder FW: Liquid nitrogen
cryotherapy for surface eye disease (an AOS thesis). Trans Am
Ophthalmol Soc. 106:301–324. 2008.PubMed/NCBI
|
|
23
|
Sullivan JH: Cryosurgery in ophthalmic
practice. Ophthalmic Surg. 10:37–41. 1979.PubMed/NCBI
|
|
24
|
Wilkes TD and Fraunfelder FT: Principles
of cryosurgery. Ophthalmic Surg. 10:21–30. 1979.PubMed/NCBI
|
|
25
|
Ebrahimi KB, Green WR, Grebe R and Jun AS:
Acanthamoeba sclerokeratitis. Graefes Arch Clin Exp Ophthalmol.
247:283–286. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Meisler DM, Ludwig IH, Rutherford I, Bican
FE, Langston RH and Visvesvara GS: Susceptibility of acanthamoeba
to cryotherapeutic method. Arch Ophthalmol. 104:130–131. 1986.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Matoba AY, Pare PD, Le TD and Osato MS:
The effects of freezing and antibiotics on the viability of
Acanthamoeba cysts. Arch Ophthalmol. 107:439–440. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Binder PS: Cryotherapy for medically
unresponsive acanthamoeba keratitis. Cornea. 8:106–114. 1989.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Chen Y, Yang W, Gao M, Belin MW, Yu H and
Yu J: Experimental study on cryotherapy for fungal corneal ulcer.
BMC Ophthalmol. 15:292015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Woo HM, Kim MS, Kweon OK, Kim DY, Nam TC
and Kim JH: Effects of amniotic membrane on epithelial wound
healing and stromal remodelling after excimer laser keratectomy in
rabbit cornea. Br J Ophthalmol. 85:345–349. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Dua HS and Azuara-Blanco A: Amniotic
membrane transplantation. Br J Ophthalmol. 83:748–752. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kubo M, Sonoda Y, Muramatsu R and Usui M:
Immunogenicity of human amniotic membrane in experimental
xenotransplantation. Invest Ophthalmol Vis Sci. 42:1539–1546.
2001.PubMed/NCBI
|
|
33
|
Akle CA, Adinolfi M, Welsh KI, Leibowitz S
and McColl I: Immunogenicity of human amniotic epithelial cells
after transplantation into volunteers. Lancet. 2:1003–1005. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tseng SC, Li DQ and Ma X: Suppression of
transforming growth factor-beta isoforms, TGF-beta receptor type
II, and myofibroblast differentiation in cultured human corneal and
limbal fibroblasts by amniotic membrane matrix. J Cell Physiol.
179:325–335. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Meller D, Pires RT and Tseng SC: Ex vivo
preservation and expansion of human limbal epithelial stem cells on
amniotic membrane cultures. Br J Ophthalmol. 86:463–471. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bauer D, Hennig M, Wasmuth S, Baehler H,
Busch M, Steuhl KP, Thanos S and Heiligenhaus A: Amniotic membrane
induces peroxisome proliferator-activated receptor-γ positive
alternatively activated macrophages. Invest Ophthalmol Vis Sci.
53:799–810. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dua HS, Gomes JA, King AJ and Maharajan
VS: The amniotic membrane in ophthalmology. Surv Ophthalmol.
49:51–77. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Uhlig CE, Frings C, Rohloff N,
Harmsen-Aasman C, Schmitz R, Kiesel L, Eter N, Busse H and Alex AF:
Long-term efficacy of glycerine-processed amniotic membrane
transplantation in patients with corneal ulcer. Acta Ophthalmol.
93:e481–e487. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim JS, Kim JC, Hahn TW and Park WC:
Amniotic membrane transplantation in infectious corneal ulcer.
Cornea. 20:720–726. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Grau AE and Durán JA: Theatment of a large
corneal perforation with a multilayer of amniotic membrane and
Tachosil. Cornea. 31:98–100. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Hsu M, Jayaram A, Verner R, Lin A and
Bouchard C: Indications and outcomes of amniotic membrane
transplantation in the management of acute Stevens-Johnson Syndrome
and toxic epidermal necrolysis: A case-control stydy. Cornea.
31:1394–1402. 2012. View Article : Google Scholar : PubMed/NCBI
|