Introduction
Gan-Dan-Liang-Yi-Tang (GDLYT) is a prescription drug
used for the treatment of insomnia and terrified or sleepless, as
described in the Chinese Traditional Medicine book “Bian-Zheng-Lu”.
GDLYT is composed of 3 ingredients: Yuan Zhi (Polygala
tenuifolia), Bai Shao (Paeonia lactiflora Pall.) and
Chao Zao Ren [Ziziphus jujuba Mill. var. spinosa (Bunge) Hu
ex H. F. Chow]. Yuan Zhi has been used to treat insomnia, anxiety,
restlessness and disorientation (1,2).
Polygalasaponins, the primary constituents of Yuan Zhi, are able to
enhance pentobarbital-induced sleeping behaviors via γ-aminobutyric
acid (GABA)-ergic systems in mice (3). Bai Shao, the processed root portion of
Paeonia lactiflora Pall (Ranunculaceae), is a component of
numerous Chinese medicinal formulae prescribed for the treatment of
depression-like syndromes; Bai Shao functions by modifying the
levels of serotonin [5-hydroxytryptamine (5-HT)] and its metabolite
5-hydroxyindoleacetic acid in the hippocampus (4–6). Chao
Zao Ren has been reported to be a component of various Chinese
Medicinal Herbs (7). Chao Zao Ren is
able to improve sleep quality in patients and inhibit motion
sickness effectively by reducing blood hormones levels in the
hypothalamic-pituitary-adrenocortical axis (7–9).
Insomnia is a sleep disorder prevalent in women and
the elderly (10). Currently, drugs
used for the treatment of insomnia predominantly target the
γ-aminobutyric acid (GABA) receptor, melatonin receptor, histamine
receptor, orexin and serotonin receptor (10). Herbal medicine and Complementary and
Alternative Medicine, such as Piper methysticum and the seed of
Ziziphus jujuba Mill var. spinosa, have been widely used in
phytotherapy for insomnia (11).
GDLYT has been used for thousands of years as a Traditional Chinese
Medicine; however, few studies have been conducted on its potential
therapeutic effects in animal models. The present study
investigated the sedative and hypnotic activities of GDLYT and
explored the mechanisms underlying the sedative and hypnotic
effects of GDLYT in various mouse models of insomnia. These effects
were also investigated by analyzing monoamine neurotransmitters and
inflammatory cytokines in the mouse models.
Materials and methods
Extraction of GDLYT
Plant materials were purchased from Beijing Tong Ren
Tang Medicinal Materials Co., Ltd. (Beijing, China) in October
2012. Shade-dried Yuan Zhi, Chao Zao Ren and Bai Shao were
extracted 3 times with H2O for 1 h using a circumfluence
extraction method, mixed with 50% EtOH, and the extracts were then
filtered prior to the removal of EtOH insoluble parts. The soluble
part was concentrated using a rotary vacuum evaporator. The residue
was stored for 1 week at room temperature to obtain a dry solid
mass.
Animals and ethical approval
A total of 420 Kunming mice weighing 20–24 g and 56
adult male Sprague-Dawley rats weighing 180–200 g were used for the
behavioral experiments. All animals were obtained from the Animal
Breeding Center of the PLA General Hospital (Beijing, China). The
animals were housed in cages (45×60×25 cm) with water and food
available ad libitum at a constant temperature (22±2°C),
under an 12-h light/dark cycle (lights on at 7:00). All animal
experiments were carried out in accordance with the Principles of
Laboratory Animal Care, the China legislation for the use and care
of laboratory animals and the Institutional Animal Care and Use
Committee of General Hospital of Chinese PLA.
Sub-hypnotic dosage of sodium
pentobarbital treatment
Animals in the control and model groups were
administered distilled water, while the drug treatment groups were
administered sodium pentobarbital (1.3 mg/kg/day), and the animals
in experimental groups were administered GDLYT at doses of 0.65,
1.3, 2.6 and 5.2 g/kg/day. Drugs were administered orally once
daily for various durations, sodium pentobarbital i.p. (25 mg/kg)
was carried on after 50 min of the last administration, the onset
of sleep was observed in each mouse. Mice were considered to be
asleep when they lost righting reflex for >1 min. In the
sub-hypnotic dosage of sodium pentobarbital treatment test
(12), the percentage of sleep onset
was calculated as follows: Sleep onset (%) = (No. falling
asleep/total no.) × 100.
Anticonvulsant experiments
The modified methods previously outlined by Chindo
et al (13) and Ngo Bum et
al (14) were used to estimate
the anticonvulsant effect of GDLYT in mice. Briefly, the mice
acclimatized to their new environment prior to the start of each
experiment, and were kept in individual transparent mouse cages
(25×15×15 cm) for 30 min. Pentetrazole (PTZ; 100 mg/kg) was used to
induce convulsions (seizures) in the mice. Time latency between the
administration of PTZ and myoclonic convulsive behavior was scored
as follows: 0, no response; 1, ear and facial twitching; 2, axial
convulsive waves observed through the body; 3, body jerks; 4,
generalized clonic convulsions with turning over into side
position; 5, generalized convulsions with tonic extension episodes
and status epilepticus; 6, mortality (15). Following an additional 30 min, the
lethality of treatment on the mice was recorded.
Parachlorophenylalanine (PCPA)
pretreated model
For the PCPA pretreatment test, rats received
intraperitoneal injection of PCPA (300 mg/kg) between 08:00 and
09:00 once a day for 2 days. At 2 days after PCPA injection, GDLYT
at various doses were administered for 7 days, observers were
blinded to the treatment. Following PCPA injection, each mouse was
observed and total movement distance was recorded, and rats were
subsequently anesthetized by chloral hydrate (350 mg/kg) and
sacrificed. The hippocampus, piriform cortex, hypothalamus, corpus
striatum and brain stem were dissected from the brain on ice and
the tissue samples were immediately immersed in liquid nitrogen and
stored until further use.
Determination of 5-HT, NE and DA
levels by high-performance liquid chromatography (HPLC)
The level of 5-HT, NE and DA were determined by
reverse-phase HPLC with electrochemical detection, as previously
described (16). The 5-HT, DA and NE
levels were determined as mg/g wet weight tissue. Identification
(by retention times) and content of the compounds (by peak areas)
was determined in the tissue samples by comparing 0.2–4 ng/ml 5-HT,
NE and DA standard solutions.
Serum cytokine measurements
Serum tumor necrosis factor (TNF)-α, interleukin
(IL)-1β, IL-2, IL-4 and IL-6 concentrations were quantified by
specific rat ELISA sandwich assays, performed using antibodies
(R&D Systems China Co. Ltd., Shanghai, China) according to the
manufacturer's protocol. Absorbance was read at 450 nm on a
microtitre plate reader (PerkinElmer, Inc., Waltham, MA, USA) and
results were presented as ng/l of serum.
Statistical analysis
The results are presented as means ± standard error
of the mean, indicating the number of animals per group for each
experiment. Data were analyzed by one-way analysis of variance
followed by Dunnett's post-hoc test for multiple comparisons. In a
sub-hypnotic dosage of sodium pentobarbital treatment test, a
χ2 test was used to compare number of mice that fell
asleep. P<0.05 was considered to indicate a statistically
significant result.
Results
GDLYT induces hypnotic and sedative
effects in mice
On the subhypnotic dosage of pentobarbital-treated
mice, GDLYT raised the rate of sleep onset in 5 and 7 days with
significant effects at 1.3 and 0.65 mg/kg (P<0.01; Table I). Following the PTZ-induced seizures
experiment, GDLYT was observed to prolong the latency of
convulsions in the treated groups after 5 and 7 days, as compared
with the control group at 1.3 mg/kg doses and also protect against
the mice mortality in 7 days (P<0.05; Table II).
 | Table I.Effect of GDLYT on the sleep onset of
mice treated with a sub-hypnotic dose of sodium pentobarbital
(n=10–14). |
Table I.
Effect of GDLYT on the sleep onset of
mice treated with a sub-hypnotic dose of sodium pentobarbital
(n=10–14).
|
|
| 3 days | 5 days | 7 days |
|---|
|
|
|
|
|
|
|---|
| Group | Dosage (g/kg) | No. of mice asleep
(/10) | Sleep onset (%) | No. of mice asleep
(/10) | Sleep onset (%) | No. of mice
asleep/total | Sleep onset (%) |
|---|
| Control | – | 1 | 10 | 1 | 10 | 1/12 | 8 |
| Diazepam | 0.0013 | 9 | 90a | 9 | 90a | 9/13 | 69a |
| GDLYT | 5.2 | 3 | 30 | 4 | 40 | 4/13 | 31 |
|
| 2.6 | 5 | 50 | 5 | 50 | 5/13 | 31 |
|
| 1.3 | 5 | 50 | 6 | 60b | 7/11 | 39 |
|
| 0.65 | 4 | 40 | 4 | 40 | 4/14 | 64a |
 | Table II.Effect of GDLYT on
pentetrazole-induced convulsions and mortality in mice (n=10). |
Table II.
Effect of GDLYT on
pentetrazole-induced convulsions and mortality in mice (n=10).
|
|
| 3 days (mouse
number) | 5 days (mouse
number) | 7 days (mouse
number) |
|---|
|
|
|
|
|
|
|---|
| Group | Dosage (g/kg) | Latency (sec) | Mortality (%) | Latency (sec) | Mortality (%) | Latency (sec) | Mortality (%) |
|---|
| Control | – |
57.2±20.2 | 90 |
59.7±17.8 | 90 | 54.5±13.3 | 91 |
| Diazepam | 0.0013 |
128.0±34.7a |
30b |
129.0±61.3a | 10b |
80±15.4a |
16b |
| GDLYT | 5.2 |
68.6±22.1 | 80 |
66.0±13.6 | 80 |
57±13.2 | 58 |
|
| 2.6 | 53.2±9.6 | 80 |
131.1±115.8 | 70 |
56±6.5 | 58 |
|
| 1.3 |
55.7±11.3 | 60 |
164.6±108.9a | 50 |
89±16.0a |
36a |
|
| 0.65 |
57.3±13.7 | 70 |
144.9±123.9 | 90 |
57±10.1 | 75 |
Effect of GDLYT on PCPA-induced
insomnia in pentobarbital-treated mice
Consistent with the results of our previous
unpublished study, the results of the present study indicated that
treatment with PCPA (300 mg/kg) successfully induced insomnia.
GDLYT significantly reversed the insomnia effects in PCPA-treated
mice, by decreasing the total movement distance on days 2 and 7
(P<0.05; Table III) compared
with the PCPA mice.
 | Table III.Effect of GDLYT on movement in
PCPA-treated rats (n=8). |
Table III.
Effect of GDLYT on movement in
PCPA-treated rats (n=8).
|
|
| Total movement
distance (m) |
|---|
|
|
|
|
|---|
| Group | Dosage (g/kg) | 2-day PCPA
treatment | 7-day GDLYT
treatment |
|---|
| Control | – | 16.786±5.981 | 12.915±2.649 |
| PCPA | 0.3 |
27.348±10.235a |
21.791±7.453b |
| Diazepam | 0.001 |
25.180±7.002a |
12.694±1.344a |
| GDLYT | 3.7 |
24.076±7.262a |
9.445±3.296c |
|
| 1.85 |
27.421±9.515a |
9.069±2.846c |
|
| 0.925 |
23.481±5.989a |
7.648±2.606c |
Effect of GDLYT on monoaminergic
neurotransmitter and cytokines in rats
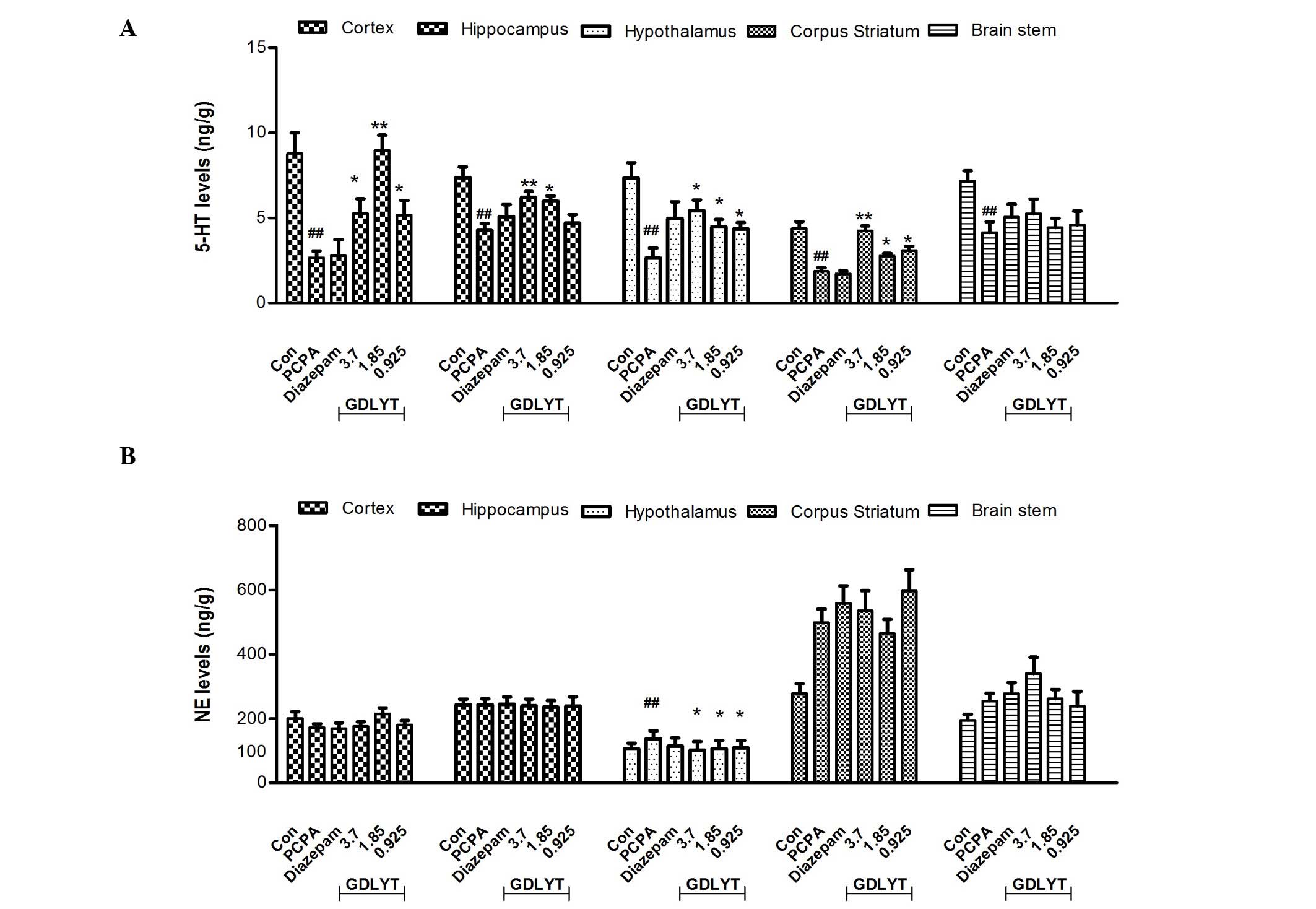
As shown in Fig. 1A,
after 7 days treatment, PCPA treatment induced a significant
decrease in 5-HT levels (P<0.05) in several areas of the brain,
including the prefrontal cortex, hippocampus, hypothalamus, corpus
striatum and brain stem, whereas it only induced an increase in NE
levels in the hypothalamus (Fig.
1B). PCPA did not have any effect on DA levels in the brain
(data has not shown). GDLYT was able to significantly reverse the
effects of PCPA after 7 days administration in the prefrontal
cortex, hippocampus, hypothalamus and corpus striatum (P<0.05),
as compared with the PCPA group (Fig.
1A). Furthermore, GDLYT decreased NE levels in the hypothalamus
(Fig. 1B) and there were no
significant changes in DA levels (data not shown). In addition, a
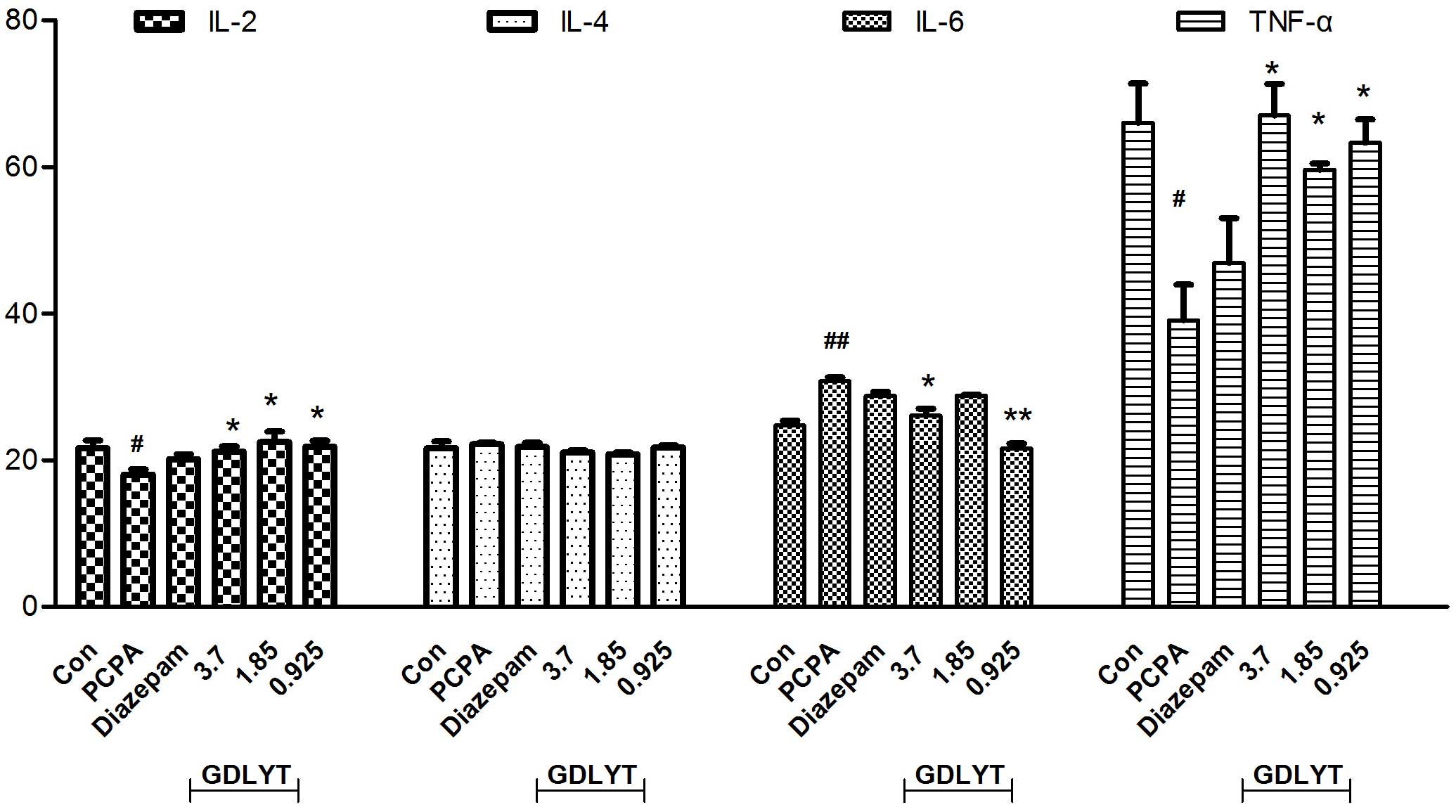
decrease in TNF-α and IL-2 levels and an increase in IL-6 levels
were observed following treatment with PCPA, although IL-4 levels
were unaffected (Fig. 2). Following
treatment with GDLYT at doses of 3.7, 1.85 and 0.925 g/kg, TNF-α
and IL-2 levels were significantly increased, and those of IL-6
were significantly decreased, as compared with the PCPA group
(P<0.05; Fig. 2).
Discussion
Insomnia is an common phenomenon causing both
physical and mental health impairments (17). However, evidence for treatment
options is limited. Insomnia and anxiety are typically treated with
adjunctive benzodiazepines, which risk abuse and dependency if used
chronically (18). In the current
study, the results demonstrated that a traditional herbal medicine,
GDLYT, exerts hypnotic-sedative and anticonvulsant effects in
mice.
In the brain, endogenous neurotransmitters such as
asdopamine, norepinephrine, acetylcholine, serotonin, GABA,
histamine and neuropeptides have been suggested to have important
roles in sleep mechanisms (19–22).
GDLYT decreased the sleep onset in an animal model treated with a
subhypnotic dosage of pentobarbital, a classic behavioral
pharmacology methods, shown its hypnotic-sedative effect. As PTZ
has been reported to interact with the GABA neurotransmitter
(11,13,23), the
restrain of PTZ-induced seizures suggests that GDLYT possesses
anticonvulsant properties through some of bioactive constituents
adjusts GABA-ergic neurotransmission.
In addition, chronic administration of PCPA, a
serotonin 5-HT synthesis inhibitor, may induce complete insomnia or
substantially reduced sleep (24).
These insomnia effects were resisted by treatment with GDLYT, which
may involve the restoration of serotonin synthesis and thus
restored sleep. Therefore, it was hypothesized that the serotonin
system was hypnogenic, due to the serotonin-sleep connection. A
previous study demonstrated that when serotonin concentration is
decreased or following the destruction of the dorsal raphe nuclei
in the brainstem, which contain the majority of the serotonergic
cell bodies of the brain, sleep is also reduced (25). As reviewed by Dugovic (26), the complex effects of 5-HT in the
adjusting on sleep is in part due to the fact that 5-HT may be
present in different areas of the brain that are involved in the
control of sleep and wakefulness. Consistent with previous reports,
the results of the present study demonstrated that different 5-HT
levels could be observed in various areas of the brain. GDLYT was
able to reverse PCPA-induced 5-HT decrease in several parts of the
brain, including the cortex, hippocampus, striatum and
hypothalamus. Furthermore, GDLYT also modulated NE levels in the
hypothalamus. These results suggested there may be certain
activated components of GDLYT that stimulated the serotonergic
system.
Cytokines have an important role in immune
activation, but are also transported into the central nervous
system, where they influence noradrenergic, dopaminergic and
serotonergic neurotransmission (27). IL-1 and IL-6 were reported to be
associated with psychomotor, sickness behavior and sleep (28,29);
IL-2 and TNF-α partly disturb memory and are involved in cognitive
impairment. The hyper-secretion of IL-2 has been associated with
schizophrenia, and that of IL-6 with depression (30,31). As
observed in the present study, the expression levels of IL-2 and
TNF-α pro-inflammatory cytokines were decreased, and those of IL-6
anti-inflammatory cytokine were increased following treatment with
PCPA inhibitor. Treatment with GDLYT increases the expression
levels of IL-2 and TNF-α, and reduced the serum expression levels
of IL-6. Therefore, another important mechanism underlying the
effect of GDLYT on sleep disorders in animals is by adjusting
cytokine levels so that each can be maintained at normal
ranges.
In conclusion, the results of the present study
revealed that GDLYT may contain anti-psychoactive components with
potential hypnotic, sedative and anticonvulsant properties. The
results also suggested that the serotonergic and immune system may
participated in the hypnotic-sedative activity of GDLYT. Future
studies will be required in order to investigate the mechanism
underlying this hypnotic-sedative effect, and to isolate the active
ingredient from GDLYT.
Acknowledgements
The present study was supported by a grant from the
National Military Chinese Medicine Special Foundation (grant no.
10ZYZ141).
References
|
1
|
Gang-qiang HX Zhang, Dong Xian-Zhe and Liu
Ping: Sedative and Hypnotic Activities of Gandan Liangyi Tang via
the Elevation Effects on Amino Acids Neurotranmitters in Mice.
Zhong Guo Shi Yan Fang Ji Xue Za Zhi. 19:226–229. 2013.(In
Chinese).
|
|
2
|
Yao Y, Jia M, Wu JG, Zhang H, Sun LN, Chen
WS and Rahman K: Anxiolytic and sedative-hypnotic activities of
polygalasaponins from Polygala tenuifolia in mice. Pharm Biol.
48:801–807. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lee CI, Lee MK and Oh KW: Ethanol extract
of Polygalae radix augments pentobarbital-induced sleeping
behaviors through GABA(A)ergic systems. Natural Product Sciences.
19:179–185. 2013.
|
|
4
|
Lee SM, Yoon MY and Park HR: Protective
effects of Paeonia lactiflora pall on hydrogen peroxide-induced
apoptosis in PC12 cells. Biosci Biotechnol Biochem. 72:1272–1277.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mao QQ, Ip SP, Tsai SH and Che CT:
Antidepressant-like effect of peony glycosides in mice. J
Ethnopharmacol. 119:272–275. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang MZ, Zhang QY and Cui GB: Clinical
study of XiaoYao-San in the treatment of depressive neurosis. Shan
Dong Zhong Yi Yao Da Xue Xue Bao. 22:34–37. 1998.(In Chinese).
|
|
7
|
Shen HX, Jiang ZL, Dong GS, Yang HQ, Jiang
R, Li X, Yin P and Chen MM: Anti-motion sickness efficacy of the
extracted mixture of Chinese medical herbs and its influence on the
blood level of hormones. Zhongguo Ying Yong Sheng Li Xue Za Zhi.
28:398–403. 2012.(In Chinese). PubMed/NCBI
|
|
8
|
TingLi WWSWL: The antidepression effect of
ChaoZaiRen. Zhong Yi Yao Xue Bao. 42:13–15. 2014.(In Chinese).
|
|
9
|
Xing-li H: Peng's gouqizaoren decoction
intractable insomnia treatment. Shi Yong Zhong Yi Nei Ke Za Zhi.
6:58–59. 2010.(In Chinese).
|
|
10
|
Shi Y, Dong JW, Zhao JH, Tang LN and Zhang
JJ: Herbal insomnia medications that target GABAergic systems: A
review of the psychopharmacological evidence. Curr Neuropharmacol.
12:289–302. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cao JX, Zhang QY, Cui SY, Cui XY, Zhang J,
Zhang YH, Bai YJ and Zhao YY: Hypnotic effect of jujubosides from
Semen Ziziphi Spinosae. J Ethnopharmacol. 130:163–166. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao X, Cui XY, Chen BQ, Chu QP, Yao HY,
Ku BS and Zhang YH: Tetrandrine, a bisbenzylisoquinoline alkaloid
from Chinese herb radix, augmented the hypnotic effect of
pentobarbital through serotonergic system. Eur J Pharmacol.
506:101–105. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chindo BA, Ya'u J, Danjuma NM, Okhale SE,
Gamaniel KS and Becker A: Behavioral and anticonvulsant effects of
the standardized extract of Ficus platyphylla stem bark. J
Ethnopharmacol. 154:351–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ngo Bum E, Taiwe GS, Moto FC, Ngoupaye GT,
Nkantchoua GC, Pelanken MM, Rakotonirina SV and Rakotonirina A:
Anticonvulsant, anxiolytic and sedative properties of the roots of
Nauclea latifolia smith in mice. Epilepsy Behav. 15:434–440. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malhi SM, Jawed H, Hanif F, Ashraf N,
Zubair F, Siddiqui BS, Begum S, Kabir N and Simjee S: Modulation of
c-Fos and BDNF protein expression in pentylenetetrazole-kindled
mice following the treatment with novel antiepileptic compound
HHL-6. Biomed Res Int. 2014:8767122014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu Y, Liu P, Dai-Hong G, Rahman K, Wang
DX, Chen ML and Xie TT: Behavioral and biochemical effects of
Kaixin-San, a traditional Chinese medicinal empirical formula. Drug
Dev Res. 69:267–271. 2008. View Article : Google Scholar
|
|
17
|
Putnins SI, Griffin ML, Fitzmaurice GM,
Dodd DR and Weiss RD: Poor sleep at baseline predicts worse mood
outcomes in patients with co-occurring bipolar disorder and
substance dependence. J Clin Psychiatry. 73:703–708. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uzun S, Kozumplik O, Jakovljević M and
Sedić B: Side effects of treatment with benzodiazepines.
Psychiatria Danubina. 22:90–93. 2010.PubMed/NCBI
|
|
19
|
Karmann AJ, Kundermann B and Lautenbacher
S: Sleep deprivation and pain: A review of the newest literature.
Schmerz. 28:141–146. 2014.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Monti JM, BaHammam AS, Pandi-Perumal SR,
Bromundt V, Spence DW, Cardinali DP and Brown GM: Sleep and
circadian rhythm dysregulation in schizophrenia. Prog
Neuropsychopharmacol Biol Psychiatry. 43:209–216. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Roecker AJ, Mercer SP, Schreier JD, Cox
CD, Fraley ME, Steen JT, Lemaire W, Bruno JG, Harrell CM, Garson
SL, et al: Discovery of
5′-chloro-N-[(5,6-dimethoxypyridin-2-yl)methyl]-2,2′:5′,3′-terpyridine-3′-carboxamide
(MK-1064): A selective orexin 2 receptor antagonist (2-SORA) for
the treatment of insomnia. Chem Med Chem. 9:311–322. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
van Swinderen B and Kottler B: Explaining
general anesthesia: A two-step hypothesis linking sleep circuits
and the synaptic release machinery. Bioessays. 36:372–381. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Salih MA and Mustafa AA: A substance in
broad beans (Vicia faba) is protective against experimentally
induced convulsions in mice. Epilepsy Behav. 12:25–29. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Borbély AA, Neuhaus HU and Tobler I:
Effect of p-chlorophenylalanine and tryptophan on sleep, EEG and
motor activity in the rat. Behav Brain Res. 2:1–22. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gianotti M, Botta M, Brough S, Carletti R,
Castiglioni E, Corti C, Dal-Cin M, Fratte S Delle, Korajac D,
Lovric M, et al: Novel spirotetracyclic zwitterionic dual
H-1/5-HT2A receptor antagonists for the treatment of sleep
disorders. J Med Chem. 53:7778–7795. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dugovic C: Role of serotonin in sleep
mechanisms. Rev Neurol (Paris). 157:S16–S19. 2001.PubMed/NCBI
|
|
27
|
Ruiz FS, Andersen ML, Martins RC, Zager A,
Lopes JD and Tufik S: Immune alterations after selective rapid eye
movement or total sleep deprivation in healthy male volunteers.
Innate Immunity. 18:44–54. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Q, Peng C, Wu X, Chen Y, Wang C and
You Z: Maternal sleep deprivation inhibits hippocampal neurogenesis
associated with inflammatory response in young offspring rats.
Neurobiol Dis. 68:57–65. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Müller N: Role of the cytokine network in
the CNS and psychic disorders. Nervenarzt. 68:11–20. 1997.(In
German). PubMed/NCBI
|
|
30
|
Patterson ZR and Holahan MR: Understanding
the neuroinflammatory response following concussion to develop
treatment strategies. Front Cell Neurosci. 6:582012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Torabi-Nami M, Nasehi M and Zarrindast
M-R: Sleep loss and the brain vulnerability to neurodegeneration:
Behavioral, biochemical and neuro-histopathological observations in
a rat model. Excli Journal. 12:347–372. 2013.PubMed/NCBI
|